基于实验室诱导的昼夜循环缺氧和 pH 值的双壳软体动物连续气门目瞪口呆测量用应变计监测仪 (通用)
Summary
了解双壳悬浮馈线对环境变量 (如溶解氧) 的行为反应, 可以解释一些生态系统过程。我们开发了一个价格低廉, 以实验室为基础的应变计监测仪 (锦葵) 来测量牡蛎、牡蛎、昼夜循环缺氧和周期性 pH 值的气门打呵欠反应。
Abstract
研制了一种成本低廉、基于实验室的应变计阀, 用于监测双壳软体动物在昼夜循环缺氧时的气门打呵欠行为。一座惠斯通电桥连接到与牡蛎壳相连的应变计 (锦葵)。记录的信号允许的开放和关闭的双壳贝类将连续记录在两天期间的实验性昼夜循环缺氧和昼夜循环变化的 pH 值。在这里, 我们描述了一个用于开发廉价的应变计监视器的协议, 并在一个示例实验室实验中描述了如何使用它来测量东部牡蛎 (锦葵) 的气门打呵欠行为, 以应对昼夜循环缺氧和pH 值的周期性变化。对周期性严重缺氧 (0.6 毫克/升) 溶解氧条件下的牡蛎进行了气门打呵欠测量, pH 值、周期性轻度缺氧 (1.7 毫克/升) 条件和常氧 (7.3 毫克/升) 条件下无周期性变化。我们证明, 当牡蛎遇到重复的昼夜周期, 他们迅速关闭他们的壳, 以应对严重缺氧和关闭的时间滞后到轻度缺氧。当 normoxia 恢复时, 它们又迅速地打开了。牡蛎没有反应周期性的 pH 条件叠加在昼夜循环严重缺氧。在氧条件降低时, 三个以上的牡蛎同时关闭。我们证明, 牡蛎对昼夜循环缺氧的反应, 这必须考虑当评估的行为, 双壳贝类溶解氧。该阀可用于评估双壳软体动物对溶解氧或污染物的变化的反应。密封技术, 以更好地密封的阀门打哈欠应变计从海水需要进一步改进, 以提高传感器的寿命。
Introduction
缺氧,即溶解氧浓度足够低, 对生物和生态过程产生负面影响, 但通常功能上定义为 [做] < 2 毫克/升1, 缺氧 (功能上定义为 0.0-0.2 毫克/升)在世界沿海水域、河口和深海2、3发生频繁和严重的情况, 并经常因增加富营养化4、5而加剧。随着缺氧和缺氧程度的增加, 动物对生境的影响和生境质量的丧失。预计气候变化会恶化缺氧和缺氧6。
在许多分层, 营养丰富的河口, 如美国切萨皮克湾, 季节性持续缺氧可以战胜和可能发生年复一年2年。此外, 昼夜的低氧循环经常出现在诸如切萨皮克湾和其他地点的河口, 并发生在夜间或清晨7,8的凌晨时间。
大多数研究的重点是持续暴露的有机体对低 [做] 和对他们的耐受性缺氧和缺氧9,10,11,12,13,14.此外, 研究还观察了物种分布、丰度和物种组成的大规模转移, 以响应延长的低 [做]4,15。通常对低 [做] 非常敏感的物种, 死于大众,16将剩下的物种转移到一个年轻的, 规模较小, 短命的动物群中, 例如, 在路易斯安那州-德克萨斯大陆架生态系统4上发现。
行为变化通常在社区崩溃之前 17和研究报告了有机体的行为反应到延长的低 [做]4,16,17,18,19 ,20,21,22,23,24,25。然而, 这些研究并不侧重于生物体对缺氧昼夜循环暴露的反应以及河口 [做] 可用性的波动性。
昼夜在浅水河口的循环缺氧已得到越来越多的认识, 因为研究监测 [做] 更频繁的过程中的天数与 sondes 在河口16,26。水可以保持缺氧几个小时, 在夜间或清晨小时, 当没有氧气的光合作用在夜间, 但高耗氧呼吸7,16。研究还发现, 潮汐影响低条件下的昼夜循环, 当低潮与夜间27的结束相吻合时, 观测到最极端的极小值。只有在几个小时的缺氧后才会回到 normoxia7,16,28在每天的周期。
为了确定锦葵对昼夜循环缺氧和 ph 值的行为反应, 我们监测了暴露于实验室诱导的昼夜循环和周期性 pH 值的牡蛎瓣膜的开启和关闭。双壳贝类的打呵欠反应已被用来检测恶劣的环境条件。在反应污染物29、30、31、有毒藻类32、33、34、热污染35、36的情况下, 双壳贝类阀门关闭,37、食物数量减少38,39,40, 哺养的政权39,41, 再现37,42, 光周期43,44、ph 值45、46、ph 值和溶解氧的总和47均已测定。例如, 打呵欠技术包括直接观测48,49,13, 连续测量使用簧片开关和磁铁 (Dreissena 显示器)50, 或光纤传感器51需要清水。此外, 磁铁和磁场强度霍尔传感器已用于研究贻贝角52,53,54,55和高频电磁感应系统这可以测量两个电线圈之间的不同距离, 被粘在阀门已使用56,57,58,59。电磁感应系统需要高压源, 必须将电源输送到外壳52的两侧。该系统还可作为 “MOSSELMONITOR” (http://mosselmonitor.nl/) 提供商业用途。
在紧张的研究预算, 我们建立了一个廉价的应变计监测 (通用), 以持续测量牡蛎目瞪口呆的实验室诱导昼夜循环的 [做] 和 pH 值, 在低能见度条件下。我们的系统也比竞争系统简单得多, 允许许多动物在实验中进行检测。我们想确定 C 的行为反应. 锦葵昼夜循环严重 ([做] = 0.6 毫克/升) 缺氧与控制 ph 值 (ph = 7.8) 和循环 ph 值 (ph = 7.8-7.0), 并对轻度 ([做] = 1.7 毫克/升) 缺氧的目瞪口呆反应。此外, 我们想确定牡蛎是否能够迅速响应在昼夜周期的变化, 以及他们如何反应时, normoxia 返回后, 缺氧事件。也许牡蛎是最佳地适应迅速地波动的环境在许多河口16,27在他们居住的地方被发现。虽然有更多复杂的阀门目瞪口呆显示器可用, 但通用提供了一种廉价的技术, 允许连续测量阀门在水中的目瞪口呆, 即使在低能见度条件。
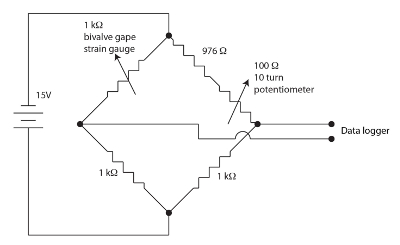
图 1。用于气门打呵欠装置的惠斯通电桥.请单击此处查看此图的较大版本.
用于监测双壳类打呵欠的应变计传感器是聚酰亚胺支持下的一种曲折模式的电阻膜。少量的应变调节传感器的电阻。当呵欠导致传感器的电阻发生变化时, 双壳贝类会弯曲应变片。我们使用了一个零位, 平衡, 惠斯通电桥每双壳的通道, 如图 1所示, 以测量传感器电阻的变化。惠斯通电桥由电位器清零, 允许数据存储器使用相当高的增益。惠斯通电桥是一种标准方法, 用于准确测量未知电阻, 使用与已知电阻标准和电压表的比值。这个非常老技术的历史在 Ekelof (2001)60被谈论。我们集成了12通道, 每一个都有自己的惠斯通电桥和零电位计, 进入应变仪显示器 (通用) 单元。
Protocol
Representative Results
Discussion
典型的研究集中在连续, 延长的时间期间低氧气条件和反应, 经常被测量作为生存, 动物。然而, 目前我们对动物对昼夜循环缺氧的行为反应的理解最小为63。因此, 更多的研究应该集中在反应昼夜循环缺氧的有机体的行为, 这在夏天经常发生在许多河口7,8。
在此, 我们提出了一种连续测量双壳贝类对昼夜循环缺氧和?…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
我们感谢梅林达 Forseth 拍摄牡蛎的照片和测量他们在 ImageJ 的打呵欠宽度。我们感谢丹尼斯 Breitburg 水族馆与昼夜循环缺氧和周期性 pH 条件的接触。我们感谢史密森环境研究中心, Edgewater, 马里兰, 为实验空间。低氧实验由国家海洋和大气管理中心资助, 为海岸海洋研究资助号。NA10NOS4780138 和史密森亨特登基金对丹尼斯 Breitburg。在缺氧实验过程中, 阀门的打呵欠测量是由华盛顿学院为埃尔卡 t. 波特提供的一项师资增强补助金资助的。
Materials
| Campbell CR 10x data logger | Campbell Scientific, Logan, Utah | Or other data logger. At Campbell the CR 10X has been replaced with the CR 1000 | |
| Campbell CR 10x multiplexer | Campbell Scientific, Logan, Utah | Data logger needs to have space for 12 channels | |
| Dsub connector male crimp pins | TE Connectivity | 205089-1 | pins for gape sensor leads |
| PCA tape | Micro Measurements Corp, NC | To seal the strain gauge | |
| Duro Quick Gel | Ace Hardware | Superglue | |
| SG13/1000-LY43 or LY41 | Omega Engineering Inc., Stanford, CT | Strain gauges | |
| 32 AWG (7/40) teflon Alpha wires | AlphaWire, Elizabeth, NJ | 2840/7 | Sensor cables, different colors are available |
| 1/16" heat shrink tubing | Qualtek | B01A3QKKO6 | To seal the leads of the sensor cable |
| Weller WES51 Analog Soldering Station | Amazon | Lots of soldering, need a good soldering iron. https://www.amazon.com/Weller-WES51-Analog-Soldering-Station/dp/B000BRC2XU/ref=sr_1_23?s=hi&ie=UTF8&qid=1505654295 &sr=1-23&keywords=soldering+iron |
|
| Rosin Soldering Flux Paste | Amazon | Needed for soldering | |
| 60-40 Tin Lead Rosin Core Solder Wire | Amazon | Needed for soldering | |
| Aquarium sealant | Home Depot | Attach sensors to bivalve | |
| PC Laptop | Any old PC to run Campbell gape program | ||
| heat gun | Amazon | shrink shrink tubing | |
| Drill | Hardware store, Amazon | for twisting wires to make sensor cables | |
| AC to DC power module | Acopian | DB15-30 | Wheatstone bridge power supply |
| Poteniometer | Clarostat | 733A | Wheatsone bridge nulling |
| isolating BNC connector | Sterren Electronics | "200-148 | Wheatstone bridge output for multimeter |
| Fused AC receptical panel module | Adam technologies | IEC-GS-1-200 | Wheatstone bridge power supply connector |
| 976 ohm 1% resistor | Vishay Dale | CMF50976R00FHEB | Wheatstone bridge resistor |
| 1 kohm 1% resistor | Vishay Dale | CMF501K0000FHEB | Wheatstone bridge resistor |
| Potentiometer scale dial | Kilo International | 462 | 10 turn dial for nulling potentiometer |
| DB25 male panel connector | TE connectivity | 1757819-8 | Data logger connector on Wheatstone bridge |
| DB25 female panel connector | TE connectivity | 1757819-8 | Sensor connector to Wheatstone bridge |
| perforated circuit board | Vector electronics | 64P44WE | circuit board for mounting of bridge components |
| enclosure | Hammond Manufacturing | 1444-29 | Enclosure for sensor readout electronics |
Riferimenti
- Vaquer-Sunyer, R., Duarte, C. M. Thresholds of hypoxia for marine biodiversity. Proceedings of the National Academy of Sciences of the United States of America. 105 (40), 15452-15457 (2008).
- Diaz, R. J., Rosenberg, R. Spreading dead zones and consequences for marine ecosystems. Science. 321, 926-929 (2008).
- Levin, L. A., Breitburg, D. L. Linking coasts and seas to address ocean deoxygenation. Nature Climate Change. 5, 401-403 (2015).
- Diaz, R. J., Rosenberg, R. Marine benthic hypoxia: A review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanography and Marine Biology: An annual Review. 33, 245-303 (1995).
- Patterson, H. K., Boettcher, A., Carmichael, R. H. Biomarkers of dissolved oxygen stress in oysters: a tool for restoration and management efforts. PLoS One. 9 (8), 104440 (2014).
- Altieri, A. H., Gedan, K. B. Climate change and dead zones. Global Change Biology. 21 (4), 1395-1406 (2015).
- Tyler, R. M., Brady, D. C., Targett, T. E. Temporal and spatial dynamics of diel-cycling hypoxia in estuarine tributaries. Estuaries and Coasts. 32 (1), 123-145 (2009).
- Breitburg, D. L., et al. Landscape-level variation in disease susceptibility related to shallow-water hypoxia. PLoS One. 10 (2), 0116223 (2015).
- Stickle, W. B., Kapper, M. A., Liu, L. -. L., Gnaiger, E., Wang, S. Y. Metabolic adaptations of several species of crustaceans and molluscs to hypoxia: toterance and microcalometric studies. Biological Bulletin. 177 (2), 303-312 (1989).
- Gamenick, I., Jahn, A., Vopel, K., Guiere, O. Hypoxia and sulphide as structuring factors in a macrozoobenthic community on the Baltic Sea shore: colonization studies and tolerance experiments. Marine Ecology Progress Series. , 73-85 (1996).
- Modig, H., Olafsson, E. Responses of Baltic benthic invertebrates to hypoxic events. Journal of Experimental Marine Biology and Ecology. 229 (1), 133-148 (1998).
- Riedel, B., Zuschin, M., Stachowitsch, M. Tolerance of benthic macrofauna to hypoxia and anoxia in shallow coastal seas: a realistic scenario. Marine Ecology Progress Series. 458, 39-52 (2012).
- Lombardi, S. A., Harlan, N. P., Paynter, K. T. Survival, acid-base balance, and gaping responses of the Asian Oyster C. ariakensis and the Eastern Oyster C. virginica during clamped emersion and hypoxic immersion. Journal of Shellfish Research. 32 (2), 409-415 (2013).
- Jansson, A., Norkko, J., Dupont, S., Norkko, A. Growth and survival in a changing environment: Combined effects of moderate hypoxia and low pH on juvenile bivalve Macoma balthica. Journal of Sea Research. 102, 41-47 (2015).
- Gooday, A. J., et al. Faunal responses to oxygen gradients on the Pakistan margin: A comparison of foraminiferans, macrofauna and megafauna. Deep Sea Research Part II: Topical Studies in Oceanography. 56 (6-7), 488-502 (2009).
- Montagna, P. A., Ritter, C. Direct and indirect effects of hypoxia on benthos in Corpus Christi Bay, Texas, U.S.A. Journal of Experimental Marine Biology and Ecology. 330 (1), 119-131 (2006).
- Villnas, A., Norkko, J., Lukkari, K., Hewitt, J., Norkko, A. Consequences of increasing hypoxic disturbance on benthic communities and ecosystem functioning. PLoS One. 7 (10), 44920 (2012).
- Breitburg, D. Effects of hypoxia, and the balance between hypoxia and enrichment on coastal fishes and fisheries. Estuaries. 25 (4), 767-781 (2002).
- Costantini, M., et al. Effect of hypoxia on habitat quality of striped bass (Morone saxatilis) in Chesapeake Bay. Canadian Journal of Fisheries and Aquatic Sciences. 65 (5), 989-1002 (2008).
- Ludsin, S. A., et al. Hypoxia-avoidance by planktivorous fish in Chesapeake Bay: Implications for food web interactions and fish recruitment. Journal of Experimental Marine Biology and Ecology. 381, 121-131 (2009).
- Zhang, H., et al. Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. Journal of Experimental Marine Biology and Ecology. 381, 80-91 (2009).
- Sparks, B. L., Strayer, D. L. Effects of low dissolved oxygen on juvenile Elliptio complanata (Bivalvia:Unionidae). Journal of the Norther American Benthological Society. 17, 129-134 (1998).
- Llanso, R. J. Effects of hypoxia on estuarine benthos: the lower Rappahannock River (Chesapeake Bay), a case study. Estuarine, Coastal and Shelf Science. 35 (5), 491-515 (1992).
- Riedel, B., Zuschin, M., Haselmair, A., Stachowitsch, M. Oxygen depletion under glass: Behavioural responses of benthic macrofauna to induced anoxia in the Northern Adriatic. Journal of Experimental Marine Biology and Ecology. 367 (1), 17-27 (2008).
- Riedel, B., et al. Effect of hypoxia and anoxia on invertebrate behaviour: Ecological perspectives from species to community level. Biogeosciences. 11 (6), 1491-1518 (2014).
- Breitburg, D. L. Near-shore hypoxia in the Chesapeake Bay: Patterns and relationships among physical factors. Estuarine Coastal and Shelf Science. 30, 593-609 (1990).
- Baumann, H., Wallace, R. B., Tagliaferri, T., Gobler, C. J. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries and Coasts. 38, 220-231 (2015).
- Breitburg, D. L., et al. Landscape-level variation in disease susceptibility related to shallow-water hypoxia. PLoS One. 10 (2), 0116223 (2015).
- de Zwart, D., Kramer, J. M., Jenner, H. A. Practical experiences with the biological early warning system “mosselmonitor”. Environmental Toxicology and Water Quality. 10 (4), 237-247 (1995).
- Kadar, E., et al. Avoidance responses to aluminum in the freshwater bivalve Anodonta cygnea. Aquatic Toxicology. 55, 137-148 (2001).
- Soliman, M. F. M., El-Shenawy, N. S., Tadros, M. M., Abd El-Azeez, A. A. Impaired behavior and changes in some biochemical markers of bivalve (Ruditapes decussatus) due to zinc toxicity. Toxicological & Environmental Chemistry. 97 (5), 674-686 (2015).
- Shumway, S. E., Cucci, T. L. The effects of the toxic dinoflagellate Protogonyaulax tamarensis on the feeding and behaviour of bivalve molluscs. Aquatic Toxicology. 10, 9-27 (1987).
- Basti, L., et al. Effects of the toxic dinoflagellate Heterocapsa circularisquama on the valve movement behaviour of the Manila clam Ruditapes philippinarum. Aquaculture. 291 (1-2), 41-47 (2009).
- Tran, D., Haberkorn, H., Soudant, P., Ciret, P., Massabuau, J. -. C. Behavioral responses of Crassostrea gigas exposed to the harmful algae Alexandrium minutum. Aquaculture. 298 (3-4), 338-345 (2010).
- Shumway, S. E., Koehn, R. K. Oxygen consumption in the American oyster Crassostrea virginica. Marine Ecology Progress Series. 9, 59-68 (1982).
- Nicastro, K. R., Zardi, G. I., McQuaid, C. D., Pearson, G. A., Serrao, E. A. Love thy neighbour: group properties of gaping behaviour in mussel aggregations. PLoS One. 7 (10), (2012).
- Dowd, W. W., Somero, G. N. Behavior and survival of Mytilus. congeners following episodes of elevated body temperature in air and seawater. Journal of Experimental Biology. 216 (3), 502-514 (2013).
- Higgins, P. J. Effects of food availability on the valve movements and feeding behavior of juvenile Crassostrea virginica (Gmelin). I. Valve movements and periodic activity. Journal of Experimental and Experimental Marine Biology and Ecology. 45, 229-244 (1980).
- Riisgård, H. U., Lassen, J., Kittner, C. Valve-gape response times in mussels (Mytilus edulis)-Effects of laboratory preceding-feeding conditions and in situ tidally induced variation in phytoplankton biomass. Journal of Shellfish Research. 25, 901-911 (2006).
- Robson, A. A., De Leaniz, C. G., Wilson, R. P., Halsey, L. G. Behavioural adaptations of mussels to varying levels of food availability and predation risk. Journal of Molluscan Studies. 76, 348-353 (2010).
- Robson, A. A., de Leaniz, C. G., Wilson, R. P., Halsey, L. G. Effect of anthropogenic feeding regimes on activity rhythms of laboratory mussels exposed to natural light. Hydrobiologia. 655, 197-204 (2010).
- Nicastro, K. R., Zardi, G. I., McQuaid, C. D., Stephens, L., Radloff, S., Blatch, G. L. The role of gaping behaviour in habitat partitioning between coexisting intertidal mussels. BMC Ecology. 10, 17 (2010).
- Loosanoff, V. S., Nomejko, C. A. Feeding of oysters in relation to tidal stages and to periods of light and darkness. Biological Bulletin. 90 (3), 244-264 (1946).
- Comeau, L. A., Mayrand, E., Mallet, A. Winter quiescence and spring awakening of the Eastern oyster Crassostrea virginica at its northernmost distribution limit. Marine Biology. 159 (10), 2269-2279 (2012).
- Pynonnen, K. S., Huebner, J. Effects of episodic low pH exposure on the valve movements of the freshwater bivalve Anodonta cygnea L. Water Research. 29 (11), 2579-2582 (1995).
- Jakubowska, M., Normant-Saremba, M. The effect of CO2-induced seawater acidification on the behaviour and metabolic rate of the baltic clam Macoma balthica. Annales Zoologici Fennici. 52 (5-6), 353-367 (2015).
- Jakubowska, M., Normant, M. Metabolic rate and activity of blue mussel Mytilus edulis trossulus.under short-term exposure to carbon dioxide-induced water acidification and oxygen deficiency. Marine and Freshwater Behaviour and Physiology. 48 (1), 25-39 (2015).
- Newell, C. R., Wildish, D. J., MacDonald, B. A. The effects of velocity and seston concentration on the exhalent siphon area, valve gape and filtration rate of the mussel Mytilus edulis. Journal of Experimental Marine Biology and Ecology. 262, 91-111 (2001).
- Maire, O., Amouroux, J. -. M., Duchene, J. -. C., Gremare, A. Relationship between filtration activity and food availability in the Mediterranean mussel Mytilus galloprovincialis. Marine Biology. 152 (6), 1293-1307 (2007).
- Borcherding, J. Ten years of practical experience with the Dreissena-monitor, a biological early warning system for continuous water quality monitoring. Hydrobiologia. 556 (1), 417-426 (2006).
- Frank, D. M., Hamilton, J. F., Ward, E. E., Shumway, S. E. A fiber optic sensor for high resolution measurement and continuous monitoring of valve gape in bivalve molluscs. Journal of Shellfish Research. 26 (2), 575-580 (2007).
- Wilson, R., Reuter, P., Wahl, M. Muscling in on mussels: New insights into bivalve behaviour using vertebrate remote-sensing technology. Marine Biology. 147 (5), 1165-1172 (2005).
- Nagai, K., Honjo, T., Go, J., Yamashita, H., Seok Jin, O. Detecting the shellfish killer Heterocapsa circularisquama (Dinophyceae) by measuring bivalve valve activity with a Hall element sensor. Aquaculture. 255 (1-4), 395-401 (2006).
- Robson, A., Wilson, R., de Leaniz, C. G. Mussels flexing their muscles: a new method for quantifying bivalve behaviour. Marine Biology. 151 (3), 1195-1204 (2007).
- Robson, A. A., Thomas, G. R., de Leaniz, C. G., Wilson, R. P. Valve gape and exhalant pumping in bivalves: optimization of measurement. Aquatic Biology. 6 (1-3), 191-200 (2009).
- de Zwart, D., Kramer, J. M., Jenner, H. A. Practical experiences with the biological early warning system “mosselmonitor”. Environmental Toxicology and Water Quality. 10 (4), 237-247 (1995).
- Jou, L. -. J., Lin, S. -. C., Chen, B. -. C., Chen, W. -. Y., Liao, C. -. M. Synthesis and measurement of valve activities by an improved online clam-based behavioral monitoring system. Computers and Electronics in Agriculture. 90, 106-118 (2013).
- Barile, N. B., Scopa, M., Recchi, S., Nerone, E. Biomonitoring of coastal marine waters subject to anthropogenic use: development and application of the biosensor Mosselmonitor. Ovidius University Annals of Chemistry. 27 (2), 81-86 (2016).
- Ballesta-Artero, I., Witbaard, R., Carroll, M. L., van der Meer, J. Environmental factors regulating gaping activity of the bivalve Arctica islandica in Northern Norway. Marine Biology. 164 (5), 116 (2017).
- Ekelof, S. The genesis of the Wheatstone bridge. Engineering Science and Education Journal. 10, 37-40 (2001).
- Keppel, A. G., Breitburg, D. L., Wikfors, G. H., Burrell, R. B., Clark, V. M. Effects of co-varying diel-cycling hypoxia and pH on disease susceptibility in the eastern oyster Crassostrea virginica. Marine Ecology Progress Series. 538, 169-183 (2015).
- Burrell, R. B., Keppel, A. G., Clark, V. M., Breitburg, D. L. An automated monitoring and control system for flow-through co-cycling hypoxia and pH experiments. Limnology and Oceanography: Methods. 14, 168-185 (2015).
- Porter, E. T., Breitburg, D. L. Eastern oyster Crassostrea virginica, valve gape behavior under diel-cycling hypoxia. Marine Biology. 163 (218), (2016).
- Bergeron, C. M. . The impact of sediment resuspension on mercury cycling and the bioaccumulation of methylmercury into benthic and pelagic organisms. , (2005).

