Microdialysis of Excitatory Amino Acids During EEG Recordings in Freely Moving Rats
Summary
Here, we describe a method for in vivo microdialysis to analyze aspartate and glutamate release in the ventral hippocampus of epileptic and non-epileptic rats, in combination with EEG recordings. Extracellular concentrations of aspartate and glutamate may be correlated with the different phases of the disease.
Abstract
Microdialysis is a well-established neuroscience technique that correlates the changes of neurologically active substances diffusing into the brain interstitial space with the behavior and/or with the specific outcome of a pathology (e.g., seizures for epilepsy). When studying epilepsy, the microdialysis technique is often combined with short-term or even long-term video-electroencephalography (EEG) monitoring to assess spontaneous seizure frequency, severity, progression and clustering. The combined microdialysis-EEG is based on the use of several methods and instruments. Here, we performed in vivo microdialysis and continuous video-EEG recording to monitor glutamate and aspartate outflow over time, in different phases of the natural history of epilepsy in a rat model. This combined approach allows the pairing of changes in the neurotransmitter release with specific stages of the disease development and progression. The amino acid concentration in the dialysate was determined by liquid chromatography. Here, we describe the methods and outline the principal precautionary measures one should take during in vivo microdialysis-EEG, with particular attention to the stereotaxic surgery, basal and high potassium stimulation during microdialysis, depth electrode EEG recording and high-performance liquid chromatography analysis of aspartate and glutamate in the dialysate. This approach may be adapted to test a variety of drug or disease induced changes of the physiological concentrations of aspartate and glutamate in the brain. Depending on the availability of an appropriate analytical assay, it may be further used to test different soluble molecules when employing EEG recording at the same time.
Introduction
To provide insight into the functional impairment of glutamate-mediated excitatory and GABAergic inhibitory neurotransmission resulting in spontaneous seizures in temporal lobe epilepsy (TLE),we systematically monitored extracellular concentrations of GABA1 and later the levels of glutamate and aspartate2 by microdialysis in the ventral hippocampus of rats at various time-points of the disease natural course, i.e., during development and progression of epilepsy. We took advantage of the TLE pilocarpine model in rats, which mimics the disease very accurately in terms of behavioral, electrophysiological and histopathological changes3,4 and we correlated the dialysate concentration of amino acids to its different phases: the acute phase after the epileptogenic insult, the latency phase, the time of the first spontaneous seizure and the chronic phass5,6,7. Framing the disease phases was enabled by long-term video-EEG monitoring and the precise EEG and clinical characterization of spontaneous seizures. Application of the microdialysis technique associated with long-term video-EEG monitoring allowed us to propose mechanistic hypotheses for TLE neuropathology. In summary, the technique described in this manuscript allows the pairing of neurochemical alterations within a defined brain area with the development and progression of epilepsy in an animal model.
Paired devices, made up of a depth electrode juxtaposed to a microdialysis cannula, are often employed in epilepsy research studies where changes in neurotransmitters, their metabolites, or energy substrates should be correlated to neuronal activity.In the vast majority of cases, it is used in freely behaving animals, but it can be also conducted in a similar way in human beings, e.g., in pharmaco-resistant epileptic patients undergoing depth electrode investigation prior to surgery8. Both EEG recording, and dialysate collection may be performed separately (e.g., implanting the electrode in one hemisphere and the microdialysis probe in the other hemisphere or even performing the microdialysis in one group of animals while performing the sole EEG in another group of animals). However, coupling the electrodes to probes may have multiple advantages: it simplifies stereotaxic surgery, limits tissue damage to only one hemisphere (while leaving the other, intact, as a control for histological studies), and homogenizes the results as these are obtained from the same brain region and the same animal.
On the other hand, the preparation of the coupled microdialysis probe-electrode device requires skills and time if it is home-made. One could spend relatively high amounts of money if purchased from the market. Moreover, when microdialysis probes (probe tips are typically 200-400 µm in diameter and 7-12 mm long)9, and EEG electrodes (electrode tips are usually of 300-500 µm in diameter, and long enough to reach the brain structure of interest10) are coupled, the mounted device represents a bulky and relatively heavy object on one side of the head, which is troublesome for animals and prone to be lost especially when it is connected to the dialysis pump and the hard-wire EEG recording system. This aspect is more relevant in epileptic animals that are difficult to handle and less adaptive to the microdialysis sessions. Proper surgical techniques and appropriate post-operative care can result in long-lasting implants that cause minimal animal discomfort and should be pursued for combinatory microdialysis-EEG experiments10,11,12.
The advantages and limitations of the microdialysis technique have been reviewed in detail by many neuroscientists. Its primary advantage over other in vivo perfusion techniques (e.g., fast flow push-pull or cortical cup perfusion) is a small diameter of the probe which covers a relatively precise area of interest13,14,15. Second, the microdialysis membrane creates a physical barrier between the tissue and the perfusate; therefore, high-molecular weight substances do not cross and do not interfere with the analysis16,17. Moreover, the tissue is protected from the turbulent flow of the perfusate18. Another important advantage is the possibility to modify the perfusate flow for maximizing the analyte concentration in the perfusate (i.e., the process of microdialysis can be well defined mathematically and can be modified to yield high concentrations of the analyte in the sample)19. Finally, the technique may be used to infuse the drugs or pharmacologically active substances into the tissue of interest and to determine their effect at the site of intervention20. On the other hand, microdialysis has a limited resolution time (typically more than 1 min due to the time needed for collecting samples) in comparison to electrochemical or biological sensors; it is an invasive technique that causes tissue damage; it compromises the neurochemical balance within the space around the membrane due to the continuous concentration gradient of all soluble substances which enters the perfusate together with the analyte of interest. Finally, the microdialysis technique is highly influenced by the limits of the analytical techniques employed for the quantification of substances in the perfusate9,21,22,23. The high-performance liquid chromatography (HPLC) after derivatization with orthophthaldialdehyde for glutamate and aspartate analysis in biological samples has been well validated24,25,26,27 and its extensive discussion is out of the scope of this manuscript, but the data produced by using this method will be described in detail.
When performed properly and without modifications of the perfusate composition, microdialysis can provide reliable information about the basal levels of neurotransmitter release. The largest portion of the basal levels is likely the result of the transmitter spillover from the synapses9. Because in many instances the simple sampling of the neurotransmitter in the extra synaptic space is not sufficient to pursue the goals of an investigation, the microdialysis technique can be also employed to stimulate neurons or to deprive them of important physiological ions such as K+ or Ca2+, in order to evoke or prevent the release of the neurotransmitter.
High K+ stimulation is often used in neurobiology to stimulate neuronal activity not only in awake animals but also in primary and organotypic cultures. The exposure of a healthy central nervous system to solutions with high concentrations of K+ (40-100 mM) evokes the efflux of neurotransmitters28. This ability of neurons to provide an additional release in response to high K+ may be compromised in epileptic animals1 and in other neurodegenerative diseases29,30. Similarly, the Ca2+ deprivation (obtained by perfusing Ca2+ free solutions) is used to establish calcium-dependent release of most neurotransmitters measured by microdialysis. It is generally believed that Ca2+ dependent release is of neuronal origin, whereas Ca2+ independent release originates from glia, but many studies raised controversy over the meaning of Ca2+-sensitive measurements of e.g. glutamate or GABA9: thus, if possible, it is advisable to support microdialysis studies with microsensor studies, as these latter have higher spatial resolution and the electrodes allows to get closer to synapses31.
Regarding microdialysis studies in epileptic animals, it is important to stress that the data obtained from most of them rely upon video or video-EEG monitoring of seizures, i.e., of the transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain32. There are some specifics of electrographic seizures in pilocarpine treated animals which should be considered when preparing the experiment. Spontaneous seizures are followed by depressed activity with frequent EEG interictal spikes3 and occur in clusters33,34. Sham operated non-epileptic animals may exhibit seizure-like activity35 and therefore the parameters for EEG recordings evaluation should be standardized36 and, if possible, the timing of microdialysis sessions should be well defined. Finally, we highly recommend following the principles and methodological standards for video-EEG monitoring in control adult rodents outlined by experts of International League Against Epilepsy and American Epilepsy Society in their very recent reports37,38.
Here, we describe microdialysis of glutamate and aspartate in parallel with the long-term video-EEG recordings in epileptic animals and their analysis in the dialysate by HPLC. We will emphasize the critical steps of the protocol that one should take care of for best result.
Protocol
All experimental procedures have been approved by the University of Ferrara Institutional Animal Care and Use Committee and by the Italian Ministry of Health (authorization: D.M. 246/2012-B) in accordance with guidelines outlined in the European Communities Council Directive of 24 November 1986 (86/609/EEC). This protocol is specifically adjusted for glutamate and aspartate determination in rat brain dialysates obtained under EEG control of microdialysis sessions in epileptic and non-epileptic rats. Many of the materials described here may be easily replaced with those that one uses in his laboratory for EEG recordings or microdialysis.
1. Assembly of the Microdialysis Probe-electrode Device
- Use a 3-channel two-twisted electrode (with at least a 20 mm cut length of the registering electrode and a 10 cm long grounding electrode) and couple it to a guide cannula to prepare the device. See examples of 3-channel electrode and guide cannula for dialysis in Figure 1A-1B.
- Remove (Figure 1C) and insert (Figure 1D) the metal guide cannula into the dummy plastic cannula a few times prior to the usage in order to ease its removal at the moment of its switch for microdialysis probe in animal.
- Bend the twisted wires of registering electrode two times (Figure 1E-1F) in order to align the wires with the dummy cannula of the guide and cut the electrode tip (Figure 1G) to be 0.5 mm longer than the tip of the guide cannula (Figure 1H) using the digital caliper.
- Have ready the 1 mm long silicon circlet (O.D. 2 mm, thickness 0.3 mm; Figure 1I) and insert the tip of the guide cannula and the tip of the twisted electrodes into the silicon circlet using the tweezers (Figure 1J). Fix it onto the foot of the guide cannula pedestal with polymer glue of rapid action or resin (Figure 1K). See the example of the completed devices in Figure 1L and Figure 2A.
- Sterilize the device under germicidal UV light for 4 h. Turn over the device four times so as each of its sides is exposed to the light for 1 h.
Note: Many home-made electrodes and microdialysis probes may be assembled in a similar way. The head of above described implant for rats has the following dimensions: 7 mm width x 5 mm depth x 10 mm length from the top to pedestal toe; the implant tip is about 11 mm long, 600 µm in diameter and all the device weights about 330-360 mg. The device may be reused two or three times if (i) a sufficient length of the ground electrode is left on the skull during the surgery for the next use and (ii) when the animal is killed, and the device recovered together with the dental cement it is left in acetone overnight, such that the cement may be mechanically disaggregated, and the device washed and sterilized again.
2. Stereotaxic Surgery
- Use a stereotaxic apparatus and probe clip holder (Figure 2B) for the device implantation following the contemporary standards for aseptic and pain-free surgeries39,40,41.
- Anesthetize the adult Sprague-Dawley rats with ketamine/xylazine mixture (43 mg/kg and 7 mg/kg, i.p.) and fix it onto the stereotaxic frame. Add isoflurane anesthesia (1.4% in air; 1.2 mL/min) to initial ketamine/xylazine injection as it allows to control the depth of anesthesia in time. Shave the fur on animal’s head.
- Swab the head skin surface by iodine-based solution followed by 70% ethanol to prepare it for aseptic surgery39.
- Implant the guide cannula-electrode device prepared in precedence (1.1 – 1.5) into the right ventral hippocampus using the following coordinates: nose bar + 5.0 mm, A – 3.4 mm, L + 4.5 mm, P + 6.5 mm to bregma1,2. Follow standard techniques for stereotaxic surgeries10,11,12.
- Ensure that it does not cover the anchoring screws. When mounting it onto the stereotaxic apparatus, grasp the device for the guide cannula head as this may be easily aligned to the probe holder.
- Anchor the device to the skull with at least four stainless screws screwing them into the skull bone (1 screw into the left and 1 screw into the right frontal bone plates, 1 screw into the left parietal and 1 screw into the interparietal bone plates). Add a drop of tissue glue to further fix each screw to the skull bone.
- Cover half of screw threads with methacrylic cement. Promote the binding of the cement by making shallow grooves in the bone to increase the adherence.
- Once the tip of the device is positioned into the brain tissue, twist the wire of the ground electrode around 3 anchoring screws. Cover all mounted screws and the device with the dental cement12,42,43.
- Monitor the animals during the surgery and for about 1 h thereafter until upright and moving around the cage. Keep them on a warming pad to avoid hypothermia. Allow the rats to recover for at least 7 days after the device implantation.
- Monitor the animals at least once daily for 3 days after the surgery for signs of pain or distress. Give the animals with the antibiotic cream (gentamycin 0.1%) close to the incised site to prevent the infection and an analgesic (tramadol 5 mg/kg, i.p.) for 3 days to prevent the post-surgical pain.
3. Temporal Lobe Epilepsy Induction by Pilocarpine and Assignment of Animals to Experimental Groups
- After a week of post-surgical recovery, assign the animals randomly to groups: (i) control animals receiving vehicle and (ii) epileptic animals that will receive pilocarpine. Use a proportionally higher number of animals for epileptic group since not all of the pilocarpine administered rats will develop the disease.
- Inject a dose of methylscopolamine (1 mg/kg, s.c.) and 30 min after, a single injection of pilocarpine (350 mg/kg, i.p.) to induce the status epilepticus (SE). Inject methylscopolamine and the vehicle (saline) to the control rats. Use 1 mL syringe with 25G needle for all i.p. administrations.
- Check visually the animals to start to have behavioral seizures (moving vibrissa within 5 min, nodding head, cloning the limbs) and within 25 min to clone continuously all the body (SE).
- Arrest the SE 2 h after the onset to have a mortality about 25% and a mean latent period of approximately 10 days by administration of diazepam (20 mg/kg, i.p.). Observe and record any seizure behavior beginning immediately after the pilocarpine injection and continue for at least 6 h thereafter.
- Give the animals saline (1 mL, i.p.) using 1 mL syringe with 25G needle and sucrose solution (1 mL, p.o.) using 1 mL syringe and flexible feeding 17G needle for 2-3 days after SE to promote the recovery of body weight loss.
- Exclude the animals that do not achieve the initial body weight within the first week after pilocarpine SE from the study (except for the acute group killed 24 h after SE, where the body weight follow up is not possible).
- Assign post-SE animals randomly to different experimental groups (Figure 3): acute phase (where the microdialysis takes place 24 h after SE), latency (7-9 days after SE), first spontaneous seizure (approximately 11 days after SE), and chronic period (starts about 22-24 days after SE, i.e. about 10 days after the first seizure). Monitor the animals for the occurrence of spontaneous seizures.
NOTE: Use the following inclusion/exclusion criteria for further experiments in epileptic rats: development of convulsive SE within 1 h after pilocarpine administration; weight gain in the first week after SE and the correct positioning of the microdialysis probe and electrode.
4. Epileptic Behavior Monitoring and Analysis
- Long-term monitoring of epileptic behavior
- Approximately 6 h after pilocarpine administration (i.e., at the end of direct observation by the researchers), place the animals into the clean home cages and start the 24 h video monitoring.
- Continue the 24 h video monitoring until day 5, using a digital video surveillance system.
- Beginning at day 5, connect the rats in their home rectangular cages to tethered EEG recording system and continue the 24 h video monitoring.
- Set the parameters on the amplifier positioned outside of the Faraday cage (set amplification factor on each channel according to the specificity of the EEG signal of each single animal) and start the EEG acquisition observing the EEG signal produced by unconnected cables. Use sampling rate 200 Hz and low pass filter set to 0.5 Hz.
- Connect the animal to cables holding an animal's head between two stretched fingers of one hand and screwing down the connectors to the electrode pedestal using the other free hand. Start the acquisition.
CAUTION: Ensure that the signal is free of artifacts. Common artifacts are the spikes greatly exceeding the scale. - A day before the microdialysis experiment, transfer the animals into the tethered EEG system equipped with plexiglass cylinders for microdialysis. Disconnect the animals from the EEG tethering system in home cage screwing up the connectors from the electrode without restrain the animal. Place the animal into the high plexiglass cylinders.
- Monitoring of epileptic behavior a day before and during the microdialysis session
- Switch on the amplifier positioned outside of the Faraday cage. Open the EEG software. Start the EEG acquisition observing the EEG signal produced by unconnected cables.
- Connect the animal to the tethered EEG recording system holding an animal's head between two stretched fingers of one hand and screwing down the connectors to the electrode pedestal using the other free hand. Set an amplification factor (gain) on each channel of amplifier according to electrode signal of single animal so the EEG signal is in scale. Let the animal explore the new cage (cylinder) for at least 1 h under the direct observation of the researcher.
- NOTE: 24 h before the microdialysis experiment, the rats are briefly anesthetized with isoflurane for the switch of guide cannulas to microdialysis probe. Take advantage of the moment when they are anesthetized to connect them to the EEG recording system.
- Shorten or prolong pendulous cable according to animal's commodity. Make sure that the cables do not interfere with animal's movements and lying posture.
- Check for the correct image framing of the video cameras. Start the video-EEG recording.
- Identification of seizures and EEG activity
- Use a software player to watch the videos. Scroll the movie 8 times faster than the real time playing and individuate the generalized seizures (see animal to rear with forelimb clonus or animal rearing and falling with forelimb clonus). Slow down the video and note the precise time of the beginning and of the end of the behavioral seizure.
- Process the data by counting the number of generalized seizures observed in 24 h of video records and express them in terms of seizure frequency and duration as mean values of all seizures observed in 24 h.
- Define the EEG seizures as the periods of paroxysmal activity of high frequency (>5 Hz) characterized by a >3-fold amplitude increment over baseline with progression of the spike frequency that lasts for a minimum of 3 s2,44 or similar28. Use EEG software to process the raw EEG recordings. Split the EEG traces into 1 h fractions. Copy the EEG tracing fractions to file for software automatic spike analysis.
- Analyze the EEG activity data using EEG software and predefined parameters (4.3.3.). Conduct all video analyses in two independent investigators who are blind for the group of analyzed animals. In case of divergence, make them re-examine the data together to reach a consensus45.
5. Microdialysis
- In vitro probe recovery
- Prepare the probe for its first use according to the manufacturer’s instructions, handling it in its protective sleeve.
- Run the experiment in triplicate: prepare three 1.5 mL test tubes loading them with 1 mL of Ringer’s solution containing the mixture of standards (2.5 µM of glutamate and 2.5 µM of aspartate). Put three loaded 1.5 mL test tubes into the block heater set to 37 °C and position it on the stirrer.
NOTE: Use the same standard solutions for chromatography calibrations. - Seal the 1.5 mL test tubes with paraffin film and puncture it by sharp tweezers to make a hole of about 1 mm in diameter.
- Take the probe and insert it into the hole made in paraffin film. Immerse the membrane at least 2 mm under the solution level. Fix further the probe to 1.5 mL test tube by paraffin film.
CAUTION: Ensure that the tip does not touch the walls of the 1.5 mL test tube. - Connect the probe inlet to the syringe mounted on the infusion pump using FEP-tubing and tubing adapters. Optionally, use fine bore polythene tubing of 0.28 mm ID and 0.61 mm OD and colored tubing adapters (red and blue tubing adapters) for connections.
- Start the pump at 2 µL/min and let the fluid appear at the outlet tip. Connect the probe outlet to the 0.2 mL collecting test tube using FEP-tubing and tubing adapters.
NOTE: Use FEP-tubing for all connections. Cut the desired length of tubing by using a razor blade. Use tubing adapters of different color for inlet and outlet of the probe. Let the pump run for 60 min. Check for leaks and air bubbles. These should not be present. - Set the pump to the flow rate 2 µL/min and start to collect the samples on the outlet side of the tubing.
- Collect three 30 min perfusate samples and three equal volume samples of the solution in the 1.5 mL test tube. Take the equal volume samples from the 1.5 mL test tube every 30 min using the microsyringe immersed into the standard solution in the 1.5 mL test tube.
- Repeat the experiment (5.1.1-5.1.8) setting up the pump to the flow rate 3 µL/min (5.1.7) to have a probe recovery comparison when using two different perfusion flow rates.
- When finished, stop the pump and rinse the tubing with distilled water, 70% ethanol and push the air into it. Store the probe in a vial filled with clean distilled water. Rinse the probe thoroughly by perfusing it at 2 µL/min by distilled water prior the storage.
- Analyze the concentration of the glutamate and aspartate in the samples by chromatography (see the details below; 6.3).
- Calculate the recovery using the following equation:
Recovery (%) = (Cperfusate /Cdialysed solution) x 100.
- Microdialysis sessions in freely moving rats
- Preparative procedures: probe insertion and testing, infusion pump setting and start
- Prepare the microdialysis probes for the first use according to the manufacturer user's guide and fill them with Ringer’s solution. Cut about 10 cm long pieces of FEP-tubing and connect them to inlet and outlet cannulas of the probe using the tubing adapters of different colors.
- Make sure that the tubing touches the adapters with no dead space in all connections.
- 5.2.1.3. 24 h before the microdialysis experiment, anesthetize briefly the animal with isoflurane (5% in air) in an induction chamber until recumbent. Remove the dummy cannula from its guide using the tweezers and holding the animal's head firmly. Insert the microdialysis probe, endowed with a dialyzing membrane, into the guide cannula and firm further the microdialysis cannulas inserted in their guide by modeling clay.
CAUTION: Do not let the probe touch the walls of the protective probe sleeve when extracting. - Put the animal into the plexiglass cylinder and let it explore the new ambience. Connect the animal to the tethered EEG recording system as described above (follow the points 4.2.2 and 4.2.3).
- Follow the awake and freely moving rat movements and connect the inlet of the probe to the 2.5 mL syringe with blunted 22G needle containing Ringer’s solution using the tubing adapters. Push Ringer’s solution inside the probe ejecting 1 mL of Ringer’s solution in 10 s pushing continuously the piston of 2.5 mL syringe. Check for the drop of the liquid appearing on the outlet. The probe is now ready for use.
- Fill up the 2.5 mL syringes connected to FEP-tubing by tubing adapters with Ringer’s solution and mount them onto the infusion pump. Start the pump at 2 µL/min. Let it run overnight.
NOTE: Use the desired length of all FEP-tubing but calculate the tubing dead volume to know when the high K+ stimulation should be started and to correlate the quantification data with neurochemical changes in animal brain. Use the air bubble created in the tubing under the working flow to calculate this time.
- Collection of samples during EEG recording and potassium stimulation
- Verify the absence of seizures in the 3 h preceding the onset of sample collection (video-EEG recordings) and continue to monitor seizure activity during microdialysis.
- Stop the pump carrying the FEP-tubing cannulated syringes filled up with Ringer’s solution. Mount onto the pump another set of 2.5 mL syringes connected to FEP-tubing with tubing adapters filled up with a modified Ringer’s solution containing 100 mM K+ solution.
- Start the pump at 2 µL/min and let it run. For more rapid filling of the tubing, set the pump at 5 µL/min for the time of filling. Check for the absence of air bubbles in the system. Ensure that the tubing touches the adapters with no dead space in all connections.
- Test the probe if ready for use in animal as described above (5.2.1.5).
NOTE: If for some reason the probe does not work, change it. For these cases, keep a few prepared microdialysis probes ready to use near the microdialysis-EEG workstation. Disconnect the animal from EEG cables and anesthetize it briefly with isoflurane if necessary to realize the change. - Connect the FEP-tubing of syringes filled up with Ringer’s solution to the inlet cannula of the probe in each animal and wait for the appearance of the liquid drop on the tip of the outlet.
- Connect the outlet of the probe to the FEP-tubing, which leads to collection in the test tube. Insert the FEP-tubing into the closed 0.2 mL test tube with a perforated cap. Ensure that the tube stays in the place by fixing with a piece of modeling clay.
- Continue to run the pump at 2 µL/min for 60 min without collecting samples to equilibrate the system (zero sample).
- Collect 5 consecutive 30 min dialysate samples (60 µL respective volume) under baseline conditions (perfusion with normal Ringer’s solution). Store samples on ice.
- Calculate the time it takes liquid to pass from the pump into the animal's head (it depends on dead volume of tubing, i.e., air bubble time) and switch the FEP-tubing that goes from the syringes containing normal Ringer’s solution to syringes containing modified (100 mM K+) Ringer’s solution at this time without stopping the pump. Check for the absence of the air bubbles in the system. Let the pump run for 10 min.
CAUTION: In 10 min of high K+ stimulation, the animals tend to move themselves frenetically and usually present a great number of wet dog shakes (control and out of seizure cluster animals) or behavioral seizures (epileptic animals), so be ready to intervene to protect the tubing and cables from twisting. - After 10 min, switch the tubing from the syringes containing 100 mM K+ Ringer’s solution to normal Ringer’s solution and let the pump run. Do not turn off the pump during the solution changes so that that there will be a drop of the liquid at the end of the tubing to be connected in line.
- From the moment at which the dialysate contains high potassium, i.e., after collection of the fifth post-equilibration dialysate, collect the dialysate fractions every 10 min (20 µL) for 1 h. Collect 3 additional 30 min dialysate samples and stop the pump. Store the samples on ice.
- Store the samples at -80 °C after the experiment until HPLC analysis.
- Repeat the microdialysis experiment for 3 consecutive days, except for the acute (24 h) and first seizure group, in which only one microdialysis session takes place 24 h after SE or within 24 h after the first spontaneous seizure (Figure 3).
- On completion of each experiment, euthanize the animal with an anesthetic overdose and remove the brain for verification of probe and electrode placement.
- Preparative procedures: probe insertion and testing, infusion pump setting and start
- Post-microdialysis procedures
- Rinse the used microdialysis probes with distilled water and store them in a vial filled with clean distilled water until next use.
NOTE: The reused membranes may have increased permeability; check for the probe recovery before its repeated use. - Rinse the entire microdialysis set up (tubing, connectors and syringes) with distilled water followed by 70% ethanol. Replace ethanol with air and store the set up in a sterile environment.
- Split the basal dialysate samples into 20 µL fractions and use only one 20 µL fraction for amino acid basal concentration analysis. Store the remaining sample volume for further or confirmatory analysis at -80 °C.
- Fix the brains in 10% formalin and preserve them by paraffin-embedding1. Coronally section the brains into slices and stain them with hematoxylin and eosin. Examine the brains for correct probe and electrode placement1,2.
NOTE: Fix the brains in cooled 2-methylbutane and store them at -80 °C. Use any other proven staining on the nervous tissue sections which permits to visualize the probe and electrode tract.
- Rinse the used microdialysis probes with distilled water and store them in a vial filled with clean distilled water until next use.
6. Chromatographic Analysis of Glutamate and Aspartate
- Preparation of derivatizing agent
- Mix the respective volumes 20:1 (v/v) of orthophthaldialdehyde reagent (OPA) and 2-mercaptoethanol (5-ME) in the vial. Close the vial using the cap and air tight septum.
CAUTION: Work under the chemical hood. - Vortex the prepared solution and put it into the autosampler into the position for derivatizing agent.
- Mix the respective volumes 20:1 (v/v) of orthophthaldialdehyde reagent (OPA) and 2-mercaptoethanol (5-ME) in the vial. Close the vial using the cap and air tight septum.
- Preparation of dialysate samples
- Put the glass insert with bottom spring into the 2 mL brown autosampler vial. Prepare the vials for all samples measured in one batch.
- Take the 20 µL dialysis samples from -80 °C freezer and let them melt. Remove 1 µL of the solution and add 1 µL of internal standard (IS) L-Homoserine (50 µM) to 19 µL of the sample, thus the sample contains 2.5 µM of IS. Pipette 20 µL of the dialysis sample into the glass insert in vial and seal it with an air tight septum.
- Place the vials containing the samples into the autosampler using the chromatographic software to label the samples in their positions.
- Chromatographic analysis of samples to determine glutamate and aspartate concentration
- Run the analyses on the liquid chromatograph system with spectrofluorometric detection. Detect the amino acids after 2 min pre-column derivatization with 20 μL of orthophthaldialdehyde/5-mercaptoethanol 20:1 (v/v) added to 20 μL of sample.
- Prepare the system for amino acids analysis. Switch on the autosampler, the pump, the degasser, the detector and controlling unit together with computer.
- Immerse the siphons into the bottles containing the mobile phase and purge the channels of the chromatographic pump to be used for the analysis.
- Start to increase the flow of the mobile phase checking the pressure on the column (e.g., start at 0.2 mL/min and increase the flow for additional 0.2 mL/min every 5 min until achieving the working flow). Let the system run at working flow 0.8 mL/min for at least 1 h to equilibrate the column.
CAUTION: Unstable pressure indicates the presence of the air in the system. The pressure should not exceed 25 MPa. - Set the gain, high sensitivity and the excitation and emission wavelengths on the detector to 345/455 nm respectively. Reset the detector signal (AUTOZERO).
- Using the chromatographic software, send the method to the instrument. Now, the chromatograph should be ready to measure.
- Separate the dialysate samples, standard spiked dialysate samples as well as standards (0.25 µM – 2.5 µM aspartate and glutamate in Ringer’s solution) on the appropriate chromatographic column. Calibrate the chromatographic method and establish the detection and quantification limits before any dialysis samples analysis.
- Activate the single analysis or create the sequence of the samples to be analyzed using the chromatography software and run the sequence.
CAUTION: Run more than one blank sample and different standard samples within the sequence of dialysate samples in order to control the method accuracy. - Once the chromatograms are acquired, analyze them with chromatography software. Check the integration of the peaks of the interest into the calibration plot. Use peak height or peak area for quantification.
- Once the sample recording is finished fill the column and the system with an organic solvent (e.g., 50% acetonitrile in ultrapure water) to prevent its aging and mold growth in it.
- Shut down the system.
Representative Results
Probe recovery
The mean recovery (i.e., the mean amino acid content in the perfusate as a percentage of the content in an equal volume of the vial solution) was 15.49 ± 0.42% at a flow rate of 2 μL/min and 6.32 ± 0.64 at 3 μL/min for glutamate and 14.89 ± 0.36% at a flow rate of 2 μL/min and 10.13 ± 0.51 at 3 μL/min for aspartate when using the cuprophane membrane probe. If using the polyacrylonitrile membrane probe, the mean recovery was 13.67 ± 0.42% at a flow rate of 2 μL/min and 6.55 ± 1.07 at 3 μL/min for glutamate and 14.29 ± 0.62% at a flow rate of 2 μL/min and 8.49 ± 0.15 at 3 μL/min for aspartate (Figure 4A-4B). As it can be clearly seen in Figure 4A, the slower flow rate (2 μL/min) enhances the dialyzing performance of both probes. For the following experiments the cuprophane membrane endowed probe perfused at a flow rate 2 μL/min was chosen, because its mean recovery was higher (even if not significantly) at this flow rate for both analytes and because of experimental continuity (these probes were used for analyzing GABA in precedence1).
Seizures development and progression of the disease after status epilepticus
The behavioral and EEG monitoring of seizures, their evaluation, was done in all the animals employed in this study to confirm the development and progression of TLE disease in these.
The robust convulsive SE, that was interrupted after 3 h using diazepam, occurred 25±5 min after the pilocarpine injection. Then, all the animals entered a latency state in which they were apparently well and they were continuously video-EEG monitored in order to verify that no spontaneous seizures occurred in the first 9 days or to identify the first spontaneous seizure, respectively for the latency and the first seizure group. The first spontaneous seizure occurred 11.3 ± 0.6 days after SE (mean ± SEM, n=21). Thereafter, seizures occurred in clusters, and aggravated in time. In late chronic phase (days 55-62 after SE) the epileptic rats experienced 3.3±1.2 (mean ± SEM, n=12) generalized seizures daily. There was a clear progression of the disease. Many, but not all EEG seizures, corresponded with behavioral seizure activity. Figure 5B shows the recorded paroxysmal epileptiform activity that was observed about 500 ms before and during behavioral seizures. Figure 5A shows control traces in non- epileptic rats.
Representative basal values of amino acids found in microdialysis perfusate and potassium stimulated release of glutamate
Basal glutamate concentration found in chronic epileptic rats (0.87 ± 0.06 µM) was significantly higher than in control animals (0.59 ± 0.03 µM; p < 0.05 vs. controls; one-way ANOVA and post hoc Dunnett's test). There was no statistically significant difference between chronic epileptic (0.31 ± 0.04 µM) and non-epileptic animals (0.30 ± 0.05 µM) in basal or high K+ evoked aspartate concentrations. See the original article for details2. The reported basal levels of glutamate in control rats are in line with those found by others in similar studies (i.e., about 0.75 µM when using a 2 µL/min flow and membranes of 2 mm effective length)46,47,48,49,50,51,52,53. However, many different factors can influence the results of microdialysis, for example the effective length of the probe and the membrane cut off.
High K+-evoked an additional release of glutamate for about 30 min in control rats and for about 60 min in chronic epileptic rats (Figure 6). See the original article for details2. As can be seen from the depicted time course, the 10 min time resolution of microdialysis was sufficient to capture the variances in glutamate release found in both groups of animals.
HPLC calibration and limits
The data were calculated based on calibration curves obtained with standard solutions of glutamate and aspartate and the internal standard L-homoserine. The concentration of the neurotransmitters glutamate and aspartate in the perfusates was expressed in absolute values (µmol/L). Each calibration plot was constructed by analysis of solutions of glutamate and aspartate at four concentration levels (five replicates at each level).Regression coefficients were calculated for calibration plots: y = kx + q, where x was the concentration ratio of aspartate or glutamate to L-homoserine (IS) and y was the corresponding peak-area ratio of aspartate or glutamate to L- homoserine (IS). The coefficient of determination (r2) was calculated. The applicability of HPLC method was within the limits; the lower limit of quantification was determined as the lowest concentration in the standard calibration curve and the upper limit of quantification as the highest used concentration of amino acid analytes for calibration, respectively. Limit of detection (LOD) was also calculated. Some of these values are delineated in Table 1. A model chromatogram of blank sample, standards sample and collected dialysis sample obtained with above described method are shown in Figure 7.
Probe localization
Microdialysis probe and recording electrode were implanted into the right ventral hippocampus and their correct placement was verified. Only those animals where the implantation was maximally in 500 μm distant from stabilized coordinates (see Figure 8) were included in analysis.
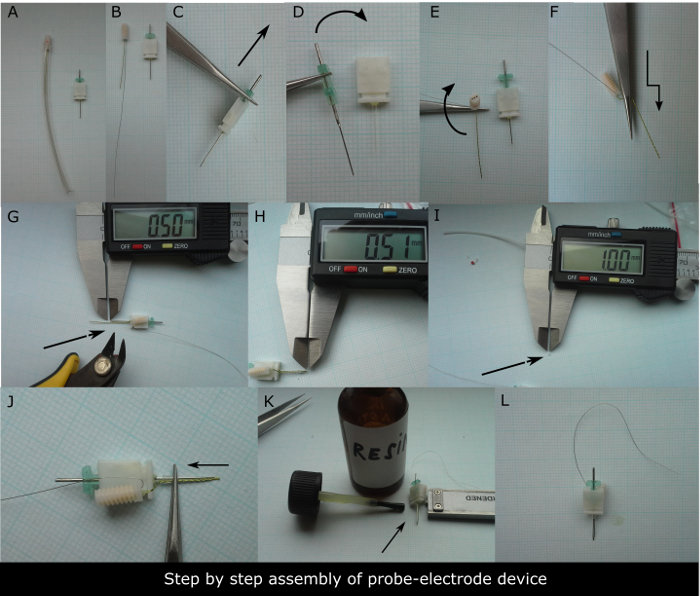
Figure 1. Step-by-step preparation of the device to be implanted. (A) 3-channel electrode with 10 cm long grounding electrode in its protective sleeve on the left and guide cannula for microdialysis on the right needed to assemble the device. (B). The bare electrode and guide cannula in detail. The first step is to remove (C) and insert (D) the metal guide cannula from and into its plastic dummy few times to ease its release once implanted into the animal's head. The second step is to bend two times the twisted registering electrode to be aligned to dialysis guide cannula (E, F). (G) The electrode tip should be cut to be 0.5 mm longer than the tip of the metal guide cannula. (H) Check for the precision of the cut using the digital caliper. Subsequently, about 1 mm long silicon circlet should be used to fix the alignment of electrode to guide cannula foot (I). (J) The photograph showing how to ring the electrode and guide cannula shaft. The final step is to put a drop of resin or glue onto the guide cannula pedestal fixing the silicon circlet to it (K). (L) Assembled device ready to be sterilized. Please click here to view a larger version of this figure.
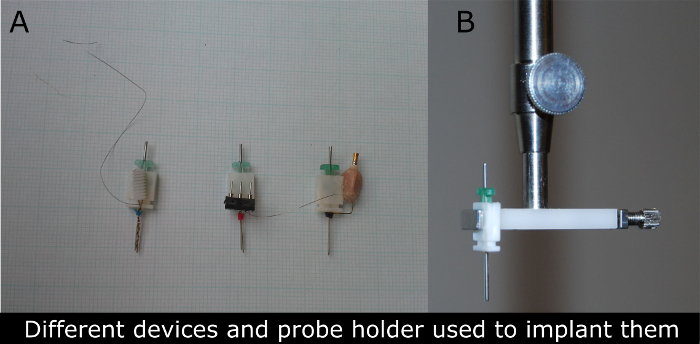
Figure 2. Photographs of different types of devices for microdialysis-EEG in rats used (A) and the photograph of the probe clip holder (B) used to implant these devices. (A) The guide cannula (in green) is replaced by a microdialysis cannula typically 24 h before the experiment. The electrical connector of the device (first left was used for the recordings described in this manuscript) permits the attachment of wires that conduct electrical signal to amplifier and data collection equipment. The device is surgically attached to the skull of anesthetized rats and recordings may be obtained later without causing pain or discomfort in freely behaving rats. Please click here to view a larger version of this figure.
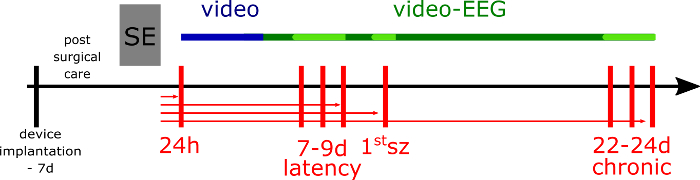
Figure 3. Experimental design. The week before status epilepticus (SE) induction, the rats are implanted with the device. SE is induced by pilocarpine and animals (if not dialyzed and killed at 24 h after SE; the rats from acute group) are video monitored for 5 days (blue line), then video-EEG monitored to assess the seizure frequency and duration in their home cages (green line). For the microdialysis experiment, the epileptic and respective non-epileptic control rats are transferred to another EEG set up equipped with cylinder cages in 24 h before the microdialysis session and still video-EEG monitored (light green line). The vertical red lines represent the dialysis sessions at different time-points of epileptic disease development. The horizontal red lines represent the different groups of epileptic animals (and respective non-epileptic animals), where the arrow indicates the last day of the microdialysis and the day of animal's death. Please click here to view a larger version of this figure.
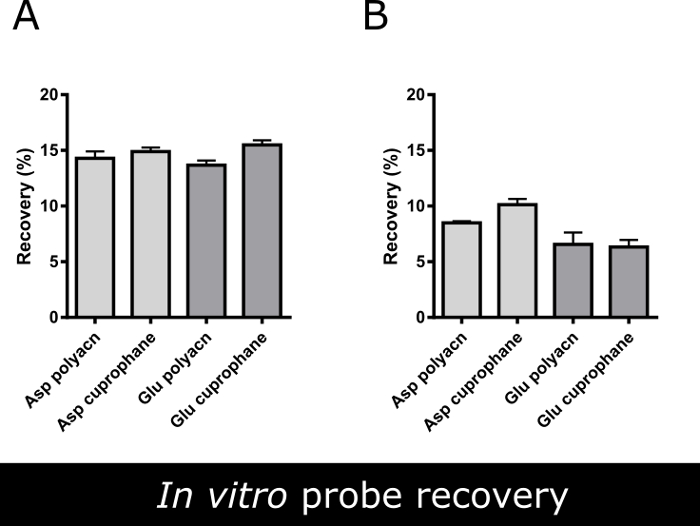
Figure 4. In vitro recovery of two dialysis probes. Mean in vitro recovery (%) of aspartate and glutamate using two different commercially available microdialysis probes (both endowed with 1 mm long dialyzing membrane) at (A) 2 μL/min and (B) 3 µL/min flow rate. Data are the mean ± SEM of 3 independent experiments run in triplicates. There are not statistically significant differences between the efficiency of various probes (Student's unpaired t-test, p<0.05).Using a flow rate 2 μL/min the glutamate recovery increased about 5% compared to 3 µL/min flow rate, thus the slower flow rate was used for microdialysis experiments. Please click here to view a larger version of this figure.
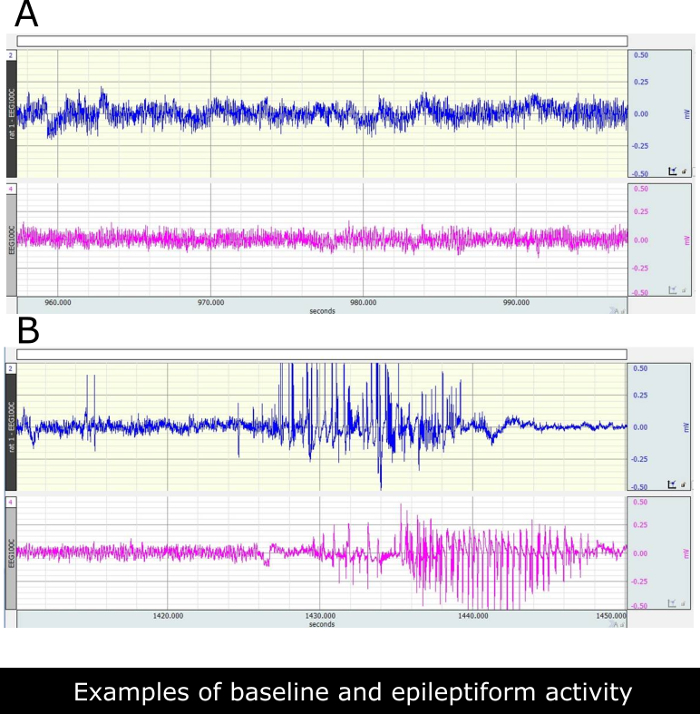
Figure 5. Illustrative EEG recordings from ventral hippocampus of paraoxystic activities as can be seen at chronic phase in control and epileptic rats. (A) Two representative traces recorded in two saline treated non-epileptic rats. (B) Traces recorded in two epileptic rats. Epileptiform discharges correspond with class 3 behavioral seizures in these rats. Please click here to view a larger version of this figure.
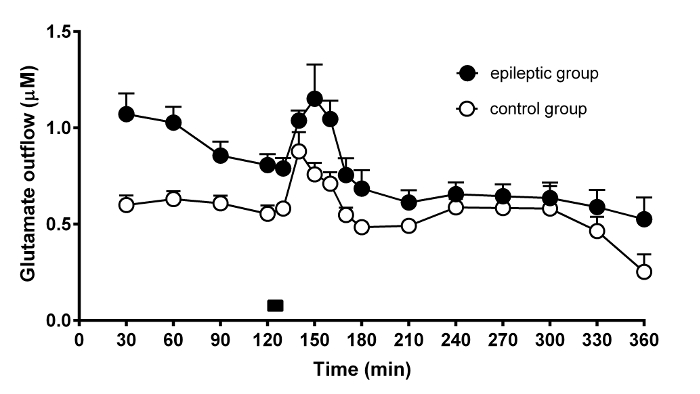
Figure 6. Time-course of the effect of potassium stimulation on glutamate release from the rat hippocampus. Representative result of the microdialysis experiment performed in 6 control (open circles) and 6 chronic epileptic rats (black circles). The graph shows the temporal changes of dialysate glutamate concentration in the course of microdialysis experiments and during high 100 mM K+ stimulation. The time of high K+ stimulus (10 min) is indicated by the black bar on bottom of the graph. The data are the means ± SEM of 6 animals per group. Please click here to view a larger version of this figure.
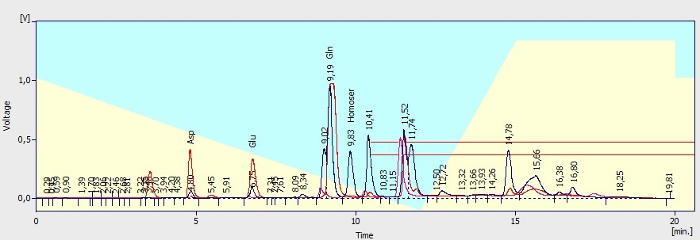
Figure 7. Illustration of chromatograms. Known peaks are labeled. Pink trace: chromatogram of Ringer's solution without intentionally added amines after OPA/5-ME derivatization (blank sample). Blue trace: chromatogram of dialysate sample after derivatization showing the peaks of amino acids: aspartate (tR 4.80 min), glutamate (tR 6.75 min) and glutamine (tR 9.19 min) and the peak of IS L-homoserine (2.5 µM, retention time, tR 9.83 min). Red trace: Chromatogram of standard of aspartate (2.5 µM) and glutamate (2.5 µM) in Ringer's solution. Azure and yellow background of the picture stands for mobile phase A (azure) and mobile phase B (yellow) portion used for analytes elution. A red rectangle indicated area (tR 10.41 min and further) shows the peaks of unknown substances and OPA degradation products. All injection volumes were 20 µL. The derivatives were separated at a flow rate of 0.8 mL/min. Please click here to view a larger version of this figure.
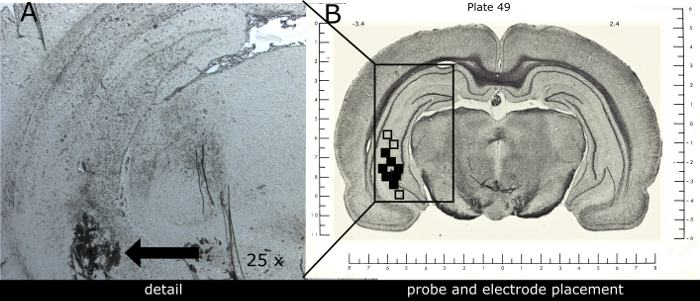
Figure 8. Representative image of combined electrode-probe placement within the ventral hippocampus. (A) Photograph shows the scare left by the device tip in detail (black arrow). (B) Schematic illustration of the electrode-probe tip positions within the implanted ventral hippocampus of 12 rats. The solid squares (some overlapping) indicate correctly localized probe-electrode tips. Open squares indicate incorrectly localized probe-electrode tips in animals excluded from the study (n=3). Coronal brain slices containing probes and recording sites were processed after experiments for histological analysis. The numbers above the illustration show the distance from Bregma (according to Pellegrino et al. 1979 atlas of rat brain; nose bar + 5.0 mm, co-ordinates used: A -3.4 mm, L+5.4 mm; P + 7.5 mm from dura). Please click here to view a larger version of this figure.
| Analyte | c (μmol/l) | k | q | r2 | LOD (pmol/l) |
| Glutamate | 0.25-2.5 | 5.215 | 1043.79 | 0.999 | 19.4 |
| Aspartate | 0.25-2.5 | 2.258 | 1994.72 | 0.998 | 31.7 |
Table 1. Quantification characteristics of HPLC method used for amino acids determination. Concentration range of standards (c), slope (k), intercept (q), coefficient of determination (r2) and limit of detection (LOD) describing the calibration plots obtained with standard solutions of glutamate and aspartate (0.25, 0.5, 1.0 and 2.5 µM) and internal standard L-homoserine (2.5 µM) using the described HPLC method with spectrofluorometric detection.
Discussion
In this work, we show how a continuous video-EEG recording coupled with microdialysis can be performed in an experimental model of TLE. Video-EEG recording techniques are used to correctly diagnose the different phases of the disease progression in animals and the microdialysis technique is used to describe the changes in glutamate release that occur in time (no changes have been found for aspartate in a previously published study2). We strongly recommend the use of a single device/implant to perform them both in each animal for the reasons discussed in the Introduction.
Whenever available, radiotelemetry should be preferred to tethered systems for chronic EEG recording as it minimizes interferences with behavior and reduces harm risk and distress for the animals54. However, the tethered EEG recording is much less expensive than telemetry.
In our laboratory, we use the connectors to the EEG recording system and microdialysis tubing in parallel, such that wires and tubings are attached to two different swivels. This is the most critical issue for these experiments: the wires and tubing tend to cross frequently due to the animal's movements. Therefore, we use connectors and tubing long enough to let the animal chase its own tail (a behavior that is typically observed with potassium stimulation) or fall down and roll during generalized epileptic seizures. It is advisable to firm further the microdialysis cannulas inserted in their guide by modeling clay, in order to strengthen their contact with the guide (sometimes, microdialysis cannulas are bumped against the walls of the cage during generalized seizures and may slip off). On the other hand, it is advisable to keep the tubing as short as possible, to minimize the delay between neurochemical time and collection time. This is particularly important when collection periods are short. In general, the microdialysis tubing should be of adequate length and capacity to ensure that the sampling time does not exceed the time between dialysis outlet and collection. It was observed that the solutes tend to diffuse more between some plugs if the tubing dead time is superior to the sampling rate55. Therefore, the experimental dead time/volume of microdialysis tubing should be reduced as much as possible and determined very precisely in order to correlate the neurochemistry data with the animal's behavior. Finally, it is important to note that both swivels and electrodes coupled with cannula for combined EEG and microdialysis studies are commercially available. Therefore, whenever possible, set up the EEG system with the option to perform the microdialysis experiments.
The minor recommendations are: (i) before beginning any experiment, check that the EEG recording system and/or microdialysis set up are functioning properly and troubleshoot any problem; we suggest that having one reserve set up ready (another pump with syringes mounted on and completed of tubing filled up with working solutions) when performing the experiment, as well as a sufficient number of ready to use microdialysis probes for changing broken ones; (ii) when transferring animal into the working EEG-microdialysis cage it is helpful to have a second person assisting and starting the acquisitions; (iii) make sure that the column and autosampler reached the appropriate temperatures before chromatography; in addition, use standards and construct the calibration plots before any dialysate samples are injected on the chromatographic column; (v) whenever needed, try to develop the chromatographic or other analytical method to measure multiple analytes at the same time.
Alterations in neurotransmission have implications in many CNS disorders (including epilepsy) and there has been a great interest over the decades to quantify these changes during the progression from a healthy to a diseased phenotype. Today, only a few techniques allow the measurement of changes in neurotransmitter levels over days or months. Microdialysis is one of these techniques. In a large number of cases, like that described here, it is performed in freely moving animals and coupled to conventional offline analytical assays like high performance liquid chromatography (HPLC) or capillary electrophoresis (CE), with which it reaches 5-30 min temporal resolution31,56. Clearly, these sampling intervals do not reflect the rapid neurotransmitter dynamics in the vicinity of synapses, but may be convenient for some long term microdialysis applications (e.g., disease development or drug effect studies) which require coupling neurochemical, EEG and behavioral data. However, other studies are primarily concerned with measuring real-time or close to real-time changes in neurotransmitter release. For these, the microdialysis technique must be refined to increase the speed of sampling (therefore decreasing sample volumes). Indeed, the classic microdialysis technique is often criticized for its poor temporal (minutes) and spatial resolution (the conventional probe is much larger than the synaptic cleft)9,21,56,57. However, it is the mass sensitivity of the analytical method coupled to microdialysis what determines the microdialysis time resolution (i.e., its resolution is equal to the time required to have enough sample to be detected by an analytical technique56). Thus, when the microdialysis produces tiny amounts of samples, the sensitivity of quantification techniques must be increased. To date, such improvements in temporal resolution of the microdialysis technique followed 3 different lines. One of these is represented by miniaturization of the columns and/or detection cells of classic HPLC methods; these are called UHPLC (ultra-performant HPLC) techniques and allow to achieve 1-10 min time resolution58,59,60. Another approach is to couple a classic HPLC to mass spectrometry (MS) or tandem (MS/MS) for multiplex analysis of neurotransmitters in brain dialysates. Combined HPLC-MS assays have an excellent sensitivity and reach about 1-5 min time resolution56,61,62,63. A third line of improvement exists in modifications of capillary electrophoresis (CE). If CE uses laser induced fluorescence detection (CE-LIFD), it enables the determination of submicromolar concentrations of various neurotransmitters in nanoliter fractions obtained every 5 min55,64,65 or even at 10 s intervals56. A clear advantage that emerges from UHPLCs or advanced CEs analytical approaches is that the sampling may be done in freely moving animals, not compromising experiments in which spontaneous behavior must be observed and analyzed. On the other hand, there are methods that permits the brain dialysate sampling at even hundred milliseconds temporal resolution (e.g., enzyme reactor based on-line assays or droplet collection of dialysate coupled to MS techniques), but these are typically used in restrained animals66 or under general anesthesia67,68,69, not allowing to couple microdialysis with behavioral studies.
When considering the second most important weakness of microdialysis, i.e., relatively low spatial resolution due to the membrane dimensions (often about 0.5 mm in diameter and 1-4 mm long), an alternative may be the microprobes developed with low-flow push-pull sampling. These probes consist of two silica capillaries (of 20 µm ID and 200 µm OD) fused side-by-side and sheathed with a polymeric tubing. During the experiment, these capillaries are perfused at very low flow rates, such that fluid is pulled out of one capillary and a sample is retrieved from the other at the same flow rate. Because the sampling occurs only at the probe tip, the spatial resolution is greater than with the probe for microdialysis70. Another possibility is to switch from miniaturized probes to microelectrode arrays (biosensors) for real-time neurotransmitter evaluation. Different electrochemical techniques (based principally on voltammetry or amperometry) permit analyte sampling very close to the synapse (micron scale) and in less than 1 s31,70,71. These devices can measure the concentration of multiple analytes from multiple brain regions. However, they also require some refinements, for example to avoid artifacts and a relatively rapid deterioration.
Considering the latest advances in in vivo neurochemical monitoring, it seems likely that the different transmitter sampling methods will be combined in one sensor in the near future. The work on microfabricated sampling probes has already started, and we believe that further progress in microfabrication technologies together with analytical advances will further facilitate in vivo neurochemical monitoring investigation. At this time, however, the conventional microdialysis correlated to EEG remains a valid method for many neuroscience applications.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors wish to thank Anna Binaschi, Paolo Roncon and Eleonora Palma for their contribution to manuscripts published in precedence.
Materials
| 3-channel two-twisted electrode | Invivo1, Plastic One, Roanoke, Virginia, USA | MS333/3-B/SPC | Material |
| guide cannula | Agn Tho's, Lindigö, Sweden | MAB 4.15.IC | Material |
| Resin KK2 Plastik | Elettra Sport, Lecco, Italy | KK2 | Material |
| Super Attack gel Loctite | Henkel Italia Srl, Milano, Italy | 2047420_71941 | Material |
| Imalgene-Ketamine | Merial, Toulouse, France | 221300288 (AIC) | Solution |
| Xylazine | Sigma, Milano, Italy | X1251 | Material |
| Isoflurane-Vet | Merial, Toulouse, France | 103120022 (AIC) | Solution |
| Altadol 50 mg/ ml – tramadol | Formevet, Milano, Italy | 103703017 (AIC) | Solution |
| Gentalyn 0.1% crm – gentamycine | MSD Italia, Roma, Italy | 20891077 (AIC) | Material |
| simplex rapid dental cement | Kemdent, Associated Dental Products Ltd, Swindon, United Kingdom | ACR811 | Material |
| GlasIonomer CX-Plus Cement | Shofu, Kyoto, Japan | PN1167 | Material |
| probe clip holder | Agn Tho's, Lindigö, Sweden | p/n 100 5001 | Equipment |
| Histoacryl® Blue Topical Skin Adhesive | TissueSeal, Ann Arbor, Michigan, USA | TS1050044FP | Material |
| Valium 10 mg/2 ml – diazepam | Roche, Monza, Italy | 019995063 (AIC) | Material |
| 1 mL syringe with 25G needle | Vetrotecnica, Padova, Italy | 11.3500.05 | Material |
| rat flexible feeding needle 17G | Agn Tho's, Lindigö Sweden | 7206 | Material |
| Grass Technology apparatus | Grass Technologies, Natus Neurology Incorporated, Pleasanton, California, USA | M665G08 | Equipment (AS40 amplifier, head box, interconnecting cables, telefactor model RPSA S40) |
| modular data acquisition and analysis system MP150 | Biopac, Goleta, California, USA | MP150WSW | Equipment |
| digital video surveillance system | AverMedia Technologies, Fremont, California, USA | V4.7.0041FD | Equipment |
| microdialysis probe | Agn Tho's, Lindigö Sweden | MAB 4.15.1.Cu | Material |
| microdialysis probe | Synaptech, Colorado Springs, Colorado, USA | S-8010 | Material |
| block heater | Grant Instruments, Cambridge, England | QBD2 | Equipment |
| stirrer | Cecchinato A, Aparecchi Scientifici, Mestre, Italy | 711 | Equipment |
| infusion pump | Univentor, Zejtun, Malta | 864 | Equipment |
| fine bore polythene tubing | Smiths Medical International Ltd., Keene, New Hampshire, USA | 800/100/100/100 | Material |
| blue tubing adapters | Agn Tho's, Lindigö Sweden | 1002 | Material |
| red tubing adapters | Agn Tho's, Lindigö Sweden | 1003 | Material |
| 2.5 mL syringe with 22G needle | Chemil, Padova, Italy | S02G22 | Material |
| vial cap | Cronus, Labicom, Olomouc, Czech Republic | VCA-1004TB-100 | Material |
| septum | Thermo Scientific, Rockwoood, Tennessee, USA | National C4013-60 8 mm TEF/SIL septum | Material |
| glass insert with bottom spring | Supelco, Sigma, Milano, Italy | 27400-U | Material |
| autosampler vial | National Scientific, Thermo Fisher Scientific, Monza, Italy | C4013-2 | Material |
| Smartline manager 5000 system controller and degasser unit | Knauer, Berlin, Germany | V7602 | Equipment |
| Smartline 1000 quaternary gradient pump | Knauer, Berlin, Germany | V7603 | Equipment |
| spectrofluorometric detector | Shimadzu, Kyoto, Japan | RF-551 | Equipment |
| chromatogrphic column | Knauer, Berlin, Germany | 25EK181EBJ | Material |
| chromatogrphic pre-column | Knauer, Berlin, Germany | P5DK181EBJ | Material |
| mobile phase solution A | 0.1 M sodium phosphate buffer, pH 6.0 | Solution | |
| mobile phase solution B | 40% 0.1 M sodium phosphate buffer, 30% methanol, 30% acetonitrile, pH 6.5 | Solution | |
| Ringer solution | composition in mM: MgCl2 0.85, KCl 2.7, NaCl 148, CaCl2 1.2, 0.3% BSA | Solution | |
| modified Ringer solution | composition in mM: MgCl2 0.85, KCl 100, NaCl 50.7, CaCl2 1.2, 0.3% BSA | Solution | |
| saline | 0.9% NaCl, ph adjusted to 7.0 | Solution | |
| sucrose solution | 10% sucrose in distilled water | Solution |
Riferimenti
- Soukupova, M., et al. Impairment of GABA release in the hippocampus at the time of the first spontaneous seizure in the pilocarpine model of temporal lobe epilepsy. Experimental Neurology. 257, 39-49 (2014).
- Soukupova, M., et al. Increased extracellular levels of glutamate in the hippocampus of chronically epileptic rats. Neuroscienze. 301, 246-253 (2015).
- Curia, G., Longo, D., Biagini, G., Jones, R. S., Avoli, M. The pilocarpine model of temporal lobe epilepsy. Journal of Neuroscience Methods. 172 (2), 143-157 (2008).
- Scorza, F. A., et al. The pilocarpine model of epilepsy: what have we learned?. Anais da Academia Brasileira de Ciencias. 81 (3), 345-365 (2009).
- Pitkanen, A., Sutula, T. P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. The Lancet Neurology. 1 (3), 173-181 (2002).
- Pitkanen, A., Lukasiuk, K. Mechanisms of epileptogenesis and potential treatment targets. The Lancet Neurology. 10 (2), 173-186 (2011).
- Reddy, D. S. Role of hormones and neurosteroids in epileptogenesis. Frontiers in Cellular Neuroscience. 7 (115), (2013).
- Engel, J. Research on the human brain in an epilepsy surgery setting. Epilepsy Research. 32 (1-2), 1-11 (1998).
- Watson, C. J., Venton, B. J., Kennedy, R. T. In vivo measurements of neurotransmitters by microdialysis sampling. Analytical Chemistry. 78 (5), 1391-1399 (2006).
- Jeffrey, M., et al. A reliable method for intracranial electrode implantation and chronic electrical stimulation in the mouse brain. BMC Neuroscience. 14, 82 (2013).
- Oliveira, L. M. O., Dimitrov, D. Chapter 2. Surgical techniques for chronic implantation of microwire arrays in rodents and primates. Methods for Neural Ensemble Recordings. , (2008).
- Fornari, R. V., et al. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. Journal of Visualized Experiments: JoVE. (59), e3528 (2012).
- Horn, T. F., Engelmann, M. In vivo microdialysis for nonapeptides in rat brain–a practical guide. Methods. 23 (1), 41-53 (2001).
- Kennedy, R. T., Thompson, J. E., Vickroy, T. W. In vivo monitoring of amino acids by direct sampling of brain extracellular fluid at ultralow flow rates and capillary electrophoresis. Journal of Neuroscience Methods. 114 (1), 39-49 (2002).
- Renno, W. M., Mullet, M. A., Williams, F. G., Beitz, A. J. Construction of 1 mm microdialysis probe for amino acids dialysis in rats. Journal of Neuroscience Methods. 79 (2), 217-228 (1998).
- Nirogi, R., et al. Approach to reduce the non-specific binding in microdialysis. Journal of Neuroscience Methods. 209 (2), 379-387 (2012).
- Zhou, Y., Wong, J. M., Mabrouk, O. S., Kennedy, R. T. Reducing adsorption to improve recovery and in vivo detection of neuropeptides by microdialysis with LC-MS. Analytical Chemistry. 87 (19), 9802-9809 (2015).
- Wisniewski, N., Torto, N. Optimisation of microdialysis sampling recovery by varying inner cannula geometry. Analyst. 127 (8), 1129-1134 (2002).
- Morrison, P. F., et al. Quantitative microdialysis: analysis of transients and application to pharmacokinetics in brain. Journal of Neurochemistry. 57 (1), 103-119 (1991).
- Westerink, B. H., De Vries, J. B. A method to evaluate the diffusion rate of drugs from a microdialysis probe through brain tissue. Journal of Neuroscience Methods. 109 (1), 53-58 (2001).
- Chefer, V. I., Thompson, A. C., Zapata, A., Shippenberg, T. S. Overview of brain microdialysis. Current Protocols in Neurosciences. , (2009).
- Westerink, B. H. Brain microdialysis and its application for the study of animal behaviour. Behavioural Brain Research. 70 (2), 103-124 (1995).
- Zhang, M. Y., Beyer, C. E. Measurement of neurotransmitters from extracellular fluid in brain by in vivo microdialysis and chromatography-mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 40 (3), 492-499 (2006).
- Allison, L. A., Mayer, G. S., Shoup, R. E. o-Phthalaldehyde derivatives of amines for high-speed liquid chromatography/electrochemistry. Analytical Chemistry. 56 (7), 1089-1096 (1984).
- Boyd, B. W., Witowski, S. R., Kennedy, R. T. Trace-level amino acid analysis by capillary liquid chromatography and application to in vivo microdialysis sampling with 10-s temporal resolution. Analytical Chemistry. 72 (4), 865-871 (2000).
- Hanczko, R., Jambor, A., Perl, A., Molnar-Perl, I. Advances in the o-phthalaldehyde derivatizations. Comeback to the o-phthalaldehyde-ethanethiol reagent. Journal of Chromatography A. 1163 (1-2), 25-42 (2007).
- Molnar-Perl, I. Quantitation of amino acids and amines in the same matrix by high-performance liquid chromatography, either simultaneously or separately. Journal of Chromatography A. 987 (1-2), 291-309 (2003).
- Solis, J. M., et al. Variation of potassium ion concentrations in the rat hippocampus specifically affects extracellular taurine levels. Neuroscience Letters. 66 (3), 263-268 (1986).
- Boatell, M. L., Bendahan, G., Mahy, N. Time-related cortical amino acid changes after basal forebrain lesion: a microdialysis study. Journal of Neurochemistry. 64 (1), 285-291 (1995).
- Sutton, A. C., et al. Elevated potassium provides an ionic mechanism for deep brain stimulation in the hemiparkinsonian rat. The European Journal of Neuroscience. 37 (2), 231-241 (2013).
- Hascup, K. N., Hascup, E. R. Electrochemical techniques for subsecond neurotransmitter detection in live rodents. Comparative Medicine. 64 (4), 249-255 (2014).
- Fisher, R. S., et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia. 46 (4), 470-472 (2005).
- Goffin, K., Nissinen, J., Van Laere, K., Pitkanen, A. Cyclicity of spontaneous recurrent seizures in pilocarpine model of temporal lobe epilepsy in rat. Experimental Neurology. 205 (2), 501-505 (2007).
- Pitsch, J., et al. Circadian clustering of spontaneous epileptic seizures emerges after pilocarpine-induced status epilepticus. Epilepsia. 58 (7), 1159-1171 (2017).
- Pearce, P. S., et al. Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy. Epilepsy and Behavior. 32, 121-131 (2014).
- Twele, F., Tollner, K., Bankstahl, M., Loscher, W. The effects of carbamazepine in the intrahippocampal kainate model of temporal lobe epilepsy depend on seizure definition and mouse strain. Epilepsia Open. 1 (1-2), 45-60 (2016).
- Kadam, S. D., et al. Methodological standards and interpretation of video-electroencephalography in adult control rodents. A TASK1-WG1 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 58, 10-27 (2017).
- Hernan, A. E., et al. Methodological standards and functional correlates of depth in vivo electrophysiological recordings in control rodents. A TASK1-WG3 report of the AES/ILAE Translational Task Force of the ILAE. Epilepsia. 58, 28-39 (2017).
- Bernal, J., et al. Guidelines for rodent survival surgery. Journal of Investigative Surgery: the official journal of the Academy of Surgical Research. 22 (6), 445-451 (2009).
- Flecknell, P. Rodent analgesia: Assessment and therapeutics. Veterinary Journal. , 70-77 (2018).
- Miller, A. L., Richardson, C. A. Rodent analgesia. The Veterinary Clinics of North America. Exotic Animal Practice. 14 (1), 81-92 (2011).
- Geiger, B. M., Frank, L. E., Caldera-Siu, A. D., Pothos, E. N. Survivable stereotaxic surgery in rodents. Journal of Visualized Experiments: JoVE. (20), (2008).
- Gardiner, T. W., Toth, L. A. Stereotactic Surgery and Long-Term Maintenance of Cranial Implants in Research Animals. Contemporary Topics in Laboratory Animal Science. 38 (1), 56-63 (1999).
- Williams, P. A., et al. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. The Journal of Neuroscience: The official journal of the Society for Neuroscience. 29 (7), 2103-2112 (2009).
- Paradiso, B., et al. Localized overexpression of FGF-2 and BDNF in hippocampus reduces mossy fiber sprouting and spontaneous seizures up to 4 weeks after pilocarpine-induced status epilepticus. Epilepsia. 52 (3), 572-578 (2011).
- Kanamori, K. Faster flux of neurotransmitter glutamate during seizure – Evidence from 13C-enrichment of extracellular glutamate in kainate rat model. PLoS One. 12 (4), e0174845 (2017).
- Kanamori, K., Ross, B. D. Chronic electrographic seizure reduces glutamine and elevates glutamate in the extracellular fluid of rat brain. Brain Research. 1371, 180-191 (2011).
- Kanamori, K., Ross, B. D. Electrographic seizures are significantly reduced by in vivo inhibition of neuronal uptake of extracellular glutamine in rat hippocampus. Epilepsy Research. 107 (1-2), 20-36 (2013).
- Luna-Munguia, H., Meneses, A., Pena-Ortega, F., Gaona, A., Rocha, L. Effects of hippocampal high-frequency electrical stimulation in memory formation and their association with amino acid tissue content and release in normal rats. Hippocampus. 22 (1), 98-105 (2012).
- Mazzuferi, M., Binaschi, A., Rodi, D., Mantovani, S., Simonato, M. Induction of B1 bradykinin receptors in the kindled hippocampus increases extracellular glutamate levels: a microdialysis study. Neuroscienze. 135 (3), 979-986 (2005).
- Meurs, A., Clinckers, R., Ebinger, G., Michotte, Y., Smolders, I. Seizure activity and changes in hippocampal extracellular glutamate, GABA, dopamine and serotonin. Epilepsy Research. 78 (1), 50-59 (2008).
- Ueda, Y., et al. Collapse of extracellular glutamate regulation during epileptogenesis: down-regulation and functional failure of glutamate transporter function in rats with chronic seizures induced by kainic acid. Journal of Neurochemistry. 76 (3), 892-900 (2001).
- Wilson, C. L., et al. Comparison of seizure related amino acid release in human epileptic hippocampus versus a chronic, kainate rat model of hippocampal epilepsy. Epilepsy Research. 26 (1), 245-254 (1996).
- Lidster, K., et al. Opportunities for improving animal welfare in rodent models of epilepsy and seizures. Journal of Neuroscience Methods. 260, 2-25 (2016).
- Parrot, S., et al. High temporal resolution for in vivo monitoring of neurotransmitters in awake epileptic rats using brain microdialysis and capillary electrophoresis with laser-induced fluorescence detection. Journal of Neuroscience Methods. 140 (1-2), 29-38 (2004).
- Kennedy, R. T., Watson, C. J., Haskins, W. E., Powell, D. H., Strecker, R. E. In vivo neurochemical monitoring by microdialysis and capillary separations. Current Opinion in Chemical Biology. 6 (5), 659-665 (2002).
- Kennedy, R. T. Emerging trends in in vivo neurochemical monitoring by microdialysis. Current Opinion in Chemical Biology. 17 (5), 860-867 (2013).
- Ferry, B., Gifu, E. P., Sandu, I., Denoroy, L., Parrot, S. Analysis of microdialysate monoamines, including noradrenaline, dopamine and serotonin, using capillary ultra-high performance liquid chromatography and electrochemical detection. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 951, 52-57 (2014).
- Jung, M. C., Shi, G., Borland, L., Michael, A. C., Weber, S. G. Simultaneous determination of biogenic monoamines in rat brain dialysates using capillary high-performance liquid chromatography with photoluminescence following electron transfer. Analytical Chemistry. 78 (6), 1755-1760 (2006).
- Parrot, S., Lambas-Senas, L., Sentenac, S., Denoroy, L., Renaud, B. Highly sensitive assay for the measurement of serotonin in microdialysates using capillary high-performance liquid chromatography with electrochemical detection. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 850 (1-2), 303-309 (2007).
- Hershey, N. D., Kennedy, R. T. In vivo calibration of microdialysis using infusion of stable-isotope labeled neurotransmitters. ACS Chemical Neuroscience. 4 (5), 729-736 (2013).
- Vander Weele, C. M., et al. Rapid dopamine transmission within the nucleus accumbens: dramatic difference between morphine and oxycodone delivery. The European Journal of Neuroscience. 40 (7), 3041-3054 (2014).
- Zestos, A. G., Kennedy, R. T. Microdialysis Coupled with LC-MS/MS for In Vivo Neurochemical Monitoring. The AAPS journal. 19 (5), 1284-1293 (2017).
- Benturquia, N., Parrot, S., Sauvinet, V., Renaud, B., Denoroy, L. Simultaneous determination of vigabatrin and amino acid neurotransmitters in brain microdialysates by capillary electrophoresis with laser-induced fluorescence detection. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 806 (2), 237-244 (2004).
- Chefer, V., et al. Repeated exposure to moderate doses of ethanol augments hippocampal glutamate neurotransmission by increasing release. Addiction Biology. 16 (2), 229-237 (2011).
- Morales-Villagran, A., Pardo-Pena, K., Medina-Ceja, L., Lopez-Perez, S. A microdialysis and enzymatic reactor sensing procedure for the simultaneous registration of online glutamate measurements at high temporal resolution during epileptiform activity. Journal of Neurochemistry. 139 (5), 886-896 (2016).
- Petit-Pierre, G., et al. In vivo neurochemical measurements in cerebral tissues using a droplet-based monitoring system. Nature Communication. 8 (1), 1239 (2017).
- Renaud, P., Su, C. K., Hsia, S. C., Sun, Y. C. A high-throughput microdialysis-parallel solid phase extraction-inductively coupled plasma mass spectrometry hyphenated system for continuous monitoring of extracellular metal ions in living rat brain. Nature Communication. 1326, 73-79 (2014).
- Zilkha, E., Obrenovitch, T. P., Koshy, A., Kusakabe, H., Bennetto, H. P. Extracellular glutamate: on-line monitoring using microdialysis coupled to enzyme-amperometric analysis. Journal of Neuroscience Methods. 60 (1-2), 1-9 (1995).
- Ngernsutivorakul, T., White, T. S., Kennedy, R. T. Microfabricated Probes for Studying Brain Chemistry: A Review. Chemphyschem: a Eurepean journal of chemical physics and physical chemistry. 19 (10), 1128-1142 (2018).
- Mirzaei, M., Sawan, M. Microelectronics-based biosensors dedicated to the detection of neurotransmitters: a review. Sensors. 14 (10), 17981-18008 (2014).

