Establishing Mouse Models for Zika Virus-induced Neurological Disorders Using Intracerebral Injection Strategies: Embryonic, Neonatal, and Adult
Summary
Here we describe a method for establishing a model of Zika virus-induced microcephaly in mouse. This protocol includes methods for embryonic, neonatal, and adult-stage intracerebral inoculation of the Zika virus.
Abstract
The Zika virus (ZIKV) is a flavivirus currently endemic in North, Central, and South America. It is now established that the ZIKV can cause microcephaly and additional brain abnormalities. However, the mechanism underlying the pathogenesis of ZIKV in the developing brain remains unclear. Intracerebral surgical methods are frequently used in neuroscience research to address questions about both normal and abnormal brain development and brain function. This protocol utilizes classical surgical techniques and describes methods that allow one to model ZIKV-associated human neurological disease in the mouse nervous system. While direct brain inoculation does not model the normal mode of virus transmission, the method allows investigators to ask targeted questions concerning the consequence after ZIKV infection of the developing brain. This protocol describes embryonic, neonatal, and adult stages of intraventricular inoculation of ZIKV. Once mastered, this method can become a straightforward and reproducible technique that only takes a few hours to perform.
Introduction
Microcephaly is a condition resulting from defective brain development characterized by smaller than average head size in newborns. Children with microcephaly exhibit a range of symptoms which can include developmental delay, seizure, intellectual disability, hearing loss, vision problems, and problems with movement and balance, among others, depending on the severity of the disease and cause1,2,3. This condition is multifactorial in nature, with genetic, infectious agent, and environmental factors linked to causing microcephaly4,5,6,7,8,9. Prior to the 2015 – 2016 ZIKV outbreak, 8 children out of 10,000 births were diagnosed with microcephaly in the United States according to the CDC10. On February 1st of 2016 the World Health Organization declared the Zika virus a Public Health Emergency of International Concern due to the alarming increase in microcephaly diagnoses associated with ZIKV infection in mothers11,12. A recent study from the CDC on ZIKV cases in the United States suggests that maternal ZIKV infection results in a 20-fold increased risk for a child to develop microcephaly compared to uninfected individuals, and 4% of ZIKV infected mothers from the USA have resulted in children with microcephaly11. The rate of microcephaly-associated birth defects during pregnancy from ZIKV infection in Brazil have been reported to have affected up to 17% of babies in infected mothers, indicating that other factors in Latin America may be contributing to the increased risk13. While we know that the ZIKV can cause microcephaly and pathogenesis in the neural progenitor cell (NPC) population7,8,14, the complete pathogenesis of ZIKV in the developing brain remains elusive. It is important to develop animal models to further investigate the disease mechanisms underlying the brain abnormalities associated with ZIKV infection.
To directly study the effect that the ZIKV has on brain development, we first developed a mouse model using intracerebral inoculation of embryonic day 14.5 (E14.5) brain with ZIKV7. This stage was chosen as it is considered representative of the end of the first trimester in human gestation14. Pups can survive up to postnatal day 5 (P5) with this embryonic intracerebral injection method (~ 1 µL of 1.7 x 106 tissue culture infective dose (TCID50/mL)). These postnatal pups exhibit a range of phenotypes similarly observed in infected human infants including enlarged ventricles, neuronal loss, axonal rarefaction, astrogliosis, and microglial activation12,15. A newborn mouse brain is relatively immature, akin to the developmental stage of the human brain at mid-gestation16, and mouse brain development includes a major postnatal component. To study later gestation stage infections, a method for postnatal infection is also described. Neonates infected with ZIKV at P1 are able to survive up to 13 days post-injection. Blood-born adult stage infection has been described in the mouse previously17 but requires the use of the interferon (IFN) regulatory factor (IRF) transcription factors IRF-3, -5, -7 triple knockout strain. This protocol describes a method of inoculating ZIKV intraventricularly to circumvent disabling the antiviral response of the murine model in the adult. While this circumvents the murine immune system, this route of injection does not directly mimic the typical route of infection. To address this discrepancy directly, the experimenter can perform an intrauterine infection of ZIKV instead of the intracranial route. Adopted from previous work18, we have briefly described this technique in this embryonic infection protocol.
The Zika virus strains implemented with this technique include the Mexican isolate MEX1-447,19 and the African isolate MR-766 isolated in 194720. Zika MEX1-44 was isolated in Chiapas, Mexico in January of 2016 from an infected Aedes aegypti mosquito. We obtained this virus with permission through the University of Texas Medical Branch at Galveston (UTMB). In addition, the Dengue virus serotype 2 (DENV2) was inoculated using this technique in a comparison study. DENV2, strain S16803 (sequence GenBank GU289914), was isolated from a patient sample from Thailand in 1974 and passaged in C6/36 cells. The virus was passaged twice in Vero cells by the World Reference Center for Emerging Viruses and Arboviruses (WRCEVA) before mouse injections. This demonstrates that this technique works equally well for diverse strains of ZIKV and other flaviviruses that may have an impact on brain development.
Protocol
All animal use protocols follow the animal care guidelines of the University of Southern California and the University of Georgia. Euthanasia methods for pregnant dams and adults are performed according to approved protocols: carbon dioxide asphyxiation, followed by cervical dislocation as a secondary method to ensure euthanasia. Neonatal pups are euthanized by decapitation.
Caution: The following protocol involves handling a pathogenic virus. Proper precaution should be taken while handling the virus. All protocols must be approved by the appropriate institutional committee prior to use.
1. Embryonic Intracerebral Inoculation of Zika Virus
- For timed pregnant mating, consider noon of the day after mating as E0.5. Pair mice at the end of the day and check plugs in the early morning to reduce variability of mating time.
- Prepare the glass needle prior to surgery. Pull the needle and cut at 1/3 its length, then sharpen at a 45° angle using a micropipette grinder with a water drip until the pore is sized to 50 – 70 µm. Check needle tips under a stereoscope for quality and breakage.
- Keep the virus aliquot on ice. Just prior to surgery, load the ZIKV injection mix into the prepared glass injection needle. Assemble the gas-tight injection syringe (50 µL volume), luer-lock attachment, and tubing, and saturate the assembled syringe with the tubing with mineral oil. Once saturated, attach the needle, and draw ~ 6 – 7 µL ZIKV into the needle (~ 1 µL is 1.7 × 106 TCID50/mL).
- Inject pregnant C57BL/6J or 129S1/SvImJ mice with embryonic day E14.5 embryos (for intracranial injection) or E10.5 embryos (for intrauterine injection) with ketamine hydrochloride (80-100 mg/kg) and xylazine (5-10 mg/kg) diluted in sterile saline solution intraperitoneally to induce anesthesia in the mother. Alternatively, anesthetize mothers with monitored isoflurane isoflurane provided by inhalation, with waste gas scavenging, if appropriate. Inject buprenorphine-SR (0.5-1.0mg/kg) subcutaneously to induce analgesia.
- Pinch test anesthetized mothers on the toe tip or tail and then lay them supine on an animal-approved heating pad (thermal pad of 100 x 200 2.5 mm). The toe pinch forceps are not sterile and are not used to handle tissue in surgery. Alternatively, gloved hands may be used to test the toe pinch withdrawal reflex.
NOTE: Refer to your institutional veterinary staff for the preferred perioperative heating method. A cover was placed between this heating pad and the animal. - Add ophthalmic ointment to the eyes.
- Shave the surface of the abdomen and sterilize with iodine and alcohol three times. Alternate the iodine and alcohol wipes; wipe in a circular motion away from the surgical site.
- Drape the skin surrounding the surgical site with sterile cloths to avoid contamination of the incision and the instruments.
NOTE: The drape opening should not be larger than the prepared surgical site; no hair should be seen in the drape opening. - Pinch the mother's skin away from her abdomen with forceps and cut the lower abdomen in a 1 – 1.5 cm line at the medial sagittal line with sterile surgical scissors. This cuts into the skin and further into the abdominal cavity so that the embryos are now exposed.
NOTE: Surgical scissors are used to incise the skin and peritoneal layer. Sterile instruments and gloves are used for each surgery. - Cut a slit in the middle of a small sterile gauze and apply on top of the surgical opening. Hydrate with sterile saline. Pull the embryos through the slit to rest on the sterile gauze, with care to not remove more than 4 – 5 embryos focusing on one uterine horn at one time.
- Hydrate the embryos with sterile saline prior to and throughout the inoculation to ensure they do not dry out. Keep track of which embryos are injected and their location within the uterine horn. Individual embryos are thus treated as separate experiments, as embryos do not change position while developing in utero. (Continue to step 1.15 for intrauterine injection).
- Gently place a spatula under the head of the embryo. Illuminate the embryos well with a lamp to visualize the head and skull sutures.
NOTE: Aseptic technique is followed, including sterile surgical gloves and instruments. After non-sterile items (such as the lamp) are manipulated, gloves should be replaced with new sterile gloves prior to handling sterile instruments and areas. - Position the head by manipulating the embryo with both the lamp (behind the head for visibility) and free fingers until the head of the embryo is pushed up directly against the uterine wall and held in place with the non-dominant hand.
NOTE: Positioning the embryo is a critical part and the technique that takes the most practice. Too much finger pressure can damage the embryonic membranes leading to lethality and not enough pressure can make the injection difficult. - Use the blood vessels running along the skull suture as a guide. Inject ZIKV virus (~ 1 µL, 1.7 × 106 TCID50/mL Mexican isolate MEX1-44) into the lateral ventricles of E14.5 embryo brains with the assembled syringe and needle. Use control media as a sham injection.
- To improve retention of the pregnancy, avoid viral injection of the two embryos next to the ovaries and the two embryos next to the upper vagina (Figure 1A). Overall, no more than 6 embryos are injected per litter to reduce the time of the surgery and prevent embryo loss.
- Place the injected embryos back into the pregnant dams, and fill the abdominal cavity with ~ 0.5 mL sterile saline. To close, first suture both the abdominal peritoneal muscle and then secondly suture the external skin layer with 4.0 sterile sutures. It is preferable to use interrupted sutures; there is possibility of wound dehiscence if the mouse chews the suture.
- Place the mouse in a cage partially resting on a heating pad to allow the mouse to escape to a non-heated area if needed. Monitor the mothers while they recover (1 – 2 h) from anesthesia. Develop the embryos after surgery for varied times according to individual experiments.
- Intrauterine injection
- With one uterine horn and embryos exposed, use a 1 mL syringe and 27G needle to inject 100 μL Zika virus (106 TCID50 units suspended in 100 μL DMEM), or control medium, into the intrauterine space or into the placental tissue. Note which embryos have been injected for embryonic-stage dissections. See previously published work for more details18.
- Place injected embryos back into the pregnant dams, and fill the abdominal cavity with ~ 0.5 mL sterile saline. To close, first suture the abdominal peritoneal muscle and then secondly suture the external skin layer with 4.0 sterile sutures. It is preferable to use interrupted sutures; there is possibility of wound dehiscence if the mouse chews the suture.
- Place the mouse in a cage partially resting on a heating pad to allow the mouse to escape to a non-heated area if needed. Monitor the mothers while they recover (1 – 2 h) from anesthesia. Develop the embryos after surgery for varied times according to individual experiments.
2. Neonatal Intracerebral Inoculation of Zika Virus: P0/P1
- Ensure that the gas-tight injection syringes (10 µL volume) and needles are not clogged. Clean and test by loading three 20 mL disposable syringes with 26-gauge needles: one with saline, one with 70% ethanol, and one with air to clear the solutions. Sterilize with 10% bleach if previously used for virus injection.
- Keep virus on ice. Load the syringe by filling it with saline to reduce dead volume in the syringe, and draw 0.75 µL of air to separate the saline solution from the injection material.
- Set up a warmed humidified recovery chamber for injected pups. Using a heating block, place a closeable container (such as an empty tip box) with a sterile gauze and humidify with saline. Preheat it prior to starting injections.
- Set up the microinjector including the pump and the controller. Determine the device type code for the specific syringe (i.e., for a 10 µL syringe, the device type is "D"). Determine the speed of injection.
- Collect the postnatal day 0 or 1 pups (P0/P1) near the surgical setup. Load the injection syringe with virus. Disinfect the pup head mount with 70% EtOH.
- Wrap the pups in a thin layer of gauze and bury them completely in ice to achieve an anesthetic state. Pups are cryoanesthetized for 5 min. Ensure that the pups are sufficiently anesthetized for injection by performing a tail/toe pinch without response. This roughly yields 15 min of an anesthetic state.
- Place the pup on head mount, sterilize the surface of the head with iodine and, ethanol (three times) and then wipe dry with a sterile wipe (isopropanol or 70% EtOH).
- Mark lambda with a black marker and record the coordinates at lambda using the stereotaxic instrument. Measure the distance from lambda to the edge of the eye and beginning from the stereotaxic coordinates of lambda calculate the 2/5 distance from lambda to the eye (for both hemispheres if you are injecting both).
- Re-check the anesthetic depth prior to creating a hole for the injection site. Use a 26-gauge needle to carefully and superficially puncture the scalp and skull at the injection location (the skull is very soft in neonates) to create an opening for the injection needle.
- Lower the injection needle into the punctured location, and once the needle has just breached the skin, calculate a 1 mm depth for the injection site (lateral ventricle) using the stereotaxic instrument.
- Once the needle is lowered to the injection depth, program the microinjector to inject: 1 µL of virus, at a rate of 10 nL/s, injecting approximately 1 µL over 1.5 min (~ 1 μl 3.4 x 105 TCID50/mL ZIKV).
- When the injection finishes, wait 30 s, and retract the needle in 0.5 mm increments by rotating the screw (dorsally), waiting 30 s after each increment.
NOTE: This reduces leakage of injected virus. - Once removed, either quickly load the needle and syringe with more virus (different viruses or mock controls should be loaded into separate syringes/needles) or remove the pup from the head mount to the pre-warmed humidified chamber. Monitor the pup every few minutes to gauge recovery. Replace the pups with its litter.
3. Adult Intracerebral Inoculation of Zika Virus
- Inject adult mice intraperitoneally with ketamine hydrochloride (80-100 mg/kg) and xylazine (5-10 mg/kg) diluted in sterile saline to induce anesthesia. Inject buprenorphine-SR (0.5-1.0mg/kg) subcutaneously to induce analgesia. Toe/tail pinch the mouse to ensure its anesthetic state, and subsequently mount it on the stereotaxic instrument (with a heating pad) to immobilize the head during surgery.
- Add ophthalmic ointment to the eyes to prevent the eyes of the mouse from drying out during surgery. Shave the scalp starting behind the eyes to the beginning of the ears. Sterilize the exposed skin with iodine and 70% EtOH three times. Alternate the iodine and alcohol wipes; wipe in a circular motion away from the surgical site.
- Re-check the anesthetic depth prior to incising the skin. Using a scalpel, make a 0.5 cm incision along the medial sagittal line of the head in the sterilized location, exposing bregma. Using a rolling motion with a cotton-tipped applicator, clear the surface of the skull of meningeal tissues, while simultaneously pushing the skin away from the injection sites.
- Starting from bregma, align the surgical drill bit and identify the injection sites using the following coordinates: AP -0.5 mm, ML: +/-1.5 mm. Using the control foot pedal, drill into skull slowly as to only drill bone until a hole has been cleared for the needle.
- Replace the drill with the microinjector pump and gas-tight syringe (10 µL volume) on the stereotaxic. Leaving a small air bubble between the saline solution and the virus, draw ~ 4-5 µL of virus. Determine the device type code for the specific syringe volume (ie, D for 10 μL volume syringe). Lower the needle to DV: -1.5 mm from the brain surface. To inject 1 µL of virus, program a rate of 10 nL/s, injecting approximately 1 µL over 1.5 min.
- When the injection finishes, wait 30 s, and remove the needle in 0.5 mm increments, waiting 30 s after each turn. This reduces backflow and leakage of the injected virus.
- When the injection(s) are finished, use forceps to loosen the skin and recover the exposed skull. Suture the scalp back together using 4.0 sutures and remove the mouse from the stereotaxic instrument. Place the mouse in a cage partially resting on a heating pad to allow the mouse to escape to a non-heated area if needed and monitor while they recover (1 – 2 h) from anesthesia.
Representative Results
Representative images of our injection methods for the ZIKV inoculation of embryonic brain are shown in diagrams depicting intracerebral injections (Figure 1A) and intrauterine and intraplacental injections (Figure 1B), illustrating the way the pregnant dam and embryos should be viewed and oriented for surgery (embryonic inoculation protocol). Figure 2A exhibits ZIKV (MEX1-44) infection (immunostained with the antibody against flavivirus group antigen, green) in the E18.5 cerebral cortex. Pax6 (red) labels NPCs in the developing cortex. ZIKV was inoculated into E14.5 embryonic brains. Using the TCID50 assay from tissue samples7, our growth curve analyses show that the ZIKV can efficiently replicate and grow in the developing brains (Figure 2B, 2C), as published7. Figure 2D shows successful infection with DENV2 in the developing cortex using the embryonic inoculation method. DENV2 is detected using an antibody against flavivirus group antigen (green). Figure 2E demonstrates infection with a different strain of ZIKV, the African lineage (MR-766). Figure 2F demonstrates a representative infection for the alternative-route, intraplacental inoculation of ZIKV-Asia (MEX1-44) at stage E10.5.
Figure 3A is a diagram depicting the landmarks used to identify the location of injection into the lateral ventricles for ZIKV inoculation of the P0/1 pups. Figure 3B shows the ZIKV (MEX1-44) infection at P13 cerebral cortex after P0/1 injection. ZIKV (MEX1-44) is detected using antibody against flavivirus group antigen (red). Figure 4 shows the fluorescent beads (red) that were used to practice injection location, and lateral ventricle injection success in adults.
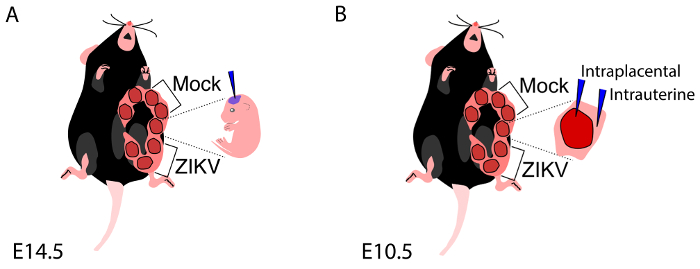
Figure 1: Embryonic inoculation of the ZIKV. (A) Diagram representing exposure of one uterine horn, with a note to avoid injection of embryos adjacent to vagina and the most lateral embryo along the horn. (B) Diagram representing exposure of one uterine horn, depicting the location of intraplacental and intrauterine injections. These diagrams have been modified from their original publication21. Please click here to view a larger version of this figure.
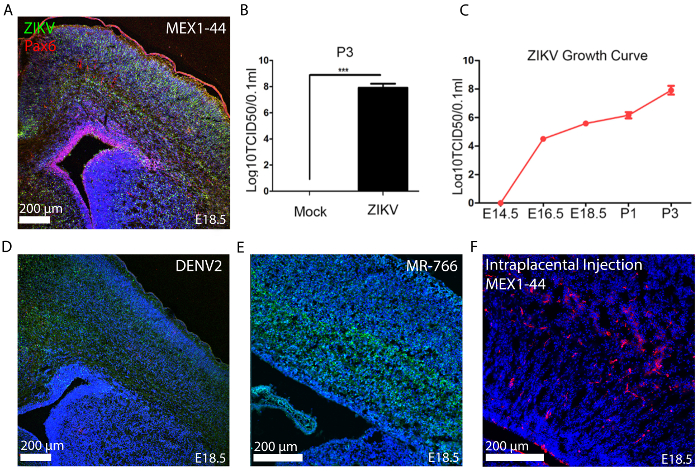
Figure 2: ZIKV Embryonic Inoculation at E14.5. (A) At E18.5 the embryonic brain is infected with ZIKV-Asia (MEX1-44) across all layers of the developing neocortex (scale bar is 200 µm). Neural progenitor cells are immunolabeled with Pax6 (red) and ZIKV via flavivirus antigen (green). (B) TCID50 results from Postnatal Day 3 (P3) brain tissue inoculated at E14.5 with ZIKV-Asia (MEX1-44). Error bars indicate s.e.m. of three independent measurements with one mock and one ZIKV-infected brain in each measurement (***p <0.0001, Student's t-test)7. (C) TCID50 results describing typical increased growth curve of ZIKV viremia in infected fetal brain tissue. Error bars indicate the s.e.m. of three independent measurements with one mock and one ZIKV-Asia (MEX1-44) infected brain in each measurement. Analysis of variance (ANOVA) detects a significant increase in viral titer as development proceeds7. (D) Dengue virus (DENV2) infected representative histology (E14.5 embryos inoculated with ~ 1 µL of 3.4 x 105 TCID50/mL, scale bar is 200 um) and (E) ZIKV-Africa (MR766) infected representative histology (scale bar 200 µm). (F) ZIKV-Asia (MEX1-44) infected representative histology from the intraplacental injection strategy (scale bar is 200 µm). In all images, Hoechst stains nuclei. These figures have been modified from their original publication7. Please click here to view a larger version of this figure.
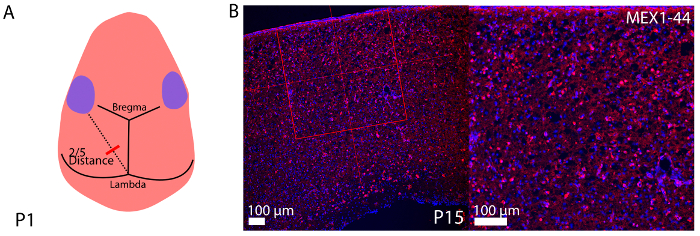
Figure 3: Representative histology of P1 inoculation of ZIKV. (A) Diagram demonstrating the method to determine the injection location into the lateral ventricles of P1 pups. (B) Representative coronal cryosections immunostained for flavivirus group antigen with low (on left, scale bar is 100 µm) and high (on right, scale bar is 50 µm) magnification of P1 inoculation of ZIKV at P15 (~ 1 μl 3.4 x 105 TCID50/mL ZIKV). Hoechst stains nuclei. Please click here to view a larger version of this figure.
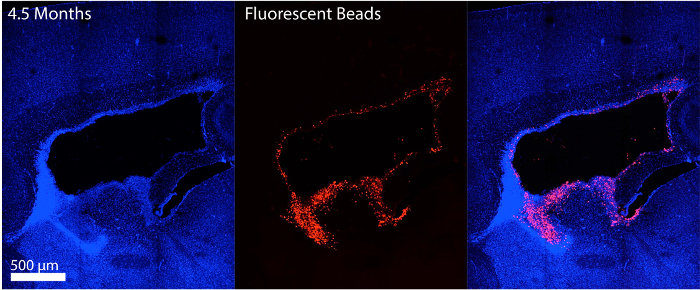
Figure 4: Adult stage intraventricular injection. Adult mouse injected with fluorescent beads was sacrificed shortly after surgery to determine the injection location success (scale bar is 200 µm). Sagittal cryosection could be viewed immediately after sectioning as beads do not require further staining. Hoechst stains nuclei. Please click here to view a larger version of this figure.
Discussion
Described here is a method for intracerebral inoculation of the ZIKV at embryonic, neonatal, and adult stages for the investigation of ZIKV-induced damages in brain development. While straightforward, there are a few considerations that investigators should take to ensure the quality of the study and the safety of those involved.
DENV is closely related to ZIKV in the flavivirus genus. DENV has not been causally linked with pediatric brain disorders in humans. DENV2 can successfully infect and replicate in the developing brain using the embryonic inoculation method (~ 1 µL of 3.4 x 105 TCID50/mL) (Figure 2D). Therefore, in addition to uninfected Vero cell media (mock), DENV infection can be used as a negative control in order to study ZIKV-specific pathogenesis in the developing brain. It is important to avoid virus leakage in utero, which can result in unwanted contamination of other embryos. Therefore, it is necessary to verify infected and non-infected individuals through isolating brain tissue from each embryo followed by qRT-PCR or the TCID50 assay. A dye may be added to the viral medium to visually confirm the injection, but test to ensure that the dye does not cause damage itself.
Mouse strains exhibit growth variability, and therefore P1 injection locations need to be verified for each mouse line. Perform a mock neonate injection with fluorescent beads to visualize the injection site. This preliminary study will inform whether the injection reaches the ventricle or goes too deep into the brain tissue. For ideal results, pups can be sacrificed shortly after injection to identify the location of the fluorescent beads in the ventricle. The entire head of the pup can be fixed overnight, embedded, and cryosectioned to observe the precise injection location. Injection with the oil-saturated syringe requires experience and it is recommended to practice using sham injections with dye onto parafilm to verify that the injection volume is accurate.
Biosafety measures are critical in these studies to avoid accidental infection of laboratory personnel working with the virus. Biosafety approvals must be made in advance at the facility where the work is being completed. ZIKV is considered a Biosafety Level 2 (BSL2) pathogen by the United States Centers for Disease Control and Prevention. All individuals working with the virus or working in areas where the virus is being handled should be aware and familiarized with biosafety protocols for their institution. All tools and surfaces exposed to the ZIKV or DENV2 should be disinfected with 10% bleach to destroy remaining viral particles after use. Women who are pregnant or are attempting to conceive are recommended to not interact with research areas exposed to ZIKV or DENV2. All tissue used for histology are fixed in 4% PFA to inactivate the viruses for analysis. Storing and culturing the virus should be done in Vero cells in a hood designated for BSL2 work. Stock viruses and tissue titration can be quantified using the TCID50 protocol.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Dr. Abdellatif Benraiss at the University of Rochester for his mentorship and discussions relevant to learning the adult surgical and neonate techniques. The authors would also like to acknowledge Dr. James Lauderdale at UGA for the use of his stereotaxic equipment and discussions related to setting up the methodology for this technique, and the Advancement for Research College Scientists (ARCS) foundation for their support and our NIH support (NINDS grants R01NS096176-02, R01NS097231-01, & F99NS105187-01).
Materials
| Flexible Drive Shaft Drill Hanging Motor | Leica | 39416001 | |
| Mouse Stereotax | Kopf | 04557R | |
| Micro4 Microsyringe Pump Controller | WPI | SYS-MICRO4 | |
| UMP3 UltraMicroPump | WPI | UMP3 | |
| Modulamp | Schott | – | |
| Luer-lock tubing (19-gauge) | Hamilton | 90619 | |
| Melting Point Capillary | Kimble | 34500-99 | Glass needle |
| Fluoro-Max: Red Fluorescent Microspheres | Thermo Scientific | R25 | No dilution; Use for practice injections |
| 10 µL, Model 1701 LT SYR | Hamilton | 80001 | for embryonic inoculation |
| 10 µL, Model 1701 RN SYR, Small Removable NDL, 26s ga, 2 in, point style 2 | Hamilton | 80030 | for neonate/adult |
| 4-0 Ethilon Nylon Sutures | Ethicon | – | |
| Mineral Oil | VWR | – | |
| micropipette puller | Sutter Instruments | P-1000 | |
| Micropipette Grinder | Narishige | EG-44 | |
| Fastgreen FCF Dye | Sigma | F7252 | inject with 0.5% Dye |
| Antibodies | |||
| Flavivirus group antigen antibody | Millipore | MAB10216 | ms IgG2a 1:400 (Figure 2, Figure 3) |
| Pax6 | DBHB | Pax6-s | ms IgG1 1:20 |
Riferimenti
- Dreher, A. M., et al. Spectrum of Disease and Outcome in Children with Symptomatic Congenital Cytomegalovirus Infection. J of Pediatr. 164 (4), 855-859 (2014).
- Lanzieri, T. M., et al. Long-term outcomes of children with symptomatic congenital cytomegalovirus disease. J of Perinatol. 37 (7), 875-880 (2017).
- Naseer, M. I., et al. A novel WDR62 mutation causes primary microcephaly in a large consanguineous Saudi family. Ann Saudi Med. 37 (2), 148-153 (2017).
- Abuelo, D. Microcephaly Syndromes. Semin Pediatr Neurol. 14 (3), 118-127 (2007).
- Nicholas, A. K., et al. WDR62 is associated with the spindle pole and is mutated in human microcephaly. Nat Genet. 42 (11), 1010-1014 (2010).
- Pulvers, J. N., et al. Mutations in mouse Aspm (abnormal spindle-like microcephaly associated) cause not only microcephaly but also major defects in the germline. Proc Natl Acad Sci U S A. 107 (38), 16595-16600 (2010).
- Shao, Q., Herrlinger, S., et al. Zika virus infection disrupts neurovascular development and results in postnatal microcephaly with brain damage. Development. 143 (22), 4127-4136 (2016).
- Li, C., et al. Zika Virus Disrupts Neural Progenitor Development and Leads to Microcephaly in Mice. Cell Stem Cell. 19 (5), 672 (2016).
- Miki, T., Fukui, Y., Takeuchi, Y., Itoh, M. A quantitative study of the effects of prenatal X-irradiation on the development of cerebral cortex in rats. Neurosci Res. 23, 241-247 (1995).
- Cragan, J. D., et al. Population-based microcephaly surveillance in the United States, 2009 to 2013: An analysis of potential sources of variation. Birth Defects Res Part A Clin Mol Teratol. 106 (11), 972-982 (2016).
- Cragan, J. D., et al. Baseline Prevalence of Birth Defects Associated with Congenital Zika Virus. MMWR Morb Mortal Wkly Rep. 66 (8), 219-220 (2017).
- Mlakar, J., et al. Zika Virus Associated with Microcephaly. N Engl J Med. 374 (10), 951-958 (2016).
- Jaenisch, T., Rosenberger, D., Brito, C., Brady, O. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ. 95 (3), 191-198 (2017).
- Clancy, B., Darlington, R. B., Finlay, B. L. Translating developmental time across mammalian species. Neuroscienze. 105 (1), 7-17 (2001).
- Driggers, R. W., et al. Zika Virus Infection with Prolonged Maternal Viremia and Fetal Brain Abnormalities. N Engl J Med. 374 (22), 2142-2151 (2016).
- Semple, B. D., Blomgren, K., Gimlin, K., Ferriero, D. M., Noble-Haeusslein, L. J. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 106, 1-16 (2013).
- Li, H., et al. Zika Virus Infects Neural Progenitors in the Adult Mouse Brain and Alters Proliferation. Cell Stem Cell. 19 (5), 593-598 (2016).
- Vermillion, M., et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat. Commun. 8, 14575 (2017).
- Goodfellow, F., et al. Zika Virus Induced Mortality and Microcephaly in Chicken Embryos. Stem Cells Dev. 25 (22), 1-27 (2016).
- Dick, G. W. A., Kitchen, S. F. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 46 (5), 509-520 (1952).
- Shao, Q., Herrlinger, S., et al. The African Zika virus MR-766 is more virulent and causes more severe brain damage than current Asian lineage and Dengue virus. Development. , (2017).

