Assessment of Antibody-based Drugs Effects on Murine Bone Marrow and Peritoneal Macrophage Activation
Summary
Antibody-based drugs have revolutionized treatment for inflammatory diseases. In addition to having direct effects on specific targets, antibodies can activate macrophages to become anti-inflammatory. This protocol describes how anti-inflammatory macrophage activation can be assessed in vitro, using mouse bone marrow macrophages, and in vivo, using peritoneal macrophages.
Abstract
Macrophages are phagocytic innate immune cells, which initiate immune responses to pathogens and contribute to healing and tissue restitution. Macrophages are equally important in turning off inflammatory responses. We have shown that macrophages stimulated with intravenous immunoglobulin (IVIg) can produce high amounts of the anti-inflammatory cytokine, interleukin 10 (IL-10), and low levels of pro-inflammatory cytokines in response to bacterial lipopolysaccharides (LPS). IVIg is a polyvalent antibody, primarily immunoglobulin Gs (IgGs), pooled from the plasma of more than 1,000 blood donors. It is used to supplement antibodies in patients with immune deficiencies or to suppress immune responses in patients with autoimmune or inflammatory conditions. Infliximab, a therapeutic anti-tumor necrosis factor alpha (TNFα) antibody, has also been shown to activate macrophages to produce IL-10 in response to inflammatory stimuli. IVIg and other antibody-based biologics can be tested to determine their effects on macrophage activation. This paper describes methods for derivation, stimulation, and assessment of murine bone marrow macrophages activated by antibodies in vitro and murine peritoneal macrophages activated with antibodies in vivo. Finally, we demonstrate the use of western blotting to determine the contribution of specific cell signaling pathways to anti-inflammatory macrophage activity. These protocols can be used with genetically modified mice, to determine the effect of a specific protein(s) on anti-inflammatory macrophage activation. These techniques can also be used to assess whether specific biologics may act by changing macrophages to an IL-10-producing anti-inflammatory activation state that reduces inflammatory responses in vivo. This can provide information on the role of macrophage activation in the efficacy of biologics during disease models in mice, and provide insight into a potential new mechanism of action in people. Conversely, this may caution against the use of specific antibody-based biologics to treat infectious disease, particularly if macrophages play an important role in host defense against that infection.
Introduction
Macrophages are innate immune cells, which play multiple roles in the immune response to infection or injury. Macrophages are responsible for initiating an immune response to infection or tissue damage, stopping the inflammatory response, and promoting the healing response1. Examples of the three best studied macrophage activation states are: 1) macrophages treated with interferon gamma (IFNγ) and bacterial lipopolysaccharide (LPS), designated M(IFNγ + LPS), which contribute to the inflammatory response; 2) macrophages stimulated with interleukin 4 (IL-4), M(IL-4), which are associated with the healing response; 3) macrophages stimulated with immune complexes (IC) and LPS, M(IC + LPS), which have the ability to turn off the inflammatory response2,3. M(IC + LPS) are distinct from M(IL-4) wound healing macrophages, and do not express the enzyme arginase (Arg-1) or FIZZ14. The best marker for these anti-inflammatory macrophages is their cytokine production5. Macrophages have multiple roles in maintaining health, but also contribute to inflammatory diseases and cancer3. Because of this, macrophages are a key therapeutic target for the treatment of a wide variety of diseases. It is important to investigate the effects of antibodies on their activation state to develop macrophage-based treatments for disease.
The focus of this paper is on the use of murine bone marrow derived macrophages (BMDMs) and peritoneal macrophages to test the effect of antibody drugs on inflammatory responses in vitro and in vivo. Recently, there have been multiple studies showing the effects of antibodies on macrophage activation6,7,8. Macrophages co-activated with immune complexes, which are antibodies complexed with an antigen, and LPS, a normally inflammatory stimulus, produce very high levels of the anti-inflammatory cytokine, IL-10, and very low levels of the pro-inflammatory cytokine, interleukin 12 (IL-12)9. In addition, infliximab, a monoclonal antibody against TNFα, has been found to work, in part, by inducing anti-inflammatory macrophages through its fragment crystallizable (Fc) region7. We have reported that IVIg + LPS induce anti-inflammatory macrophage activation that is similar to M(IC + LPS), wherein co-stimulated macrophages produce large amounts of IL-10 and low amounts of the pro-inflammatory cytokine subunit Interleukin 12 or 23 p40 (IL-12/23p40), interleukin- 6 (IL-6), and TNF8. IVIg is a drug comprised of polyclonal antibodies, primarily IgG, which has been pooled from the blood of more than 1,000 donors10. It is used to treat a wide variety of immunological diseases, such as idiopathic thrombocytopenic purpura and chronic demyelinating polyneuropathy, but its mechanism of action is not completely understood11. The effects of antibody based drugs on macrophage activation can be assessed using methods described herein.
The effects of specific biologics on macrophage activation can be tested in BMDMs and peritoneal macrophages. Using these macrophage sources permits assessment of primary cells. Preliminary testing of antibodies on cultured primary cells requires less time and monetary investment than other time consuming and expensive disease models. By injecting a drug into a healthy mouse in vivo, and isolating the cells and analyzing them ex vivo, one can determine if studies are warranted to assess whether treatment with biologics affects macrophage activation in disease models.
With few studies testing the effect of biologic therapies on macrophage activation in vitro and in vivo directly, our techniques provide an advantage over alternative techniques. Current techniques involve testing biologic drug effects on mixed cell populations in vitro, such as the effect of infliximab in a mixed lymphocyte reaction (MLR) or IVIg on human macrophages in peripheral blood mononuclear cells, where the effect cannot be attributed to a specific cell type7,12. Using BMDMs and peritoneal macrophages is advantageous over using cell lines, such as RAW264.7 cells, which do not produce the pro-inflammatory cytokine, IL-12, in response to LPS8,13. Testing the effect of an antibody-based drug on macrophage responses ex vivo has advantages because cytokine responses can be attributed directly to macrophages, rather than inferring macrophage responses by measuring serum cytokine levels14. BMDMs and peritoneal macrophages can be derived and isolated from genetically modified mice to determine the specific role of a protein in anti-inflammatory macrophage activation. For example, we have used Il10 deficient(-/-) BMDMs to demonstrate that IVIg-induced reduction of pro-inflammatory cytokine production is partially dependent on IL-108. A drug's mechanism of action can be investigated using western blotting, where the role of specific proteins and signaling events can be determined. Quantitative polymerase chain reaction (qPCR) can be performed on BMDMs or peritoneal macrophages to show patterns of gene expression that result from antibody activation. Disease models in mice can provide information on the potential efficacy of antibody-based biological therapies in models for diseases like inflammatory bowel disease, rheumatoid arthritis, and cancer15,16,17. However, the techniques described here will provide information on the mechanism of action of these biologics by determining whether they induce anti-inflammatory macrophage activity.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of British Columbia.
1. Bone Marrow Macrophage Derivation and Activation with Antibodies
- Perform euthanasia using CO2 asphyxiation.
- Place mouse in induction chamber. Euthanize mouse with 5% isoflurane anesthesia, for 60 – 90 s, until immobile and breathing is deep and slow.
- Turn off isoflurane anesthesia and administer 6 – 8 L/min of CO2 until mouse has stopped breathing. Leave mouse with CO2 on for at least another 5 min. Verify that there is no longer a heartbeat or respiration. Perform a cervical dislocation to ensure death.
NOTE: 8 – 12 weeks old mice provide the highest yield of macrophages.
- Spray mouse legs with 70% ethanol, and pin them with arms and legs stretched out in the supine position over a foam board. With scissors and forceps to hold the skin, make a shallow cut (0.2 cm) to remove the skin and fur from the surface of each of the hind legs.
NOTE: Perform this procedure and all tissue culture manipulations in a sterile hood. - Trim muscle to make tibias and femurs visible. Remove the tibias and femurs, by cutting the bone and muscles just below the hip joint and above the ankles. Trim as much muscle off as possible.
- Spray bones with 70% ethanol and wait 1 min for it to evaporate, then place them in 6 well plate of bone flush medium (Iscove's modified Dulbecco's medium (IMDM), 10% fetal bovine serum (FBS)).
- Trim the ends of the bones by cutting 0.1 cm of bone off the ankle and top of the femur so that the bone cavity is exposed. Ensure that the marrow is visible and a 26 gauge needle can be placed in the bone cavity.
- Cut the bones and muscles to separate the tibias and femurs from the knee joints. Insert a 26 gauge needle attached to a 10 mL syringe into the cavity. Flush the bone marrow into a 50 mL conical tube with 5 mL of bone flush medium. Flush two tibias and two femurs, from one mouse, into each 50 mL tube.
- Pipette up and down several times or vortex the marrow at a slow speed to gently disperse clumps. Strings of marrow should be broken up into small (less than 0.5 mm) flecks of marrow. Fill the conical tube to 50 mL with bone flush medium. Pipette contents of the conical tube into a 75 cm2 tissue culture treated flask, and incubate at 37 °C, 5% CO2 for 1 h.
NOTE: Mature macrophages and mesenchymal cells will become adherent to the flask and be discarded, while the desired hematopoietic progenitors remain in suspension. - Pipette the medium with hematopoietic progenitors into a 50 mL conical tube. Centrifuge the tube at 300 x g at room temperature for 5 min. Discard the supernatant and resuspend the pellet in 5 mL of macrophage colony stimulating factor (MCSF) culture medium (IMDM, 10% FBS, 5 ng/mL MCSF, 100 U/mL penicillin/streptomycin, and 150 μM monothioglycerol (MTG)).
- Count cells using a hemocytometer18. Add medium to resuspend cells to a concentration of 0.5 x 106 cells/mL, 1.5 x 107 cells/flask in 30 mL of medium, and pipette up and down gently. Pipette 30 mL of the suspension into a new 75 cm2 tissue culture treated flask.
- On day 4 and day 7 after initial culture in step 1.9, verify that the cells are adherent and slightly branched using an inverted phase contrast bright field microscope. Discard the medium containing the non-adherent cells. Wash the cells with IMDM one time and add 30 mL of MCSF culture medium.
- When the cells are mature by day 10 (>95% positive for F4/80 and Mac-1), verify again that the cells are adherent and slightly.
- To plate cells for stimulations, remove the culture medium from the flask and add 8 mL of enzyme free, EDTA-based cell dissociation buffer (Table of Materials). Incubate the cells for 5 min at 37 °C, 5% CO2.
- Scrape cells gently, with a small amount of pressure, using a sterile cell scraper. Check in the microscope that cells have detached from the flask surface. Pipette dissociated cells into a 50 mL conical tube. Rinse the flask 3 times with 5 mL of IMDM and pool the rinse solution with the cells that were harvested. Centrifuge cells at 300 x g at room temperature for 5 min.
- Resuspend the cell pellet in 3 mL of MCSF culture medium per 50 mL conical tube. Count viable cells using a hemocytometer18. Resuspend cells at a concentration of 1 x 106 cells/mL and plate 100 μL of cell suspension (1 x 105 cells/well) per well of a 96-well tissue culture treated, flat bottom plate.
NOTE: Bones from one mouse will generate enough cells for 100 wells. If desired, 1 mL of cells can be plated in a 6-well plate (tissue culture treated, flat bottom). A larger number of cells (1 x 106 cells) may be useful for western blot analyses. - Once adherent, stimulate duplicate or triplicate wells each with 10 ng/mL of LPS in IMDM, 30 mg/mL of IVIg, or IVIg + LPS. Doses of LPS or IVIg can be titrated to optimize responses. Leave duplicate or triplicate wells as unstimulated controls. Incubate them for 24 h (37 °C, 5% CO2).
NOTE: Re-plated cells should adhere to tissue culture wells within 1 h. - Collect cell supernatants from each well into individual 1.7 mL microcentrifuge tubes and remove any particulate matter by spinning at 10,000 x g for 5 min.
- Remove the cell supernatant and avoid disturbing the pellet. Place the clarified cell supernatant in a sterile microcentrifuge tube.
NOTE: Cell supernatants can be assayed for cytokines, IL-10, and cytokine subunit, IL-12/23p40, by enzyme-linked immunosorbent assay (ELISA) immediately, or stored at -80 °C long term. Follow ELISA protocol from commercially available kit (Table of Materials). Other pro-inflammatory cytokines can be assayed, such as IL-6 and TNF, as they are also reduced by co-treatment with IVIg + LPS stimulation compared to stimulation with LPS alone. After stimulation, adherent cells can be prepared for techniques such as western blotting or quantitative polymerase chain reaction (Q-PCR) 19,20.
2. Challenging Mice In Vivo with IVIg and Harvesting Peritoneal Macrophages
- Weigh each mouse. Calculate the volume of IVIg (100 mg/mL stock concentration) needed to provide a final dose of 2.5 g/kg body weight for each mouse.
NOTE: For example, for a 20 g mouse, 500 µL of IVIg is required. - Draw the required amount of IVIg into a sterile 1 mL syringe fitted with a sterile 26 gauge needle. For control mice that will not receive IVIg, administer an equivalent dose volume of sterile phosphate buffered saline (PBS), pH 7.4 instead of IVIg.
- Scruff the mouse with one hand by grabbing its skin at the back of the neck with your thumb and index finger and holding its tail and hind legs with your remaining fingers. Tilt its body towards the ground.
- Place the needle at a 30 – 40° angle relative to the mouse's abdomen, in the lower right quadrant, bevel up, and insert 1.5 cm of the needle. Inject the IVIg or PBS intraperitoneally. Wait 1 h.
- Euthanize mice with 5% isoflurane anesthesia followed by 6 – 8 L/min CO2 asphyxiation (see steps 1.1.1 – 1.1.2), or according to institution's ethical guidelines.
- Pin the mouse to a foam board, with limbs spread out. Spray the parts with 70% ethanol.
NOTE: Perform the procedure in a sterile hood. - Make a shallow cut in the skin (0.2 cm) with scissors along the midline of the mouse, to avoid cutting the peritoneal cavity. Pull the abdominal skin off the mouse's belly using forceps, so that the lining of the intact peritoneal wall is visible.
- Fill a 5 mL syringe with 5 mL of sterile PBS (pH 7.4). Insert a 25 or 27 gauge needle at the top of the cavity from either the left or right side toward the center of the mouse with the bevel of the needle up.
NOTE: Place the needle carefully in the mouse so that you do not inject PBS into the organs, but rather, into the peritoneal space. - Push the fluid into the peritoneal cavity. Pull the needle out of the cavity and massage the peritoneum for 10 s to dislodge any cells. Insert needle at the top of the cavity and avoid organs. Collect and place the lavage fluid recovered into a 15 mL conical tube on ice.
NOTE: For every 5 mL of fluid injected, approximately 3.75 mL will be recovered. - Repeat injection and recovery (steps 2.8 – 2.9) 3 more times (total injection volume of 20 mL),to recover 15 mL of lavage fluid in total. Collect lavage from each mouse into a separate 15 mL conical tube.
NOTE: After the final injection, it may be easier to extract the fluid from the far left and right sides of the mouse, where fluid has accumulated. The organs will cause less interference with the needle at this position. - Spin cells down at 300 x g for 5 min at 4 °C. Resuspend the cells in 500 μL of plating medium (IMDM, 10% FBS, and 100 U/mL penicillin/streptomycin) per mouse flushed. Count viable macrophages using a hemocytometer18.
NOTE: The macrophages will be slightly larger than the other peritoneal cells. Typical yield is 5 x 105 – 1 x 106 macrophages/mouse using a wild type C57BL/6 mouse.
Optional: If a large number of red blood cells are present in the cell pellet, perform a lysis step to remove them, using the 1x red blood cell lysis buffer according to manufacturer's instructions. - Resuspend cells in plating medium at 1 x 106 macrophages/mL and plate 100 μL per well in a 96-well flat bottom tissue culture treated plate.
NOTE: It may be necessary to pool cells from more than one mouse for an experiment if using triplicate wells or titrating stimulants. For example, if one mouse had 5 x 105 macrophages, 500 μL of plating medium would be required. There would be enough cells for 5 wells, or 1 experiment in duplicate, with one LPS dose. - Incubate cells for 1 h at 37 °C, 5% CO2 to allow macrophages to become adherent. The adherent cells are the peritoneal macrophages. Remove cell supernatants and non-adherent cells. Rinse the wells twice with 200 μL IMDM (pre-warmed to 37 °C), wait 10 s and slowly tilt the plate. Replace the plating medium. Incubate the cells at 37 °C, 5% CO2 for 30 min prior to stimulation.
- Stimulate the macrophages with 10 ng/mL of LPS in IMDM, or leave unstimulated, as a control. Incubate for 24 h, collect and clarify cell supernatants (see steps 1.16 – 1.17) for IL-10 cytokine analysis by ELISA. Follow ELISA protocol from commercially available kit (Table of Materials).
NOTE: After stimulation, adherent cells can be prepared for techniques such as western blot or PCR.
3. Challenging Mice in Vivo with IVIg + LPS and Harvesting Peritoneal Macrophages
- Weigh each mouse. Calculate the volume of IVIg (100 mg/mL stock concentration) needed to provide a final dose of 2.5 g/kg body weight. Calculate the amount of LPS (100 μg/mL stock concentration in IMDM) needed to provide a final dose of 0.2 μg/g body weight.
NOTE: For example, for a 20 g mouse, 500 µL of IVIg stock solution and 40 μL of a 100 μg/mL solution of LPS is required. - Draw the required amount of IVIg and LPS into a sterile 1 mL syringe fitted with a sterile 26 gauge needle. For control mice that will not receive IVIg, replace an equivalent volume of IVIg with sterile PBS pH 7.4.
- Scruff the mouse with one hand by grabbing its skin at the back of the neck with your thumb and index finger and holding its tail and hind legs with your remaining fingers. Tilt its body towards the ground.
- Place the needle at a 30 – 40° angle relative to the mouse's abdomen, in the lower right quadrant, bevel up, and insert 1.5 cm. Inject the IVIg + LPS, or PBS + LPS, intraperitoneally as in step 2.4.
- After 1h, harvest peritoneal macrophages by performing steps 2.5 – 2.10.
- Spin cells down at 300 x g for 5 min at 4 °C. Remove 1 mL of recovered lavage fluid and use for IL-10 and IL-12/23p40 analyses by ELISA according to manufacturer's instructions or freeze at -80 °C for future analyses.
NOTE: To ensure accurate comparison between treatments for lavage fluid cytokine analysis, perform 5 mL flushs of the peritoneal cavity 4 times for a final volume of 15 mL. Not all fluid will be recovered from each flush. - As in step 2.11, resuspend and count viable macrophages.
NOTE: The macrophages will be slightly larger than the other peritoneal cells. Typical yield is 5 x 105 - 1 x 106 macrophages/mouse using a wild type C57BL/6 mouse.
Optional: If a large number of red blood cells are present in the cell pellet, perform a lysis step according to manufacturer's instructions to remove them, as in step 2.11. - As in step 2.12, re-suspend cells in plating medium.
NOTE: It may be necessary to pool cells from more than one mouse for an experiment. For example, if one mouse had 5 x 105 macrophages, 500 μL of plating medium would be required. - As in 2.13, incubate cells for 1 h at 37 °C 5% CO2 to allow macrophages to become adherent.
- Collect and clarify conditioned medium, as per steps 1.14 – 1.15, from all peritoneal cells.
NOTE: Samples may be used immediately or frozen and stored at -80 °C for cytokine analysis by ELISA. - Perform step 2.11, but incubate cells for 24 h unstimulated. Collect and clarify cell supernatants, as per steps 1.16 – 1.17, for cytokine analysis by ELISA.
NOTE: Cells can be prepared for techniques such as western blot or PCR, as in steps 1.16 – 1.17 and 2.14.
Representative Results
Murine bone marrow derived macrophages can be cultured from hematopoietic cell precursors in bone marrow aspirates. Bone marrow aspirates pooled from femurs and tibias of one C57BL/6 mouse typically yield 107 bone marrow derived macrophages, making them a convenient source of macrophages for experiments. BMDMs can be used to test antibody based drug responses when challenged with an inflammatory stimulus in vitro. Figure 1 shows that IVIg brand, Gammunex (GX), + LPS increases the production of the anti-inflammatory cytokine, IL-10, 7-fold compared to LPS stimulation alone (Figure 1A) and decreases the production of IL-12/23p40 (Figure 1B). Figure 1 also demonstrates that different IVIg preparations perform differently. Although the three different preparations of IVIg are able to decrease IL-12/23p40 significantly (Figure 1B), Octagam (OG) and Gammagard liquid (GL), do not significantly increase IL-10 production in response to LPS (Figure 1A). OG and GL are able to significantly reduce IL-12/23p40 production in response to LPS, but to a lesser degree than GX. These results demonstrate that antibody affects bone marrow derived macrophage activation, and that there are differences between preparations of the same drug that can cause changes in responses.
BMDMs can be used to test the mechanistic effects of IVIg, by western blotting. Macrophages activated with IC + LPS have increased phosphorylation of the mitogen activated protein (MAP) kinases, p38 and Erk1/2, which are responsible for increased IL-10 production21. Results in Figure 2 show a typical western blot to detect MAP kinase activation required for IL-10 production by macrophages that are unstimulated, or have been stimulated with LPS, IVIg, or IVIg + LPS. IVIg stimulation alone or IVIg + LPS co-stimulation increased activation of the MAP kinases, Erk1/2, with earlier and prolonged phosphorylation compared to that seen with LPS alone. Activation of p38 occurred earlier in macrophages stimulated with IVIg + LPS compared to those stimulated with LPS alone. These results show that methods, such as western blotting, can be used to show cell signaling effects of antibody drugs on BMDMs. In addition to cytokine production, MAP kinase activation can be used to show that anti-inflammatory macrophage activation has occurred.
Mature, tissue resident macrophages can also be isolated from mice to assess responses to IVIg 5.0 x 105 – 1.0 x 106 peritoneal macrophages can be isolated from one healthy C57BL/6 mouse. In Figure 3, peritoneal macrophages from mice injected with IVIg do not significantly increase IL-10 production in the absence of stimulation in vitro, compared to PBS injected mice. When peritoneal macrophages from IVIg injected mice are stimulated with LPS ex vivo, they produce 6-fold more IL-10 than mice injected with PBS. They do not, however, produce detectable amounts of IL-12/23p40 when stimulated with LPS ex vivo. These results demonstrate that antibody effects can be tested on macrophages by injecting the drug in vivo and culturing cells ex vivo with this method.
Macrophage responses to antibodies and inflammatory stimuli can be assessed in vivo. Cytokine production can be assessed in lavage fluid, and in supernatants from ex vivo stimulated peritoneal macrophages. Figure 4 shows the anti-inflammatory cytokine IL-10 is increased in lavage fluid (Figure 4A), peritoneal cells (Figure 4B), and peritoneal macrophages (Figure 4C) when mice are challenged with IVIg + LPS compared to PBS + LPS. Production of the pro-inflammatory cytokine subunit, IL-12/23p40, is significantly reduced in cultured peritoneal macrophages, but not in lavage fluid. This method allows examination of an antibody drug's impact on inflammatory responses in vivo as well as ex vivo.
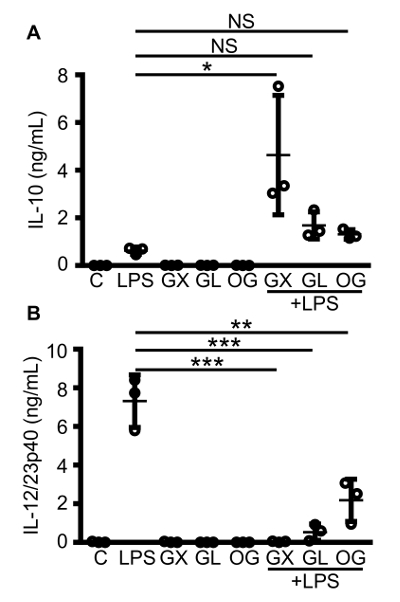
Figure 1: Macrophage IL-10 and IL-12/23p40 production in response to co-stimulation with different brands of IVIg and LPS. C57BL/6 MCSF bone marrow derived macrophages were either unstimulated (C), or stimulated with LPS (10 ng/mL), IVIg (30 mg/mL), or IVIg + LPS. Three different brands of IVIg were tested: Gammunex (GX), Gammagard liquid (GL), and Octagam (OG). Macrophage supernatants were collected 24 h after stimulation, and clarified by centrifugation. ELISAs for IL-10 (A) and IL-12/23p40 (B) were performed. Data are for macrophages from n = 3 independent experiments, with cells from 1 individual mouse per experiment, with ELISAs assayed in duplicate. Data are means ± SD. *P <0.05, ** P <0.001, ***P <0.0001 for comparison between LPS stimulation and IVIg + LPS co-stimulation. One-way analysis of variance (ANOVA) with Tukey's post-test for multiple comparisons were used for statistical analyses. Please click here to view a larger version of this figure.
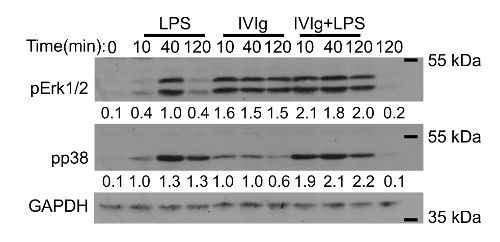
Figure 2: Western blot analysis of MAP kinase activation in IVIg-activated macrophages. MCSF derived bone marrow macrophages were unstimulated or stimulated with LPS (10 ng/mL), GX IVIg (30 mg/mL), or GX IVIg + LPS; for 0, 10, 40, or 120 min. Whole cell lysates (1.0 x 106 macrophages/lane) were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and western blotting with phospho-specific antibodies for p38 and extracellular signal-regulated kinase (Erk1/2), as well as glyceraldehyde 3-phosphate dehydrogenase (GAPDH), as a loading control. Results shown are representative of n = 3 independent experiments, with cells from 1 individual mouse per experiment. Densitometry for pp38 and pErk1/2 protein levels, normalized to GAPDH, averaged from 3 independent experiments are shown below each band. This figure has been modified from Kozicky et al8. Please click here to view a larger version of this figure.
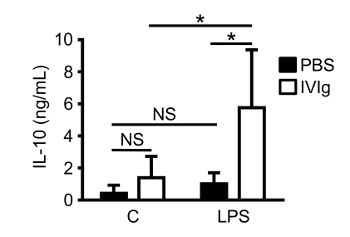
Figure 3: IL-10 production by macrophages from mice challenged with IVIg in vivo. 8-week-old C57BL/6 mice were given sterile PBS (injection control) or GX IVIg (2.5 g/kg) intraperitoneally. After 1 h, mice were euthanized and peritoneal lavages were performed. Peritoneal macrophages were isolated using adherence selection. Macrophages were either unstimulated (C), or stimulated with LPS (10 ng/mL). Cell supernatants were harvested and clarified after 24 h for IL-10 ELISAs. Data are from n = 5 individual mice per group performed in 3 independent experiments, with ELISAs assayed in duplicate. Data are means ± SD. * P <0.01 and NS = not significant. Statistical analyses were performed using a Two-way ANOVA with Sidak's posttest for multiple comparisons. This figure has been modified from Kozicky et al8. Please click here to view a larger version of this figure.
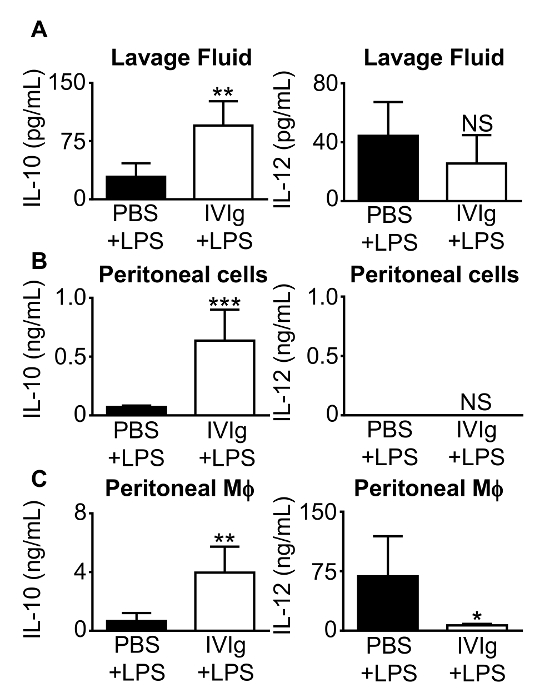
Figure 4: IL-10 and IL-12/23p40 production from mice challenged with IVIg + LPS in vivo. 8-week-old C57BL/6 mice were injected intraperitoneally with either PBS + LPS (0.2 µg/g body weight), as a control; or GX IVIg (2.5 g/kg body weight) + LPS (0.2 µg/g body weight). After 1 h, mice were euthanized and peritoneal lavages were performed. (A) Clarified lavage fluid, (B) clarified conditioned supernatants from peritoneal cells during a 1 h macrophage adherence step, and (C) clarified 24 h peritoneal macrophage (Mφ) supernatants were assayed for IL-10 and IL-12/23p40 by ELISA. Data are means ± SD for n = 5 mice in 3 independent experiments, with ELISAs assayed in duplicate. *P <0.05, **P <0.01, ***P <0.001, and NS = not significant. Mice injected with PBS + LPS were compared to mice injected with IVIg + LPS using a Student's t test. This figure has been modified from Kozicky et al8. Please click here to view a larger version of this figure.
Discussion
Macrophage activation states play an important role in both tissue homeostasis and disease22. Macrophages can have distinct as well as overlapping activation states, depending on cues in their microenvironment3. They have distinct roles in all stages of the inflammatory response: defense against pathogens, wound healing and tissue restitution, and also have a distinct anti-inflammatory activation state that is important for turning off the inflammatory response2. Anti-inflammatory macrophage activation requires two external stimuli for macrophages to produce high amounts of the anti-inflammatory cytokine, IL-10, and low amounts of pro-inflammatory cytokines, such as IL-1223. One signal comes from antibodies acting through Fc gamma receptors, and the second signal is a pro-inflammatory stimulus, such as LPS or IFNγ24,25. Serum, immune complexes, and anti-TNFα antibodies, have all been found to induce a high IL-10-produing anti-inflammatory activation state in macrophages8,23,25,26. Our group has previously published that murine bone marrow derived macrophages and peritoneal macrophages stimulated with IVIg also produce high amounts of IL-10 and little to no pro-inflammatory IL-12/23p40 in response to LPS8. We have also found that other pro-inflammatory cytokines, TNF and IL-6 are also reduced by IVIg in bone marrow derived macrophages8. The methods described here can be applied to test other antibody based drug responses in mouse BMDMs and peritoneal macrophages in vitro and in vivo.
There are many critical steps in testing antibody-based therapeutics on mouse bone marrow and peritoneal macrophages. Maintaining sterility is important for each protocol. If macrophage cultures are contaminated with microorganisms from the air, fur, or feces of mice, the macrophages will respond to those stimuli by producing pro-inflammatory cytokines. Performing all experiments in a biosafety cabinet, spraying the mouse liberally with ethanol, and ensuring integrity of the intestine during peritoneal flushes are essential. The antibody must also be kept sterile and stored according to the manufacturer's instruction. It cannot be used if it is contaminated, expired, or turbid. Turbidity will decrease the effectiveness of the drug, and can occur if the drug is not stored at the proper temperature or has been stored for too long. IVIg can be stored at 4 °C and only used before the expire date is reached, since the levels of IL-10 production can be affected. Alternatively, we have seen that it can be frozen at -20 °C with no effect on responses. Different lots of IVIg lead to similar experimental results, whereas different types of IVIg brand preparations, as in Figure 1, can cause different responses in macrophages. Essential controls, such as unstimulated cells, and cells stimulated with antibody alone, must be included in each experiment to rule out the contamination of macrophages or the drug.
Several modifications can be made to these protocols to customize an experiment. In the non-inflamed state, it can be difficult to isolate enough peritoneal macrophages for large experiments. Peritoneal macrophages can be elicited by intraperitoneal injection of thioglycollate (TG). An immune response occurs, and after 4 days, elicited peritoneal macrophages can be harvested27. The recruited macrophages may be less mature and do not represent resident cells, as we have used here, which are important considerations. TG-elicited macrophages may also have internalized agar from the TG broth, which may be a complication, particularly if doing experiments on phagocytosis27. Mice from different genetic backgrounds, C57BL/6 or BALB/c, can also be used for experiments and may be chosen to examine the impact of antibodies on macrophage responses that will be assessed in disease models requiring a specific genetic background. In addition, genetically modified mice, such as Il10-/- mice, may be used to assess the function of specific proteins in the mechanism of action of a drug, as we have done in our own work8. Different inflammatory stimuli can also be used to assess the impact of antibodies on macrophage activation. IFNγ, and host derived toll like receptor (TLR) agonists (e.g. hyaluronic acid), have been used to activate IL-10-producing macrophages25,28. An important consideration is the dose of the antibody. The dose should be titrated to obtain the optimal dose in culture and in animal experiments, as it will differ between antibodies as well as suppliers. The dose chosen should also reflect the dose given to humans, and will differ between drugs. IVIg is given as an anti-inflammatory therapy at high doses (25 – 35 mg/mL or 2 g/kg body weight), which reflects the titrated doses used in these protocols29,30.
The use of bone marrow and peritoneal macrophages have limitations. Although an improvement over macrophage cell lines, mouse BMDMs are still artificially derived cells from hematopoietic precursors. Testing immune responses of macrophages in vitro is an important step to understanding antibody responses, but in vivo experiments need to be performed as well. Injecting stimuli in vivo followed by culturing cells ex vivo, can provide more information for future studies to investigate whether antibody-based drugs can also activate anti-inflammatory macrophages in a disease state. Conclusions on what occurs in people receiving antibody-based biologics cannot be drawn from these experiments. Few studies have been published to assess the effects of IVIg on human monocyte derived macrophages in vitro. A reduction in numbers of IL-6 producing human monocytes in vitro have been reported, when IVIg is co-stimulated with LPS compared to stimulation with LPS alone12. IVIg reduces TNFα and IL-6 production when stimulated with procalcitonin (PCT) in THP-1 human monocytic cells31.
The protocols within this paper have advantages over existing methods. Bone marrow derived macrophages and peritoneal macrophages are primary cells, isolated directly from mice, rather than being immortalized cell lines. Mouse macrophage-like cell lines, such as RAW264.7 cells, do not produce the pro-inflammatory cytokine, IL-12, in response to LPS, which is an important cytokine that is regulated by the IL-10 that is produced in response to IVIg8,13. Testing a drug in vivo with LPS permits examination of the effect of the drug on the local peritoneal environment through analysis of the lavage fluid, peritoneal cells, and specifically, peritoneal macrophages. It is a short term, simple model that can provide important information to justify the examination of the non-specific effects of antibodies on macrophage activation in longer and more costly animal models of disease. It has an advantage over endotoxemia models where the experimental measure is often only mouse survival or cytokines in serum14. Survival rates and serum cytokines are valuable measures of the immune response, but they do not show the contribution that macrophages can have specifically. Isolating and culturing the peritoneal macrophages from a challenge experiment shows a direct and measurable effect that antibody therapeutics can have on macrophage function.
These methods have applications for testing and designing biologic therapies. The use of antibodies as therapeutics has dramatically increased in recent years. However, these therapies may have additional unknown effects on macrophages that can recognize the Fc portion of the antibody and change their cytokine production. The anti-TNFα antibody, infliximab, has been shown to induce IL-10 and suppress T cell proliferation through its Fc portion, and that has been proposed as one of its mechanisms of action7,15,26. Using genetic models, specific proteins can be implicated in the mechanism of how these drugs are affecting macrophage activation. Classes of IgG antibodies and preparations of antibodies can be changed to alter how macrophages are affected by biologics. Our data indicates the preparation and/or storage of the same antibody drug (IVIg) can impact its effects on macrophage activation. The methods within this paper can be used to design therapies that do not have these effects, which may be critical to maintain immunosurveillance during cancer therapies, where anti-inflammatory macrophages might promote tumor progression. These techniques could also be used to design and evaluate drugs that do have these effects, if desirable. For example, it might be beneficial to reduce macrophage inflammatory responses and create anti-inflammatory IL-10-producing macrophages to enhance treatment for inflammatory bowel diseases or rheumatoid arthritis.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
L.K. is the recipient of the University of British Columbia 4-year fellowship (4YF) graduate studentship award. L.M.S. is the recipient of a Canadian Association of Gastroenterology/ Crohn’s and Colitis Canada/ CIHR New Investigator Salary Award and is a Biomedical Scholar of the Michael Smith Foundation for Health Research. This work was supported by a Project Grant from Canadian Blood Services, in collaboration with the Canadian Institutes of Health Research (grant # CIHR2016-LS).
Materials
| Iscove’s modified Dulbecco’s medium (IMDM) | Life technologies | 12440053 | |
| Fetal Bovine Serum (FBS) | Life technologies | 12483-020 | |
| Recombinant murine macrophage colony stimulating factor (MCSF) | Stemcell technologies | 78059 | |
| Penicillin-streptomycin | Life technologies | 15140148 | |
| Monothioglycerol (MTG) | Sigma | 88640 | |
| 1X red blood cell lysis buffer | eBioscience | ||
| Cell dissociation buffer | Life technologies | 13150016 | Enzyme-Free, Hanks's-based, EDTA |
| Lipopolysaccharide (LPS) | Sigma aldrich | L 4516 | From E. coli 0127:B8 |
| IVIg (Gammunex) | Grifols | Received from BC Children's Hospital, Transfusion Medicine | |
| IVIg (Gammagard liquid) | Baxter Healthcare Corporation | Received from BC Children's Hospital, Transfusion Medicine | |
| IVIg (Octagam) | Octapharma | Received from BC Children's Hospital, Transfusion Medicine | |
| Phosphate buffered saline (PBS) (sterile), pH 7.4 | Life technologies | 10010023 | |
| mouse IL-10 ELISA | BD biosciences | 555252 | |
| mouse IL-12/23p40 ELISA | BD biosciences | 555165 | |
| anti-Erk1/2 antibody | Cell signalling technology | 9101 | |
| anti-pp38 antibody | Cell signalling technology | 9211 | |
| anti-GAPDH antibody | Fitzgerald industries | 10R-G109A | |
| 26 g needle | BD biosciences | 305110 | |
| 1 mL syringe | BD biosciences | 309659 | |
| 10 mL syringe | BD biosciences | 309604 | |
| 15 mL conical tube | BD biosciences | 352096 | |
| 50 mL conical tube | BD biosciences | 352070 | |
| microcentrifuge tube (1.7 mL) | Diamed | SPE155-N | |
| 75 cm2 tissue culture treated flask | BD biosciences | 353136 | |
| Cell scraper | BD biosciences | 353085 | |
| Forcepts | VWR | 82027-386 | fine tip, dissecting |
| Scissors | VWR | 82027-582 | Delicate, 4 1/2" |
| Brightfield microscope | Motic | AE31 | Inverted phase contrast |
| Scale | Mettler | PE 3000 |
Riferimenti
- Murray, P. J., Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 11 (11), 723-737 (2011).
- Martinez, F. O., Gordon, S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6, 13 (2014).
- Mosser, D. M., Edwards, J. P. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 8 (12), 958-969 (2008).
- Edwards, J. P., Zhang, X., Frauwirth, K. A., Mosser, D. M. Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol. 80 (6), 1298-1307 (2006).
- Mosser, D. M., Zhang, X. Activation of murine macrophages. Curr Protoc Immunol. , (2008).
- Gallo, P., Gonçalves, R., Mosser, D. M. The influence of IgG density and macrophage Fc (gamma) receptor cross-linking on phagocytosis and IL-10 production. Immunol Lett. 133 (2), 70-77 (2010).
- Vos, A. C., et al. Anti-tumor necrosis factor-α antibodies induce regulatory macrophages in an Fc region-dependent manner. Gastroenterology. 140 (1), 221-230 (2011).
- Kozicky, L. K., et al. Intravenous immunoglobulin skews macrophages to an anti-inflammatory, IL-10-producing activation state. J Leukoc Biol. 98 (6), 983-994 (2015).
- Sutterwala, F. S., Noel, G. J., Salgame, P., Mosser, D. M. Reversal of proinflammatory responses by ligating the macrophage Fcgamma receptor type I. J Exp Med. 188 (1), 217-222 (1998).
- Nimmerjahn, F., Ravetch, J. V. The antiinflammatory activity of IgG: the intravenous IgG paradox. J Exp Med. 204 (1), 11-15 (2007).
- Gelfand, E. W. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med. 368 (8), 777 (2013).
- Andersson, J., Skansén-Saphir, U., Sparrelid, E., Andersson, U. Intravenous immune globulin affects cytokine production in T lymphocytes and monocytes/macrophages. Clin Exp Immunol. 104, 10-20 (1996).
- Saito, S., Matsuura, M., Hirai, Y. Regulation of lipopolysaccharide-induced interleukin-12 production by activation of repressor element GA-12 through hyperactivation of the ERK pathway. Clin Vaccine Immunol. 13 (8), 876-883 (2006).
- Cao, S., Zhang, X., Edwards, J. P., Mosser, D. M. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J Biol Chem. 281 (36), 26041-26050 (2006).
- Neurath, M. F. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 7 (1), 6-19 (2014).
- Bryant, A., Moore, J. Rituximab and its potential for the treatment of rheumatoid arthritis. Ther Clin Risk Manag. 2 (2), 207-212 (2006).
- Weiner, L. M., Surana, R., Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 10 (5), 317-327 (2010).
- Louis, K. S., Siegel, A. C. Cell viability analysis using trypan blue: manual and automated methods. Methods Mol Biol. 740, 7-12 (2011).
- Liu, Z. Q., Mahmood, T., Yang, P. C. Western blot: technique, theory and trouble shooting. N Am J Med Sci. 6 (3), 160 (2014).
- Nolan, T., Hands, R. E., Bustin, S. A. Quantification of mRNA using real-time RT-PCR. Nat Protoc. 1 (3), 1559-1582 (2006).
- Lucas, M., Zhang, X., Prasanna, V., Mosser, D. M. ERK activation following macrophage FcgammaR ligation leads to chromatin modifications at the IL-10 locus. J Immunol. 175 (1), 469-477 (2005).
- Murray, P. J., et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 41 (1), 14-20 (2014).
- Anderson, C. F., Gerber, J. S., Mosser, D. M. Modulating macrophage function with IgG immune complexes. J Endotoxin Res. 8 (6), 477-481 (2002).
- Sutterwala, F. S., Noel, G. J., Clynes, R., Mosser, D. M. Selective suppression of interleukin-12 induction after macrophage receptor ligation. J Exp Med. 185 (11), 1977-1985 (1997).
- Riquelme, P., et al. IFN-γ-induced iNOS expression in mouse regulatory macrophages prolongs allograft survival in fully immunocompetent recipients. Mol Ther. 21 (2), 409-422 (2013).
- Vos, A. C., et al. Regulatory macrophages induced by infliximab are involved in healing in vivo and in vitro. Inflamm Bowel Dis. 18 (3), 401-408 (2012).
- Zhang, X., Goncalves, R., Mosser, D. M. The isolation and characterization of murine macrophages. Curr Protoc Immunol. , (2008).
- Gerber, J. S., Mosser, D. M. Reversing lipopolysaccharide toxicity by ligating the macrophage Fc gamma receptors. J Immunol. 166 (11), 6861-6868 (2001).
- Durandy, A., et al. Intravenous immunoglobulins–understanding properties and mechanisms. Clin Exp Immunol. 158, 2-13 (2009).
- Jolles, S., Sewell, W. A., Misbah, S. A. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 142 (1), 1-11 (2005).
- Murakami, K., et al. Intravenous immunoglobulin preparation prevents the production of pro-inflammatory cytokines by modulating NFκB and MAPKs pathways in the human monocytic THP-1 cells stimulated with procalcitonin. Inflamm Res. 63 (9), 711-718 (2014).

