HUVEC Tube-formation Assay to Evaluate the Impact of Natural Products on Angiogenesis
Summary
Here, we evaluate the effects of the water extract of Ruta graveolens on vessel network formation by using a tube formation assay on a gelled basement matrix.
Abstract
Angiogenesis is a phenomenon that includes different processes, such as endothelial cell proliferation, differentiation, and migration, that lead to the formation of new blood vessels and involve several signal transduction pathways. Here we show that the tube formation assay is a simple in vitro method to evaluate the impact of natural products on angiogenesis and to investigate the molecular mechanisms involved. In particular, in the presence of the water extract of Ruta graveolens (RGWE), endothelial cells are no longer able to form a cell-cell network and that the RGWE effects on human umbilical vein endothelial cell (HUVEC) tube formation is abolished by the constitutive activation of MEK.
Introduction
Angiogenesis is a physiological process that leads to the formation of new blood vessels from preexisting ones and occurs during embryogenesis and organ growth. In adulthood, angiogenesis is activated only in the cycling ovary, in the placenta during pregnancy, and during wound healing and repair. Angiogenesis depends on the ability of endothelial cells to proliferate, differentiate, and migrate to form an intact vascular network1. However, in several disorders, such as inflammatory, metabolic, and rheumatic diseases, angiogenic processes are altered and angiogenesis becomes excessive. Moreover, uncontrolled angiogenic processes also stimulate tumor progression and metastasis1. For these reasons, in the last decade, research studies are focused on the development of new therapeutic strategies aimed at the inhibition of excessive angiogenesis in cancer, ocular, joint, or skin disorders2,3.
Vascular endothelial growth factor (VEGF) represents the main target of current antiangiogenic therapies4, and several anti-VEGF monoclonal antibodies have been developed and synthesized to prevent excessive angiogenesis. However, these synthetic drugs show severe side effects and have an unfavorable cost-to-benefit ratio5,6. Therefore, it is imperative to find new therapeutic strategies to limit excessive angiogenesis with minimal side effects to complement and combine with currently used drugs. These new drugs can be found among natural products that are characterized by a high chemical diversity and biochemical specificity.
In this article, we propose a simple method to evaluate the impact of the RGWE on the ability of HUVECs to form tubules on a gelled basement matrix in vitro5. Indeed, RGWE is a mixture of secondary metabolites such as flavonoids and polyphenols among which rutin is the major component5. Many of them have been already tested as anti-inflammatory and vasoprotective agents7,8,9,10,11. Moreover, we have recently demonstrated that RGWE, but not rutin, is able to inhibit the HUVEC ability to form tubules on a gelled basement matrix and that this phenomenon is mediated by the MEK-ERK pathway, indicating RGWE as a potential therapeutic tool able to prevent excessive new blood vessel formation5.
Protocol
1. RGWE Preparation
- Collect R. graveolens leaves from plants during the spring/summer months under the supervision of a botanist.
NOTE: In this case, leaves were collected at the Experimental Section of Medicinal Plants in the Botanical Garden of Naples, Italy5. The plant is spontaneous, perennial, and is present in the Mediterranean regions (Figure 1). - Weigh 250 g of leaves and finely chop them with scissors.
- Put the leaves in a conical flask and add 1 L of distilled water to 250 g of chopped leaves and bring that to a boil at 110 °C for 60 min. Cover the flask with aluminum foil to avoid excessive evaporation.
- Use 3 mm-funnel filter paper to separate the boiled leaves from the liquid phase that represents the water extract.
- Filter the liquid phase through a 0.22 µm filter, collecting it in a beaker. Cover the beaker with parafilm and freeze the extract at -80 °C overnight.
- Make 10 – 15 holes with a needle in the parafilm and lyophilize the extract in a lyophilizer. Note that it can take a couple of days to obtain the powder.
- Weigh the powder obtained, divide it into aliquots, and store it at 4 °C until necessary. It is possible to store it for a year.
- When necessary for the experiments, prepare a stock solution, diluting the powder with distilled water to a standard concentration of 50 mg/mL. Separate it into small-volume (e.g., 1 mL) aliquots. This preparation can be stored at -20 °C until used but cannot be stored at 4 °C for more than a couple of days because of the oxidations of the molecules. Avoid freeze and thaw.
2. Cell Culture
- Cultivate HUVECs in endothelial cell growth medium: endothelial basal medium supplemented with 1 μg/mL hydrocortisone and 1 ng/mL epidermal growth factor, 10% FBS, and penicillin/streptomycin at 37 °C in an atmosphere of 5% CO2.
- When 80% confluent, split the cells at the ratio of 1:3 every passage. Use cells from two to five passages, that do not show apparent differences in response to growth factors. After the sixth passage, cells become senescent and do not form tubes on the gelled basement matrix.
3. Transfection
- When the HUVECs are 70% confluent, transfect them with the desired gene.
- For a 100 mm plate, use 1 µg of empty pCDNA3 vector as control and 1 µg of pCDNA3 vector containing the gene of interest (constitutively active MEK [caMEK], in this case).
- Complex 1 µg of DNA with 3 µL of lipofectamine 2,000, diluted with reduced serum medium (see Table of Materials) to the final volume of 200 µL. Remove the medium from the cells and replace it with 1 mL of fresh complete medium; then, add the transfecting solution to the cells. Incubate the cells in the incubator at 37 °C, with 5% CO2. It is possible to also use other transfection agents different from lipofectamine 2,000, following the manufacturer’s instructions.
- Add 7 mL of complete fresh medium 3 h after the transfection and, then, further incubate the cells at 37 °C for 24 – 48 h before proceeding with the next assays. The day after the transfection, replace the medium with fresh complete medium.
4. Tube Formation Assay
- Cool a 96-well plate and pipette tips at 4 °C for 1 h before preparing the gelled basement.
- At the moment of the basement preparation, put the plate on ice (0 °C) and add 50 μL of cold matrix in each well while avoiding the formation of bubbles. Note that the matrix-containing vial should be kept on ice during the procedure since the matrix becomes solid at room temperature.
- Put the plate in the incubator at 37 °C with 5% CO2 for at least 30 min to allow the basement to polymerize.
- In the meantime, harvest the cells. Wash the HUVECs with 1 mL of a 0.25% trypsin/0.53 mM EDTA solution; then, add 2 mL of the trypsin-EDTA solution and incubate at 37 °C for 5 min. Observe the cells under an inverted microscope to verify that the cell layer is completely dispersed. Then, collect the medium in which the cells are suspended and harvest the detached cells by centrifugation at 264 x g for 3 min. Resuspend them in 1 mL of phosphate-buffered saline (PBS).
- To count the cells, add 0.2 mL of the cell suspension to 0.5 mL of PBS and 0.3 mL of 0.4% trypan blue solution. After 5 min at room temperature, count the cells in a Bürker chamber.
- Resuspend the cells in complete medium at the concentration of 2 x 104 cells/50 µL. Seed them onto the gelled basement at the concentration of 2 x 104 cells per well and let them settle on it.
- Pay attention to the number of the cells. Too many or too few cells may not form tubes in the right way on the gelled basement.
- Prepare increasing doses of RGWE (0.01, 0.1, and 1 mg/mL), diluting the stock solution in culture medium or rutin (12, 120, and 300 µg/mL), all in the final volume of 50 µL/well, and add them to 50 μL/well of cell suspension. The final volume in each well will be 100 μL. Prepare four to six wells for each experimental condition. As a control, dilute distilled water (vehicle) in the culture medium at a ratio of 1:50.
- Incubate the cells in the incubator at 37 °C and 5% CO2 for a time ranging from 6 to 24 h.
- Observe the cells within a time range spanning from 6 to 24 h after seeding, using a phase-contrast microscope at a magnification of 10X. HUVECs are able to form tube-like structures within 6 h but it is possible to observe better results after 12 – 18 h. Photograph the tube-like structures on each well and image an average from three to five random fields in each well.
- After the image acquisition, the cells can be detached from the basement matrix and counted to evaluate the effect of the treatment on the number of cells. For each well, remove the medium, add dispase at a concentration of 24 U/mL, and put the plate at 37 °C for 1 h. Then, from each well, collect the medium in which the cells are suspended and harvest the detached cells by centrifugation at 264 x g for 3 min. Resuspend them in 0.2 mL of PBS. To count the cells, add 0.2 mL of cell suspension to 0.5 mL of PBS and 0.3 mL of 0.4% trypan blue solution. After 5 min at room temperature, count the cells in the Bürker chamber.
- To quantify the results of the tube formation assay, it is possible to count the number of branch points in which at least three tubes join. Using the appropriate image software, count for each field in each micrograph the number of branch points and calculate the mean ± the standard error (SE) for each experimental condition. Use a two-tailed t-test to evaluate statistical significance.
Representative Results
To evaluate the influence of RGWE on angiogenesis, we carried out a tube formation assay on a gelled basement matrix. When cultivated on it, HUVECs form tube-like structures that originate from cells that appear elongated and that connect each other to form a cell-cell network (Figure 2). In Figure 3, we show that the number of branches in HUVECs treated with RGWE was significantly lower as compared to the control conditions. Notably, in the presence of 0.1 mg/mL and 1 mg/mL RGWE, the number of the branches are lower by 40% and 60%, respectively, compared to the control condition. Since rutin has been indicated as the major component of RGWE5, we analyzed its effect on HUVEC tube-formation assay. As shown in Figure 4, rutin alone is not able to affect the HUVECs' ability to form a cell-cell network. Then, we used the tube formation assay to investigate the molecular mechanisms underlying the RGWE-induced inhibition of tube formation. The MEK/ERK intracellular signaling pathway exerts a pivotal role in angiogenic processes. HUVECs transfected by caMEK were treated with RGWE and cultivated on gelled basement matrix. As shown in Figure 5, in caMEK-transfected endothelial cells, RGWE no longer inhibits tube formation, while mock-transfected cells, used as control, still form a cell-cell network on the gelled basement matrix and are responsive to RGWE, thus indicating that the RGWE's effect in angiogenesis is mediated by the MEK-ERK pathway.

Figure 1: Ruta graveolens. A Ruta graveolens shrub. Please click here to view a larger version of this figure.
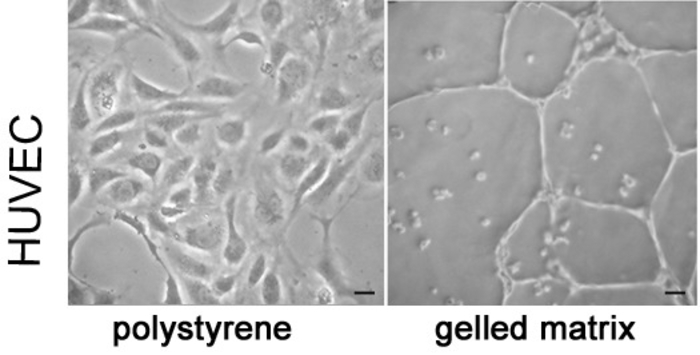
Figure 2: HUVECs form tube-like structures on a gelled basement matrix. Representative microscopic photographs of HUVECs cultured in polystyrene dish (left) and on gelled basement matrix (right). Scale bar = 10 µm.
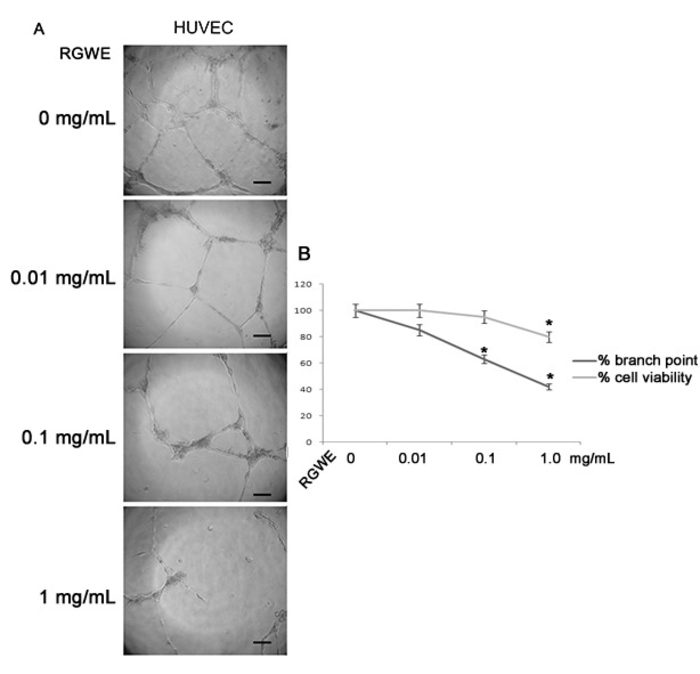
Figure 3: RGWE inhibits tube formation in HUVECs. (A) High-power microscopic photographs of HUVECs cultured on a gelled basement matrix treated with RGWE (0, 0.01, 0.1, and 1.0 mg/mL). The scale bar is 10 µm. (B) The percentage of the HUVEC branch point (dark gray) in the presence of RGWE (0.01, 0.1, and 1.0 mg/mL) compared to untreated cells (0 mg/mL) and the trypan blue exclusion test (light gray) on HUVECs treated (0.01, 0.1, and 1 mg/mL) or not (0 mg/mL) with RGWE for 24 h. *p < 0.01 vs. the control condition (0 mg/mL). The results are expressed as the mean ± the SE of three independent experiments. The statistical significance was obtained by a two-tailed t-test.
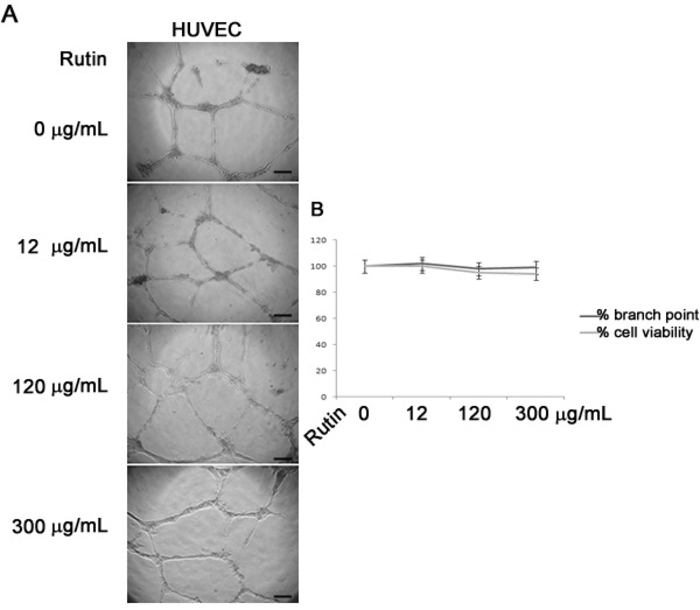
Figure 4: Rutin does not affect HUVEC tube formation. (A) High-power microscopic photographs of HUVECs seeded on a gelled basement matrix and treated (12, 120, and 300 µg/mL) or not (0 µg/mL) with rutin for 24 h. The scale bar is 10 µm. (B) The percentage of HUVEC branch point (dark gray) in the presence of increasing doses of rutin (12, 120, and 300 µ/mL) compared to control conditions (0 µg/mL) and the trypan blue exclusion test (light gray) on HUVECs treated (12, 120, and 300 µ/mL) or not (0 µg/mL) with rutin for 24 h. The results are expressed as the mean ± the SE of three independent experiments. Statistical significance was obtained by a two-tailed t-test.
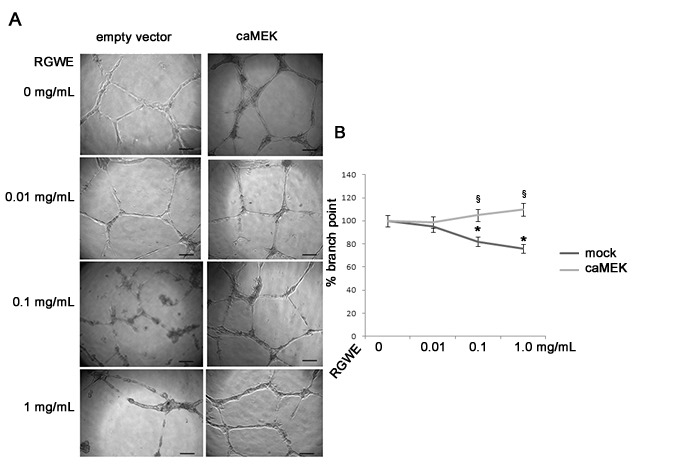
Figure 5: MEK pathway mediates RGWE effects on HUVEC tube formation. (A) High-power microscopic photographs of HUVECs transfected with empty vector (mock transfection) or with caMEK, seeded on the gelled basement matrix and treated with increasing doses of RGWE (0, 0.01, 0.1, and 1 mg/mL). The scale bar is 10 µm. (B) The percentage of the number of branch points in HUVECs mock-transfected (dark gray) and in HUVECs transfected with caMEK (light gray) and treated with increasing doses of RGWE (0.01, 0.1, and 1 mg/mL) compared to control conditions (0 mg/mL). *p < 0.01 vs. the control condition; §p < 0.05 vs. cells transfected with the empty vector and treated with the same amount of RGWE. The results are expressed as the mean ± the SE of three independent experiments. The statistical significance was obtained by a two-tailed t-test.
Discussion
Natural compounds are characterized by a high chemical diversity and biochemical specificity and represent a source of potentially therapeutic molecules. Here, we show how to obtain water extract from the plant R. graveolens and propose the tube formation assay as an easy-to-perform, reliable, and quantitative method useful to investigate RGWE's effects on angiogenesis. It is important to boil the R. graveolens leaves for 1 h to be sure to obtain the complete water extract. Boiling for less than 1 h did not allow for the extraction of all the molecules, and the extract could not exert the expected biological effect.
Tube formation assay represents an in vitro test to study the molecular mechanisms underlying the several steps that lead to the formation of new blood vessels. This assay allows researchers to identify compounds able to modulate angiogenesis, as well as the proteins and signaling cascades involved. Moreover, this assay allows researchers to test substances that can influence, at the same time, endothelial cell proliferation, adhesion, migration, and protease activity, all important mechanisms in blood vessel formation. Using only this kind of test, we show that RGWE, but not its major component rutin, is able to reduce the ability of HUVECs to form tube-like structures without affecting cell viability and that this effect depends on MEK-ERK pathway activation. However, it is important to perform the test with the right number of cells. In fact, too few or too many cells could not allow the right formation of the tubes. For this reason, it is advisable to perform a preliminary test to find the correct number of cells. Moreover, the number of cell passages is as important as the cell number since, to obtain the correct tube formation assay, cells have to be passaged twice to five times. After passage 6, senescence mechanisms occur that can impair the right tube formation.
The tube formation assay is a very reproducible assay, and it sheds light on the physiology of endothelial cells, even if it is not the gold standard for the three-dimensional study of tube formation8,9,10,11. Three-dimensional collagen and fibrin models have been demonstrated to be better for investigations of vascular tubulogenesis, sprouting, and endothelial cell-pericyte interaction. However, compared to the tube formation assay on gelled basement matrix, these tests are more time- and money-consuming11, suggesting that the first could be a good test to obtain preliminary results that can be the basis for more focused in vivo studies. Finally, the tube formation assay can be carried out in 24 h, since nontransformed HUVECs are able to form tube-like structures within 6 h12,13.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work has been funded by Fondi di Ateneo to Luca Colucci-D'Amato and VALERE Program funds to Maria Teresa Gentile and AIRC fund IG18999 to Maurizio Bifulco.
Materials
| HUVEC cells | Clontech | C2519A | |
| FBS | Invitrogen | 10270106 | |
| EBM-2 basal medium | Clontech | cc3156 | |
| Single quot kit- supplemets and growth factors | clontech | cc4147 | |
| Matrigel | Corning | 354234 | |
| 96-well plates | Thermo Scientific | 167008 | |
| 15 mL conical tubes | Sarstedt | 62,554,502 | |
| 10 mL disposable serological pipette | Sarstedt | 861,254,001 | |
| 5 mL disposable serological pipette | Sarstedt | 861,253,001 | |
| 1000 μL pipette | Gilson | Pipetman classic | |
| 100 μL pipette | Gilson | Pipetman classic | |
| 20 μL pipette | Gilson | Pipetman classic | |
| p1000 pipette tips | Sarstedt | ||
| p20-200 pipette tips | Sarstedt | 70,760,502 | |
| Burker chamber | Fortuna | ||
| Trypan blu stain | Gibco | 15250-061 | |
| DPBS | Gibco | 14190-094 | |
| mill-ex 0.22 um filters | Millipore | SLGS033SS | |
| Lyophilizer | VirTis-SP Scientific | ||
| Incubator | Thermo Scientific | ||
| CO2 | AirCos | ||
| Pen-Strep | Gibco | 15070-063 | |
| 100 mm dish | Sarstedt | 833,902 | |
| pcDNA3 | Invitrogen | v79020 | |
| Lipofectamine-2000 | Invitrogen | 11668027 | |
| Opti-MEM | Gibco | 31985070 | Reduced serum medium |
| Rutin | Sigma-Aldrich | R5143-50G | |
| Axiovert 25 microscope | Zeiss | ||
| AmScope MD500 camera | AmScope | ||
| Dispase | Thermo Scientific | D4818 | |
| Lab heater | Falc | ||
| ParaFilm | American National Can |
Riferimenti
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature. 438 (7070), 932-936 (2005).
- Carmeliet, P., Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 473 (7347), 298-307 (2011).
- Ferrara, N., Kerbel, R. S. Angiogenesis as a therapeutic target. Nature. 438 (7070), 967-974 (2005).
- Ravishankar, D., Rajora, A. K., Greco, F., Osborn, H. M. I. Flavonoids as prospective compounds for anti-cancer therapy. The International Journal of Biochemistry & Cell Biology. 45, 2821-2831 (2013).
- Gentile, M. T., et al. Ruta graveolens water extract inhibits cell-cell network formation in human umbilical endothelial cells via MEK-ERK1/2 pathway. Experimental Cell Research. 364 (1), 50-58 (2018).
- Butler, M. S. Natural products to drugs: natural product-derived compounds in clinical trials. Natural Product Reports. 25, 475-516 (2008).
- Sulaiman, R. S., Basavarajappa, H. D., Corson, T. W. Natural product inhibitors of ocular angiogenesis. Experimental Eye Research. 129, 161-171 (2014).
- Risau, W. Mechanisms of angiogenesis. Nature. 386 (6626), 671-674 (1997).
- Trung, N. X. In vitro models for angiogenesis. Journal of Science & Development. 13 (4), 850-858 (2015).
- Ucuzian, A. A., Greisler, H. P. In vitro Models of Angiogenesis. World Journal of Surgery. 31, 654-663 (2007).
- Simons, M., et al. American Heart Association Council on Basic Cardiovascular Sciences and Council on Cardiovascular Surgery and Anaesthesia. State-of-the-Art Methods for Evaluation of Angiogenesis and Tissue Vascularisation: A Scientific Statement From the American Heart Association. Circulation Research. 116 (11), e99-e132 (2015).
- Arnaoutova, I., Kleinman, H. K. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nature Protocols. 5 (4), 628-635 (2010).
- DeCicco-Skinner, K. L., et al. Endothelial cell tube formation assay for the in vitro study of angiogenesis. Journal of Visualized Experiments. (91), e51312 (2014).

