The Nematode Caenorhabditis Elegans – A Versatile In Vivo Model to Study Host-microbe Interactions
Summary
Here, we present the nematode Caenorhabditis elegans as a versatile host model to study microbial interaction.
Abstract
We demonstrate a method using Caenorhabditis elegans as a model host to study microbial interaction. Microbes are introduced via the diet making the intestine the primary location for disease. The nematode intestine structurally and functionally mimics mammalian intestines and is transparent making it amenable to microscopic study of colonization. Here we show that pathogens can cause disease and death. We are able to identify microbial mutants that show altered virulence. Its conserved innate response to biotic stresses makes C. elegans an excellent system to probe facets of host innate immune interactions. We show that hosts with mutations in the dual oxidase gene cannot produce reactive oxygen species and are unable to resist microbial insult. We further demonstrate the versatility of the presented survival assay by showing that it can be used to study the effects of inhibitors of microbial growth. This assay may also be used to discover fungal virulence factors as targets for the development of novel antifungal agents, as well as provide an opportunity to further uncover host-microbe interactions. The design of this assay lends itself well to high throughput whole-genome screens, while the ability to cryo-preserve worms for future use makes it a cost-effective and attractive whole animal model to study.
Introduction
C. elegans has been used as a powerful model organism for more than 50 years. In the 1960s, South African biologist Sydney Brenner pioneered the use of C. elegans to study neuronal development, paving the way for a long lineage of scientists to study various aspects of cell and animal biology in nematodes. This lineage includes Nobel Prize laureates Craig Mello and Andrew Fire for their RNAi work1, Robert Horvitz and John Sulston for their work on organ development and apoptosis2,3,4, and Martin Chalfie for his work on green fluorescent protein5. Although this model organism has been traditionally used to study molecular and developmental biology, over the past 15 years, researchers have begun to use C. elegans to investigate the biology of various human pathogens including Pseudomonas aeruginosa, Staphylococcus aureus, Salmonella enterica, and Serratia marcescens6,7,8,9,10. These studies revealed that many of the mechanisms involved in the human-pathogen interaction are conserved in nematodes, but also that there are some immunity mechanisms that are unique to this model organism11,12. In nature, C. elegans encounters a variety of threats from ingested pathogens present in the soil and this has provided a strong selective pressure to evolve and maintain a sophisticated innate immune system in its intestinal lumen. Many of the genes and mechanisms involved in the protection of intestinal lumen are orchestrated by highly-conserved elements that also exist in higher mammals11,13. C. elegans therefore represents a great model to study gastrointestinal pathogens like Salmonella enterica14, Shigella boydii15, or Vibrio cholera16.
Here we highlight the remarkable versatility of C. elegans as a model host to study infectious agents such as C. albicans. C. elegans as a model host allows for high throughput screening for virulence that is less expensive and time-consuming than a mouse model, which is commonly used to study candidiasis42.
In this study, we show that this model and the assosiated survival assay can be reliably used for studying host innate immune effectors important to counteract infections, pathogen determinants that drive virulence, and pharmacological compounds that can intervene in pathogenesis. Dissimilar to previously described assays, this method provides a means of studying exposure to a pathogen over the lifetime of the animal, from the larval stage to adulthood, rather than only adulthood to death43,44. In summary, our C. elegans – C. albicans model is a versatile and powerful tool that can be used not only to study the genetic bases that drive infection and immunity but also to identify new compounds for therapeutic intervention.
Protocol
1. Preparation of Nematode Growth Medium (NGM)
- For 1 L of media, combine 20 g agar, 2.5 g organic nitrogen source (e.g., bacto-peptone), and 3 g sodium chloride in a 2 L flask. Add 975 mL of sterile water.
- Add in a sterile stir bar. If using an automatic media pourer, autoclave tubing and media for 15 min; media should be autoclaved for longer if a higher volume is made.
- Set media on stir plate, and allow to cool.
- Once media is warm to touch (approximately 60 °C) aseptically add 1 mL of 1 M MgSO4● H2O, 1 mL of 1M CaCl2, 25 mL of KPO4, and 1 mL of 0.5% cholesterol in ethanol.
- Pour media (by hand, or by automatic pump) under laminar flow into 35 mm x 10 mm sterile petri dishes. Allow to dry under hood for 1-2 days before use.
NOTE: Plates should be stored at 4 °C to prevent contamination.
2. Making a Worm Pick
- Cut a 1.5 cm piece of aluminum wire. Carefully insert approximately 0.5 cm of this length into the tip of a Pasteur pipette.
- Using a Bunsen burner or alcohol lamp, melt the glass pipette tip by slowly rotating it in the flame until the wire is securely adhered to the pipette, without compromising the original shape of the pipette tip.
3. Preparation of E. coli Culture
- Using a sterile loop, inoculate single colony of E. coli strain OP50 in 200 mL of Luria Broth. Incubate overnight with shaking at 37 °C.
- The liquid culture is ready for use the following day and may be stored at 4 °C when not in use.
NOTE: The E. coli strain, OP50, is used as a food source for C. elegans. This culture may be used for up to several months when stored at 4 °C.
4. Essential C. elegans Maintenance
- Seed prepared NGM agar plates with E. coli by spotting approximately 50 – 100 µL of prepared OP50 liquid culture onto the center of the plate.
- Cover plates and allow them to dry at room temperature for 24 h. Once dry, place plates at 37 °C overnight for rapid growth or keep at room temperature, if slower growth is desired.
- Add worms to the plate by either "chunking," (see protocol step 5 "Chunking and Picking Worms"), picking worms individually, or placing worm eggs directly onto the plate (see protocol step 6 "Egg Preparation").
5. Chunking and Picking Worms
NOTE: "Chunking," refers to a practice that is common in C. elegans maintenance. This involves cutting a section of the NGM agar plate that contains worms, and transferring this piece onto a new plate, thus also transferring a large number of worms in the process. Contamination may also be cut out of the plate in this manner. When picking worms, it is important to maintain the integrity of the agar because worms can be lost in holes in the agar. In addition, it is important to allow the pick to cool before touching the plate to prevent melting the agar or burning the worms.
- In order to quickly transfer large amounts of worms onto new plates, cut a piece of NGM agar containing the selected worms using a spatula.Remove the cut piece and place it face down on the edge of the lawn of OP50 on a new plate.
- Transfer worms individually as described below in protocol step 8.2, "Survival Assay," using a worm pick. Flame the wire in the worm pick until it is red. Remove it from the flame and allow it to cool for a few seconds.
- Using the wire on the pick, gently scrape the lawn of the OP50 culture grown on the new plate, covering the wire on the pick.
- The viscous culture covering the tip of the wire makes worms stick to the tip of the pick, thus gently pick up selected worms using a swiping motion.
- Once selected worms are gathered, place them onto a new plate by gently swiping culture across the agar. Place the wire into the flame to remove remaining culture on the pick.
6. Egg Preparation
- Place approximately 20 adult animals (approximately 2 – 3 days after hatching when grown at 20 °C) by either picking or chunking as described in protocol step 5, "Chunking and Picking Worms," onto a plate seeded with 50 µL of E. coli strain, OP50.
- To collect the eggs, flood the plate with 10 mL of M9 buffer after 1-2 days. Using a glass serological pipette, swirl and gently agitate the contents on the agar plate to release eggs stuck to OP50 or adult animals. Collect all the liquid containing the eggs and adults.
- Transfer M9 and OP50 solution into a 15 mL conical tube. Centrifuge 15 mL conical tube at 2,100 x g for 2 min.
- Aspirate approximately 9 mL of supernatant without disturbing the OP50 pellet.
- Resuspend the pellet in 2 mL of sterile water, 1 mL of bleach, and 1 mL of 0.25 M NaOH.
- Mix gently by inversion until the majority (approximately 70 percent) of adult animals appear lysed; typically, eggs remain intact during this short bleach treatment because of their shell. Centrifuge the conical tube at 990 x g for 2 min.
- Aspirate supernatant without disturbing the pellet. Re-suspend the pellet in 10 mL of M9 buffer.
- Centrifuge the conical tube in 990 x g for 2 min. Aspirate supernatant without disturbing pellet. Re-suspend pellet with 200 µL of M9 buffer.
NOTE: Egg preparation may require multiple trials. Eggs may be destroyed if they are exposed to bleach for too long. If eggs do not hatch, either decrease the concentration of bleach in solution or decrease the duration of exposure to bleach.
7. Infection Plate Set-up
- Dispense 3-5 mL of YPD into a sterile test tube and inoculate it with a single colony of Candida albicans using a sterile loop.
- Place the tube on a rotatory drum for approximately 16-18 h at 30 °C.
- Label one 1.5 mL microfuge tube to contain C. albicans, and another to contain OP50. Record the weight of each empty tube.
- Place 500 µL of the overnight C. albicans culture into tube labeled C. albicans and 1,500 µL of the overnight OP50 culture (from step 3.1) into the tube labeled OP50.
- Centrifuge for 10 min at 16,100 x g. Aspirate supernatant without disturbing pellet.
- Re-suspend each pellet with 500 µL of sterile water. Centrifuge for 5 min at 16,100 x g.
- Aspirate supernatant without disturbing the pellet. Record the final weight of microfuge tubes.
- Determine the weight of each pellet by subtracting the initial weight of the microfuge tubes from the final weight.
- Using sterile water, re-suspend the C. albicans pellet to 10 mg/mL, and the OP50 pellet to 200 mg/mL. Make a master infection mix by combining 10 µL of a 50 mg/mL solution of Streptomycin, 2.5 µL of OP50 culture, 0.5 µL of C. albicans culture, and 7 µL of sterile water.
- For control plates, create a master mix by replacing the volume of C. albicans culture with sterile water. Seed 20 µL of infection mix or OP50 control solution onto the center of NGM plates using a micropipette.
NOTE: Approximately 100 worms are needed to determine statistical significance during analysis. This assay is typically run in triplicate. Thus, a 3.2x infection mix as well as a 3.2x control solution is made. These mixes are made to be slightly over 3x the original to ensure that a full 20 µL is seeded onto each plate, allowing room for error. This master mix is created because the pharynx of the worm is too small during the initial larval stages (L1-L3) to consume C. albicans. For exposure to pharmacological agents such as fluconazole (Figure 3B), the agent is introduced to the infection mixture. The concentration of the agent is empirically determined. For example, in Figure 3B, 50 µM Fluconazole is represented, however 0, 12.5, 25, 50 and 100 µM Fluconazole were also tested using the same protocol.
- For control plates, create a master mix by replacing the volume of C. albicans culture with sterile water. Seed 20 µL of infection mix or OP50 control solution onto the center of NGM plates using a micropipette.
8. Analysis of the Deformed Anal Region (Dar) Phenotype and Survival Assay
- Visualize the Dar phenotype
NOTE: Dar is best visualized at the L3 stage and beyond. Dar presents as a small protrusion in the post anal region of the worm (Figure 2A), which becomes more prominent over time.- Confirm if an animal has Dar by quickly tapping the plate on the stage of the dissection microscope; animals without Dar will move backwards immediately, while animals with Dar will either need repeated rounds of tapping to reverse their direction, or they will not do so at all.
- Survival assay
- Complete egg preparation as previously described in protocol step 6, "Egg Preparation". Count the eggs under the dissection scope, and dilute the egg solution with M9 until a concentration of approximately 5-6 healthy eggs per µL is achieved. Using a pipette, dispense 20 µL sample containing approximately 30 eggs onto prepared NGM plate, in the area between the bacterial culture and the side of plate (eggs and worm food, OP50, are in two distinct spots).
- Incubate plates at 20 °C for 48 h. Under a dissection microscope, count the number of live and dead adult worms on each plate, as well as the number of worms with Dar. Record the percent with Dar.
- Consider an animal dead if it does not respond to being tapped on the head by an aluminum wire or tapping on the plate.
- Every other day, transfer remaining adult worms by picking as described in protocol step 5, "Chunking and Picking Worms," onto a new infection or control plate. At this point, if needed, perform a survival assay by tracking the number of live, dead, and missing worms each day.
NOTE: This assay may run for two weeks, beginning with egg preparation. In addition, the egg solution should not be dispensed onto the bacterial culture, as the solution may contain traces of bleach which can kill the OP50. The solution should also be dispensed approximately 0.5 cm away from the edge of the plate, as eggs can become stuck in between the sides of the plate and the agar. Additionally, due to the rapid proliferation of worms, transferring adults onto new plates is critical to separate them from their progeny.
- Analyze Survival
- Analyze results using survival curve analysis software (refer to list of materials).
- Compare survival curves using the Grehan-Breslow-Wilcoxon method (assuming that the data from early survival times are more accurate than later times, weight the data accordingly) as well as Logrank statistical tools45.
NOTE: For example, in Figure 4B-C, the survival of worms treated with mutant C. albicans is compared to those treated with isogenic wild-type controls or complementarystrains.
Representative Results
A pathogenesis assay (Figure 1) using C. albicans and C. elegans has previously been described by our lab17,18 and other labs19,20. We demonstrate the amenability of using C. elegans to study C. albicans virulence showing that C. albicans cells are quickly ingested by the worms and accumulate in the intestinal lumen causing slower locomotion, deformed anal region (Dar) (Figure 2A), swelling of the vulva (Figure 2B), distention of the intestine (Figure 3A), and lethality (Figure 3, Figure 4). We also measure the life span of infected worms as a quantitative measure of fitness. For example, C. elegans infected with C. albicans live 10-12 days compared to 20-22 for uninfected controls. In C. elegans infected with the C. albicans double knock out mutant, efg1/efg1 cph1/cph1, which are two key virulence factors required for infection in mouse, rats and human epithelial models21,23, nematodes survived significantly longer than controls (Figure 3B). These experiments suggest that some of the lessons we learn in this simpler model about C. albicans virulence may remain valid in higher mammals and vice versa.
We also show that the nematode model system can be pharmacologically modulated (Figure 3B). In the presence of fluconazole, the most commonly prescribed antifungal drug, worms challenged with C. albicans survived significantly longer than controls. This proof of principle experiment suggests that the nematode model can be used for small molecule screening. Indeed, our C. elegans model was instrumental in identifying filastatin, a small molecule inhibiting various aspects of fungal virulence24.
Disease phenotypes and microscopic analysis of C. elegans infection
C. elegans were exposed to C. albicans over a period of six days and observed for signs of infection, progression of disease, and death. The Dar phenotype is most visible by day 4 of the survival assay, as noted by a protruding anal region that is not visible in the uninfected animal (Figure 2A). Worms infected by C. albicans are also known to exhibit swelling in the vulva region (Figure 2B). In both cases, the worm is unable to clear the infection after reaching this stage. In order to visualize colonization of C. albicans in the intestinal lumen, worms were fed wild-type C. albicans tagged with RFP, which cause areas of colonization to fluoresce red (Figure 3A). In order to produce these images, worms were anesthetized on a 2% agarose pad containing 0.01 M sodium azide. Worms were exposed to either wild-type C. albicans or RFP-tagged C. albicans, and transferred into 5 µM M9 buffer on the agarose pad. The agarose pad was then covered with a coverslip. Worms were viewed at 200X and 400X magnification using an inverted microscope with fluorescent microscopy capabilities. Images were created under differential interference contrast (Nomarski) and epifluorescence optics. The fluorescent images were enhanced using software (Figure 3A). Time series micrographs of infected worms determined that C. albicans colonized the intestinal lumen by the third day of the assay17. Infected worms showed more severe intestinal distension than observed in the uninfected control.
Genetic and pharmacological tools to study infection
Next, we tested the model using genetic and pharmacological modulation. We also tested previously documented virulence factors that regulate hyphal transition of C. albicans25 and have been shown to be important in in vivo infections of mice and nematodes20,26. We tested the ability of the worms to survive infection caused by C. albicans efg1/efg1 cph1/cph1 double mutant. Efg1 is a highly-conserved transcription factor and an essential component of the cyclic AMP/protein kinase A (PKA) metabolic pathway. In C. albicans this pathway regulates hyphal morphogenesis27, white-opaque switching28, and an arsenal of key virulence factors. These virulence factors include Hwp1 and Hwp2, two yeast-specific cell wall proteins involved in adhesion and biofilm formation, Eap1, a cell-wall adhesin involved in binding to human epithelial cells29, and Sap5, a hydrolytic enzyme involved in epithelial tissue invasion30. Cph1 is a transcription factor that regulates many metabolic processes including mating, filamentation, and biofilm formation and has been shown to play a critical role in damaging epithelial cells31 and human reconstructed epithelium21. Disruption of either of these genes has a significant impact on virulence and simultaneous disruption of both in cph1/cph1efg1/efg1 results in dramatic virulence reduction in various animal models including mice25, Drosophila32, zebrafish33,34 and moth34. The cph1/cph1efg1/efg1 double mutant is considered by the C. albicans community as the avirulent strain by definition and the gold standard for validation of novel model systems. The efg1Δ and cph1Δ single mutants showed decreased Dar (~10% and ~50%, respectively) compared with the cognate wild type, while the efg1Δ cph1Δ double mutant failed to elicit the Dar response17. These results recapitulate the pattern of virulence in mice, where the cph1∆ mutant is slightly attenuated, the efg1∆ mutant is significantly attenuated, and the double mutant is completely avirulent25. Worms infected with cph1/cph1 efg1/efg1C. albicans double mutant lived statistically significantly longer than controls (p <0.01 for both Log Rank and Breslow statistical test, n=60) suggesting that these two genes are required for C. albicans virulence against C. elegans (Figure 3B).
In order to explore the possibility of using our C. elegans model for potential drug screening, we tested the effect of the addition of fluconazole on the ultimate outcome of the infection. We used a variety of different concentrations of fluconazole and found that 50 µM gave us the most significant results. Worms infected with C. albicans and 50 µM fluconazole (Figure 3B) lived statistically significantly longer than controls (p<0.01 at both Log Rank and Breslow statistical test, n=60). This concentration was empirically determined (see the note at the end of protocol step 7, "infection plate set up"). These proof of principle experiments showed that our nematode model can be used for small molecule antifungal screening. The model was in fact instrumental in the discovery of filastatin, a small molecule inhibiting various aspects of fungal virulence that is currently undergoing further preclinical studies24. Accordingly, our assay is suitable for exploring the virulence strategies of C. albicans and pharmacological agents.
Study of innate host response
Next, we wanted to study the reciprocal host defenses against pathogens, since aspects of this innate immunity are conserved in mammals11,35. It is well known that C. elegans produces ROS upon both bacterial and fungal infections18,36,37 as part of its defense mechanism. ROS have a biocidal effect on invading organisms and play a major role in innate immunity. zcf15/zcf15 hyper susceptibility to ROS in vitro led us to hypothesize that its reduced virulence in C. elegans was due to a reduced ability to withstand the host's generated ROS. To test our hypothesis, we determined the ability of zcf15/zcf15 to kill either wild-type worms or worms with an impaired ability to produce ROS. Representative C. albicans mutant zcf15 that is sensitive to reactive oxygen species (Figure 4A) showed reduced virulence in wild-type C. elegans. C. elegans responds to ingestion of pathogen by producing extracellular ROS in the intestinal lumen via Ce-Duox1, an NADPH oxidase coded by the gene bli-3. During ROS production, the intestinal cells also produce intracellular antioxidants via DAF-16 to protect its own tissues from the ROS damaging effects36,38. Ce-Duox1 is a protein with an N-terminal peroxidase domain, a C-terminal superoxide-generating NADPH-oxidase domain and two central calmodulin-binding sites39. Upon microbial infection, this protein uses cytosolic NADPH to generate extracellular toxic ROS in the intestinal lumen to counteract the infection. Throughout a series of biochemical assays in a 2009 study, Jain et al. showed that ROS are abundantly produced upon yeast infection and that bli-3 loss of function via bli-3(e767) dramatically reduces the ability of nematodes to produce ROS. As shown in Figure 4B, worms challenged with zcf15/zcf15 survived significantly longer than wild type or complemented strains (p <0.01 by the log-rank test) indicating that ZCF15 is required for virulence. However, when we challenged ROS-deficient C. elegansbli-3(e767), we obtained kinetics of killings that were comparable between zcf15/zcf15, wild type and complemented strain (Figure 4C). This evidence indicates that zcf15/zcf15 fail to kill nematodes unless the host's ability to produce ROS is compromised. Taken together, these results suggest that ZCF15 is required for full virulence and that this gene is likely involved in the pathogen's ability to resist host generated ROS. To the best of our knowledge, this is the first time that ZCF15 has been shown to be involved in pathogenicity and ROS resistance.
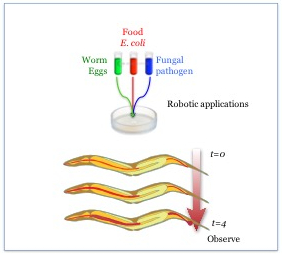
Figure 1: A schematic representation of C. elegans infection. C. elegans are exposed to a mix of E. coli strain OP50 and a pathogen and observed over a period of 4 days after reaching adulthood for signs of infection and progression of disease.
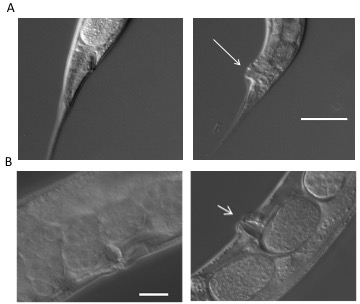
Figure 2: Disease phenotypes. (A) Deformed anal region (Dar) is visible (indicated with an arrow) as a swelling in the post anal region of infected worms (right panel) four days post exposure. The worms on the left panel are uninfected and serve as a control. (B) A subset (~15%) of infected worms show a swelling of the vulva (right panel, indicated with an arrow) compared to uninfected control worms (left panel) that represents disseminated infection resulting in matricidal death. All images were taken as described in the representative results18.
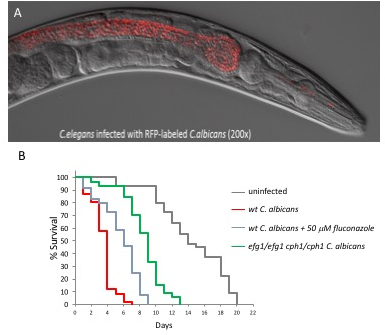
Figure 3: The C. albicans – C. elegans model is amenable to microscopic manipulation, and can be pharmacologically and genetically modulated. (A) C. albicans tagged with RFP accumulation in the nematode intestinal lumen day 3 post infection. Yeast cells are quickly ingested by the worms and accumulate in the intestinal lumen completely intact, indicating that they are able to survive the mechanical crushing of the pharynx. Images were taken as described in the representative results18. (B) Kaplan-Meier survival curves of nematodes challenged with wild-type C. albicans, cph1/cph1efg1/efg1 double mutant or wild-type C. albicans + 50 µM of fluconazole compared to uninfected control worms.
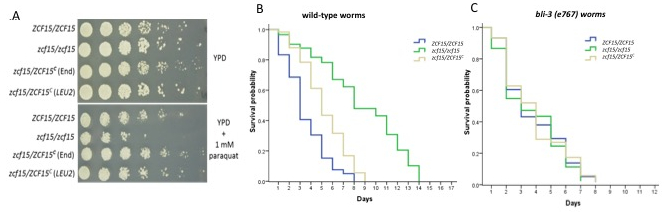
Figure 4. ZCF15 is required to withstanding C. elegans generated ROS. (A) ZCF15 is responsible for wild type resistance to paraquat, which is known to generate reactive oxygen species. Wild type, knockout or complemented strains were grown in liquid culture overnight and resuspended to OD=1. Cultures were each diluted 1:5 and plated onto YPD or YPD containing 1 mM paraquat. C. elegans produce ROS via bli-3 in order to combat pathogens that invade the intestinal lumen38. (B) Kaplan-Meier survival curves show that ZCF15 deletion limits the killing of wild-type worms. (C) The Kaplan-Meier survival curve shows similar rates of survival between zcf15/zcf15, wild type, and complemented strain when ROS-deficient C. elegansbli-3(e767) were infected.
Discussion
The methods for assaying C. elegans infection and survival over lifetime exposure to C. albicans that we have described can be modified to test another pathogen. Liquid cultures of another bacteria or fungus may be made and fed to C. elegans in a similar manner. Additionally, serial infections may be assayed by first exposing the larva to one pathogen as described, and then transferring the animals onto a new plate containing a separate pathogen after reaching adulthood.
While conducting this assay, it is important to pay careful attention to timing. First, when completing egg preparation in order to harvest eggs for the assay, it is critical to limit the amount of time the eggs are exposed to bleach. The amount of time needed to dissolve adults in the bleach solution to release the eggs varies. Immediately after administering the bleach solution, the worm solution must be watched closely under a dissection microscope. Once approximately 70% of the worms have disintegrated, then the solution should be centrifuged. If egg preparation does not yield viable eggs, decrease either the concentration of bleach or the duration of exposure to bleach. In completing this assay, it is also crucial that attention is given to transferring adult worms onto new plates as needed to avoid confusing study subjects with their progeny. Limiting the number of eggs plated at the start of the assay to 25-30 eggs should prevent this as well. Finally, transferring adult worms onto new plates frequently also decreases the chances of worms crawling up the sides of the petri dish and dying.
We have utilized Caenorhabditis elegans as a versatile model host for studying pathogenic or commensal relationships between microbes or a microbe and its host. Our system can be used as a tool to study microbial or host-microbe interaction at the cellular and molecular level using forward and reverse genetic approaches but also for exploring new molecules for therapeutic intervention. We have demonstrated the versatility of this system by conducting a genetic screen to identify genes that contribute to virulence40, those linked to microbial fitness41, those that mediate host response to microbes17,18, as well using the model for high throughput applications for drug discovery24. This is a good model for use by undergraduate students because it does not need expensive infrastructure to maintain colonies, considering that animals can be cryo-preserved for future applications. Finally, this provides a perfect "go-between" for in vitro studies and murine models.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was performed at and supported by Worcester Polytechnic Institute.
Materials
| Agar (granulated, bacterilogical grade) | Apex BioResearch Products | 20-248 | |
| Aluminum Wire (95% Pt, 32 Gauge) | Genesee Scientific | 59-1M32P | |
| Axiovision Zeiss Inverted Microscope | Axiovision Zeiss | ||
| Bacto-Peptone | Fisher BioReagants | BP1420-500 | |
| C. elegans strain Bli-3 | Caenorhabditis Genetics Center | Bli-3(e767) CB767 | |
| Calcium Chloride | Fisher Scientific | BP51-250 | |
| Cholesterol, Sigma Grade, minimum 99% | Sigma | C8667-25G | |
| Disposable Culture Tubes (20 x 150 mm) | FIsherBrand | 14-961-33 | |
| Dissection Microscope (NI-150 High Intensity Illuminator) | Nikon Instrument Inc. | ||
| E. coli | Caenorhabditis Genetics Center | OP50 | |
| GraphPad Prism (Survival Curve Analysis Software) | GraphPad Software | ||
| LB Broth (Miller's) | Apex BioResearch Products | 11-120 | |
| Magnesium Sulfate | Fisher Scientific | 10034-99-8 | |
| Medium Petri Dishes (35 X 10 mm) | Falcon | 353001 | |
| Potassium Phosphate monobasic | Sigma | P0662-500G | |
| Sodium Chloride | Fisher Scientific | BP358-1 | |
| Sodium Phosphate | Fisher Scientific | BP332-500 | |
| Wildtype C. albicans SC5314 | ATCC | SC5314 | |
| Wildtype C. elegans | Caenorhabditis Genetics Center | N2 |
Riferimenti
- Fire, A., et al. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 391 (6669), 806-811 (1998).
- Ellis, H. M., Horvitz, H. R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 44 (6), 817-829 (1986).
- Hengartner, M. O., Ellis, R. E., Horvitz, H. R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 356 (6369), 494-499 (1992).
- Sulston, J. E., Horvitz, H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 56 (1), 110-156 (1977).
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., Prasher, D. C. Green fluorescent protein as a marker for gene expression. Science. 263 (5148), 802-805 (1994).
- Kong, C., Yehye, W. A., Abd Rahman, N., Tan, M. W., Nathan, S. Discovery of potential anti-infectives against Staphylococcus aureus using a Caenorhabditis elegans infection model. BMC Complement Altern Med. 14, 4 (2014).
- Marsh, E. K., May, R. C. Caenorhabditis elegans, a model organism for investigating immunity. Appl Environ Microbiol. 78 (7), 2075-2081 (2012).
- Kaletta, T., Hengartner, M. O. Finding function in novel targets: C. elegans as a model organism. Nat Rev Drug Discov. 5 (5), 387-398 (2006).
- Sem, X., Rhen, M. Pathogenicity of Salmonella enterica in Caenorhabditis elegans relies on disseminated oxidative stress in the infected host. PLoS One. 7 (9), e45417 (2012).
- Irazoqui, J. E., et al. Distinct pathogenesis and host responses during infection of C. elegans by P. aeruginosa and S. aureus. PLoS Pathog. 6, e1000982 (2010).
- Kim, D. H., et al. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 297 (5581), 623-626 (2002).
- Bae, T., et al. Staphylococcus aureus virulence genes identified by bursa aurealis mutagenesis and nematode killing. Proc Natl Acad Sci U S A. 101 (33), 12312-12317 (2004).
- Couillault, C., et al. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 5 (5), 488-494 (2004).
- Aballay, A., Drenkard, E., Hilbun, L. R., Ausubel, F. M. Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol. 13 (1), 47-52 (2003).
- Kesika, P., Balamurugan, K. Studies on Shigella boydii infection in Caenorhabditis elegans and bioinformatics analysis of immune regulatory protein interactions. Biochem Biophys Acta. 1824 (12), 1449-1456 (2012).
- Cinar, H. N., et al. Vibrio cholerae hemolysin is required for lethality, developmental delay, and intestinal vacuolation in Caenorhabditis elegans. PLoS One. 5 (7), e11558 (2010).
- Jain, C., Pastor, K., Gonzalez, A. Y., Lorenz, M. C., Rao, R. P. The role of Candida albicans AP-1 protein against host derived ROS in in vivo models of infection. Virulence. 4 (1), 67-76 (2013).
- Jain, C., Yun, M., Politz, S. M., Rao, R. P. A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryot Cell. 8 (8), 1218-1227 (2009).
- Tampakakis, E., Okoli, I., Mylonakis, E. A C. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat. Protoc. 3 (12), 1925-1931 (2008).
- Pukkila-Worley, R., Ausubel, F. M., Mylonakis, E. Candida albicans infection of Caenorhabditis elegans induces antifungal immune defenses. PLoS Pathog. 7 (6), e1002074 (2011).
- Dieterich, C., et al. In vitro reconstructed human epithelia reveal contributions of Candida albicans EFG1 and CPH1 to adhesion and invasion. Microbiology. 148 (Pt 2), 497-506 (2002).
- Chen, C. G., et al. Non-lethal Candida albicans cph1/cph1 efg1/efg1 transcription factor mutant establishing restricted zone of infection in a mouse model of systemic infection. Int J Immunopathol Pharmacol. 19 (3), 561-565 (2006).
- Ricicova, M., et al. Candida albicans biofilm formation in a new in vivo rat model. Microbiology. 156 (Pt 3), 909-919 (2010).
- Fazly, A., et al. Chemical screening identifies filastatin, a small molecule inhibitor of Candida albicans adhesion, morphogenesis, and pathogenesis. Proc Natl Acad Sci U S A. 110 (33), 13594-13599 (2013).
- Lo, H. J., et al. Nonfilamentous C. albicans mutants are avirulent. Cell. 90 (5), 939-949 (1997).
- Koh, A. Y., Kohler, J. R., Coggshall, K. T., Van Rooijen, N., Pier, G. B. Mucosal damage and neutropenia are required for Candida albicans dissemination. PLoS Pathog. 4 (2), e35 (2008).
- McDonough, K. A., Rodriguez, A. The myriad roles of cyclic AMP in microbial pathogens: from signal to sword. Nat Rev Microbiol. 10 (1), 27-38 (2011).
- Sonneborn, A., Tebarth, B., Ernst, J. F. Control of white-opaque phenotypic switching in Candida albicans by the Efg1p morphogenetic regulator. Infect Immun. 67 (9), 4655-4660 (1999).
- Li, F., Palecek, S. P. EAP1, a Candida albicans gene involved in binding human epithelial cells. Eukaryot Cell. 2 (6), 1266-1273 (2003).
- Staib, P., Kretschmar, M., Nichterlein, T., Hof, H., Morschhauser, J. Transcriptional regulators Cph1p and Efg1p mediate activation of the Candida albicans virulence gene SAP5 during infection. Infect Immun. 70 (2), 921-927 (2002).
- Korting, H. C., et al. Reduced expression of the hyphal-independent Candida albicans proteinase genes SAP1 and SAP3 in the efg1 mutant is associated with attenuated virulence during infection of oral epithelium. J .Med Microbiol. 52 (Pt 8), 623-632 (2003).
- Chamilos, G., et al. Drosophila melanogaster as a facile model for large-scale studies of virulence mechanisms and antifungal drug efficacy in Candida species. J Infect Dis. 193 (7), 1014-1022 (2006).
- Brothers, K. M., Newman, Z. R., Wheeler, R. T. Live imaging of disseminated candidiasis in zebrafish reveals role of phagocyte oxidase in limiting filamentous growth. Eukaryot Cell. 10 (7), 932-944 (2011).
- Brennan, M., Thomas, D. Y., Whiteway, M., Kavanagh, K. Correlation between virulence of Candida albicans mutants in mice and Galleria mellonella larvae. FEMS Immunol Med Microbiol. 34 (2), 153-157 (2002).
- Mallo, G. V., et al. Inducible antibacterial defense system in C. elegans. Curr Biol. 12 (14), 1209-1214 (2002).
- Chavez, V., Mohri-Shiomi, A., Maadani, A., Vega, L. A., Garsin, D. A. Oxidative stress enzymes are required for DAF-16-mediated immunity due to generation of reactive oxygen species by Caenorhabditis elegans. Genetica. 176 (3), 1567-1577 (2007).
- Moy, T. I., Mylonakis, E., Calderwood, S. B., Ausubel, F. M. Cytotoxicity of hydrogen peroxide produced by Enterococcus faecium. Infect Immun. 72 (8), 4512-4520 (2004).
- Hoeven, R., McCallum, K. C., Cruz, M. R., Garsin, D. A. Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog. 7 (12), e1002453 (2011).
- Meitzler, J. L., Ortiz de Montellano, P. R. Caenorhabditis elegans and human dual oxidase 1 (DUOX1) "peroxidase" domains: insights into heme binding and catalytic activity. J Biol Chem. 284 (28), 18634-18643 (2009).
- Issi, L., et al. Zinc Cluster Transcription Factors Alter Virulence in Candida albicans. Genetica. 205 (2), 559-576 (2017).
- Ford, C. B., et al. The evolution of drug resistance in clinical isolates of Candida albicans. Elife. 4, e00662 (2015).
- Naglik, J. R., Fidel, P. L., Odds, F. C. Animal models of mucosal Candida infection. FEMS microbiology letters. 283 (2), 129-139 (2008).
- Garsin, D. A., et al. A simple model host for identifying Gram-positive virulence factors. Proceedings of the National Academy of Sciences. 98 (19), 10892-10897 (2001).
- Sifri, C. D., Begun, J., Ausubel, F. M., Calderwood, S. B. Caenorhabditis elegans as a Model Host for Staphylococcus aureus Pathogenesis. Infection and immunity. 71 (4), 2208-2217 (2003).
- Jain, C., Yun, M., Politz, S. M., Rao, R. P. A pathogenesis assay using Saccharomyces cerevisiae and Caenorhabditis elegans reveals novel roles for yeast AP-1, Yap1, and host dual oxidase BLI-3 in fungal pathogenesis. Eukaryotic cell. 8 (8), 1218-1227 (2009).

