Quantitative Examination of Antibiotic Susceptibility of Neisseria gonorrhoeae Aggregates Using ATP-utilization Commercial Assays and Live/Dead Staining
Summary
A simple ATP-measuring assay and live/dead staining method were used to quantify and visualize Neisseria gonorrhoeae survival after treatment with ceftriaxone. This protocol can be extended to examine the antimicrobial effects of any antibiotic and can be used to define the minimal inhibitory concentration of antibiotics in bacterial biofilms.
Abstract
The emergence of antibiotic resistant Neisseria gonorrhoeae (GC) is a worldwide health threat and highlights the need to identify individuals who fail treatment. This Gram-negative bacterium causes gonorrhea exclusively in humans. During infection, it is able to form aggregates and/or biofilms. The minimum inhibitory concentration (MIC) test is used for to determine susceptibility to antibiotics and to define appropriate treatment. However, the mechanism of the eradication in vivo and its relationship to laboratory results are not known. A method that examines how GC aggregation affects antibiotic susceptibility and shows the relationship between aggregate size and antibiotic susceptibility was developed. When GC aggregate, they are more resistant to antibiotic killing, with bacteria in the center surviving ceftriaxone treatment better than those in the periphery. The data indicate that N. gonorrhoeae aggregation can reduce its susceptibility to ceftriaxone, which is not reflected using the standard agar plate-based MIC methods. The method used in this study will allow researchers to test bacterial susceptibility under clinically relevant conditions.
Introduction
Gonorrhea is a common sexually transmitted infection (STI)1. Neisseria gonorrhoeae (GC), a Gram-negative diplococcal bacterium, is the causative agent of this disease. Symptoms of genital infection can result in pain during urination, generalized genital pain, and urethral discharge. Infection is often asymptomatic2,3,4,5, and this allows for extended colonization. These untreated infections are a major health concern, as they have the potential to facilitate transmission of the organism and this can lead to complications such as pelvic inflammatory disease (PID) and disseminated gonococcal infection (DGI)6. Antibiotic-resistant gonorrhea is a major public health crisis and an increasing socioeconomic burden7. Reduced susceptibility to cephalosporins has resulted in treatment regimen change from a single antibiotic to dual therapy, which combines azithromycin or doxycycline with ceftriaxone8. The increased failure of ceftriaxone and azithromycin9,10, in combination with asymptomatic infections, highlights the need for understanding gonorrhea treatment failures.
The minimum inhibitory concentration (MIC) test, including agar dilution and disc diffusion tests, has been used as the standard medical test for identifying resistance to an antibiotic. Nevertheless, it is unclear if the MIC test reflects bacterial antibiotic resistance in vivo. The formation of bacterial biofilms contributes to the survival of bacteria in the presence of bactericidal concentrations of antibiotic: the MIC testing is unable to detect this effect11. Because GC can form biofilms on mucosal surfaces12, we hypothesize that antibiotic susceptibility within aggregates would be different from that seen in individual GC. Additionally, studies have shown that three phase variable surface molecules, Pili, opacity-associated protein (Opa), and lipooligosaccharides (LOS), that regulate inter-bacterium interactions, lead to different sized aggregates13,14,15. The contribution of these components to antibiotic resistance has not been examined due to the lack of proper methods.
Currently, there are several methods to measure biofilm eradication. The most widely used quantitative method is by measuring the changes in biomass using crystal violet staining16. However, the method requires significant experimental manipulation, which can potentially generate errors in experiment repeats17. The live/dead staining method used here allows visualization of live and dead bacteria and their distribution within the biofilm. However, the biofilm structure can pose as a physical barrier that reduces dye penetration. Therefore, to quantify live/dead bacteria within a group, the staining is limited to small biofilms or its precursor- microcolonies or aggregations. Other methods, including the agar dilution and disc diffusion tests, are not able to measure the effects of aggregation. To examine GC susceptibility within aggregation after antibiotic exposure, an ideal method would need to have both a quantitative assay that can measure live bacteria and visualize their distribution.
The procedure described here combines an ATP-utilization measurement and a live/dead staining assay to quantitatively and visually examine GC susceptibility within aggregates in the presence of antibiotics.
Protocol
1. General maintenance of GC strains
- Streak N. gonorrhoeae strains on GCK agar with 1% Kellogg supplements18 (Table 1, Table 2) from freezer stocks and incubate 37 °C with 5% CO2 for 16-18 h. Use MS11 expressing phase-variable Opa (MS11Opa+), no Opa (MS11ΔOpa), or a truncated LOS (MS11ΔLgtE).
- Carefully pick pili negative (colony without dark edge) or positive (colony with dark edge) colonies from each strain based on colony morphology19 using a dissecting light microscope and streak onto a new GCK plate.
- Incubate at 37 °C with 5% CO2 for 16-18 h before use.
2. Viability quantification of GC aggregations
- Collect GC using a sterile applicator. Swab GC from the plate and re-suspend GC in pre-warmed broth(GCP, Table 3) supplemented with 4.2% NaHCO3 and 1% Kellogg solutions18. Use spectrophotometry at a wavelength of 650 nm (an OD650 of 1 = ~1 x 109 CFU/mL) to determine the concentration of suspended bacteria.
- Adjust the concentration of GC to ~1 x 108 CFU/mL.
- Add 99 µL of adjusted GC suspension into wells of a 96-well plate.
- Incubate the plate for 6 h at 37 °C with 5% CO2 to allow the bacteria to aggregate.
- Add 1 µL of serial diluted ceftriaxone (1000, 100, 50, 25, 12.5, 6.2, 3.1, 1.5, 0.8, 0.4, 0.2 µg/mL) into each well. Leave some wells untreated to serve as controls.
- Incubate the plate for 24 h at 37 °C with 5% CO2.
- Sonicate the suspension 3 times in each well for 5 s at 144 W and 20 kHz.
- Add 100 µL of commercially available ATP utilization glow reagent into each well, pipette up-and down for 3 times, and incubate for 15 min at 37 °C with 5% CO2.
- Carefully transfer 150 µL of mixture from each well into a new well in a 96-well black microplate and avoid introducing bubbles.
- Measure the absorbance of each well at 560 nm using the plate reader.
- Calculate the survival rate by the ratio of the reading obtained after serial ceftriaxone treatment to the reading from untreated wells.
3. Fluorescence microscopic analysis of Live/Dead of GC aggregates
- Collect GC using a sterile applicator. Swab GC from the plate and re-suspend GC in pre-warmed GCP media plus 1% Kellogg supplements.
- Determine the number of bacteria by spectrophotometry at a wavelength of 650 nm and adjust the concentration of GC to ~1 x 107 CFU/mL.
- Add 198 µL of GC suspension into in 8-well coverslip-bottom chambers.
- Incubate the chamber for 6 h at 37 °C with 5% CO2 to allow aggregation formation.
- Add 2 µL of ceftriaxone (100 µg/mL or various dilutions) into each well within each aggregation condition. Incubate for the desired time at 37 °C with 5% CO2.
- Add 0.6 µL of live/dead staining solution mixture into each well and incubate for 20 min at 37 °C with 5% CO2.
- Acquire Z-series images using a confocal microscope (an equivalent microscope can be used).
- Analyze the images using ImageJ software for measurement of the size of GC aggregates and the fluorescence intensity ratio (FIR) of live-to-dead staining in each aggregate.
4. Image analysis
- Estimation of the size of bacterial aggregates.
- Open ImageJ and open an image by dragging a raw image file to the ImageJ menu bar.
- Click Freehand Lines in the ImageJ menu bar and circle the area of each aggregation in the image.
- Click Analyze | Measure in the ImageJ menu bar.
- Obtain the number in a new window under the Area column.
- Quantification of live-to-dead ratio of aggregations
- Open ImageJ and open an image by dragging a raw image file to the ImageJ menu bar.
- Click Analyze | Set Measurements in the ImageJ menu bar.
- Check the integrated density in the pop-up window and click OK.
- Click Image | Color | Channels Tool in the menu bar and select Color.
- Check Channel 1 as the fluorescence for live bacteria staining.
- Click Freehand Lines in the ImageJ menu bar and circle the area of each aggregation.
- Click Analyze | Measure in the ImageJ menu bar.
- Obtain the number under IntDen column.
- Check Channel 2 as the fluorescence for dead bacterial staining. Repeat steps 4.2.6-4.2.8.
- Obtain the live-to-dead ratio by dividing number from step 4.2.8 by number from step 4.2.9.
- Statistical analysis
- Open GraphPad Prism and enter the numbers obtained from ImageJ to the desired column.
- Click Analyze and select t tests under Column analyses.
- Check the desired column for comparison and click OK.
- Obtain the P-Value under Analysis window.
- Select the Linear Regression under XY analyses from step 4.3.3
- Obtain the R-square and the P-Value under the Analysis window.
Representative Results
Two methods were employed: an ATP utilization assay and a live/dead staining assay. The results can either be combined or individually used for examining bacterial survival within aggregates after antibiotic treatment. The ATP utilization assay has been shown to measure accurately viable bacteria in S. aureus biofilms20,21. Here, MS11Opa+Pil+ strain was used to examine the role of GC aggregation in antibiotic susceptibility. Non-aggregated MS11Opa+Pil+, aggregated MS11Opa+Pil+, or aggregated and then disrupted by sonication MS11Opa+Pil+ were treated with serial dilutions of ceftriaxone and the ATP level measured (Figure 1A). In comparing the percent survival with and without antibiotic treatment, pre-aggregated GC had significantly higher survival than non-aggregated or aggregation-disrupted GC with equal at or above 0.015 µg/mL of ceftriaxone (MIC from agar dilution test (Table 4)). MS11Opa+Pil-, MS11ΔOpa or MS11ΔLgtE, which have the same agar dilution MIC (Table 4), but form smaller aggregates, was examined and compared to MS11Opa+Pil+ (Figure 1B). MS11Opa+Pil+, forming larger aggregates, had the higher ATP level with ceftriaxone treatment than the mutant strains.
Live/dead staining has been used in several biofilm/aggregation-related studies22,23.To determine the effect of aggregation, pre-aggregated MS11Opa+Pil+ was treated with or without ceftriaxone and imaged. This allows both visualization (which can be quantified) and the distribution of live and dead GC after antibiotic treatment (Figure 2A– left two panels). Dead bacteria (red) were largely located at the outer layers whereas live GC (green) were located mainly in the core of ceftriaxone treated aggregates. This procedure was performed with MS11Opa+Pil-, MS11ΔOpa or MS11ΔLgtE, to examine aggregation size and survival rate (Figure 2A). MS11Opa+Pil+ was shown to form the largest and MS11Opa+Pil- the smallest aggregates (Figure 2A,B). MS11Opa+Pil+ aggregates were still alive in the core layer whereas GC in the small loose aggregates of MS11ΔOpaPil+, MS11Opa+Pil-, and MS11ΔLgtEPil+ were dead (Figure 2A,C). Based on the size and survival, a correlation graph can be plotted to examine the relationship of aggregation size and antibiotic survival (Figure 2D).

Table 1: Recipe for 1 L of GCK Agar Plate.

Table 2: Recipe for 1 L of 100x Kellogg's supplement.
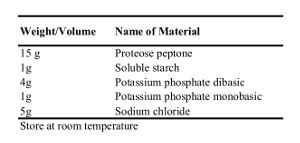
Table 3: Recipe for 1 L of GCP Bacterial Growth Media.

Table 4: Minimum inhibitory concentration of GC strains treated with ceftriaxone. MS11Opa+Pil+, MS11ΔOpa, MS11ΔLgtE, and MS11Opa+Pil- were grown and suspended in GCP. Agar dilution test was then performed with serial concentration of ceftriaxone from 0.0016 – 0.25 µg/mL.
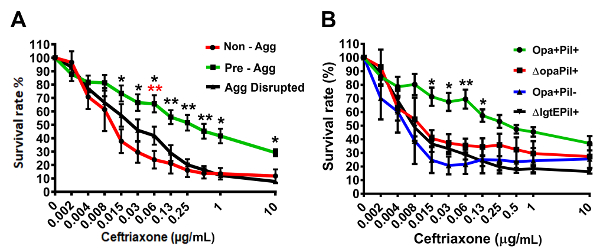
Figure 1: Representative data of survival rate of aggregated GC under ceftriaxone treatment by ATP-utilization assay. (A) Survival rate comparison of MS11Opa+Pil+ suspension without pre-aggregating, pre-aggregating for 6 h, or disrupting after pre-aggregating for 6 h. (B) Survival rate comparison of 6 h aggregated MS11Opa+Pil+ with MS11ΔOpaPil+, MS11Opa+Pil-, or MS11ΔLgtEPil+. Shown are the average values (±SD) obtained from three independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05. This figure was previously published 24 and is used with permission. Please click here to view a larger version of this figure.
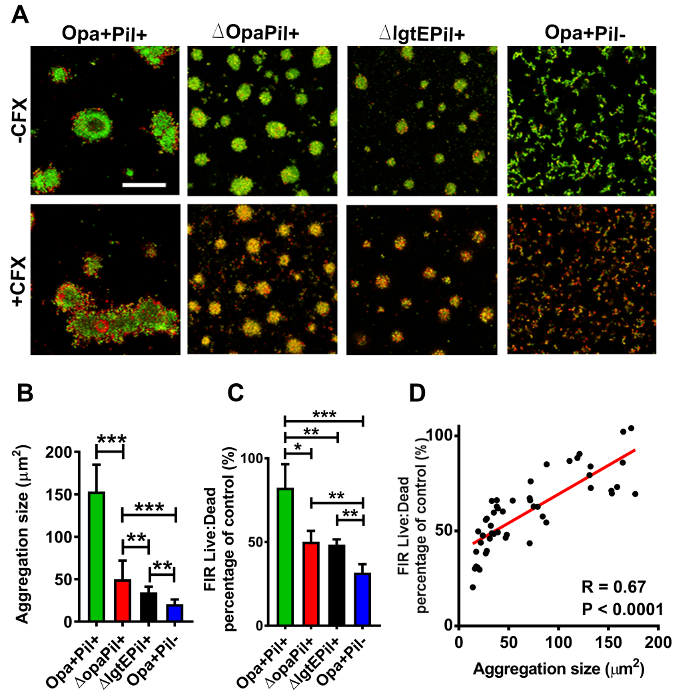
Figure 2: Representative data of live/dead bacteria distribution within aggregates under ceftriaxone treatment. (A) Pre- aggregated MS11Opa+Pil+, MS11ΔOpaPil+, MS11Opa+Pil-, or MS11ΔLgtEPil+ was either incubated in the presence or absence of 1 µg/mL ceftriaxone for 2 h. Aggregates were then stained to visualize viable (green) and dead (red) GC and visualized with confocal fluorescence microscope. Scale bar: 50 µm. Images were then analyzed for (B) aggregation size and (C) ratio of live-to-dead GC and (D) a correlation graph was then created. Shown are the average values (SD) obtained from > 40 images of three independent experiments. ***p < 0.001; **p < 0.01; *p < 0.05. This figure was previously published 24and is used with permission. Please click here to view a larger version of this figure.
Discussion
Bacteria can form biofilms during infection of the human body. Traditional MIC testing may not reflect the concentration needed to eradicate bacteria in a biofilm. To test antimicrobials effects on a biofilm, methods based on biofilm biomass as well as plating CFUs can be erroneous due to the impact of biofilm structure. For example, the plating method only works if the biofilm can be disrupted. Hence, the CFU obtained may be lower than the actual number of viable bacteria. Visualizing dead and live bacteria within the biofilm have been established for measuring the survival of bacteria within biofilm, however, depending on the structure and density of biofilm, the staining may fail to penetrate into the biofilm and result in an inaccurate and underestimated survival rate.
The method here uses both a quantitative and a visualization assay to measure bacterial survival after treatment of aggregates. The advantage of this method is that it measures the bacterial survival in an environment that is closer to what is seen in a real infection. The survival rate differences between non-aggregated and aggregated bacteria will allow us to determine if the in vitro MIC correlates with the in vivo MIC.
The ATP utilization assay, which measures ATP production, can quantitatively measure the survival of aggregates. The assay is more sensitive and can differentiate survival between small differences in antibiotic concentration, compared to other similar assays. However, due to the high sensitivity of this assay, assurance of bacterial cell lysis is critical. Therefore, a sonication step was used. In addition, this method cannot be used to measure bacterial survival in vivo due to the large amount of ATP that host cells produce that can mask the bacterial ATP level. Moreover, the interaction of host cells with bacteria may affect bacterial ATP production. Using this assay on bacteria with pigments may be limiting as the pigments may interfere with the reading.
The live/dead stain has been widely used in biofilm studies. However, the penetration of the staining dyes may stain only the outermost bacteria, whereas the core may not be stained. Therefore, it can only be used for visualization but not quantification, due to uneven distribution of the dye. We used small aggregates for this staining and demonstrated this assay can be used to quantify the overall bacterial survival within the aggregates. Furthermore, negative and positive controls are needed for adjusting the concentration of the dye for different bacteria.
In combination with the MIC protocol, the ATP utilization assay and live/dead stain can serve as an effective method to quantitatively and visually analyze N.gonorrhoeae as well as other bacterial aggregations25,26. The application of the combined protocol could provide a better understanding of disease formation along with its versatile uses in drug screening based on bacteria biofilms. This method may reflect more accurately the in vivo MIC of an antibiotic.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from National Institute of Health to D.C.S. and W.S. AI123340. L.-C.W., J.W., A.C., and E.N. were supported in part/participate in "The First-Year Innovation & Research Experience" program funded by the University of Maryland. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript. We acknowledge the UMD CBMG Imaging Core for all microscopy experiments.
Materials
| 100x Kellogg's supplement | |||
| Agar | United States Biological | A0930 | |
| BacTiter Assay | Promega | G8232 | |
| Ceftriaxone | TCI | C2226 | |
| Difco GC medium base | BD | 228950 | |
| Ferric nitrate, nonahydrate | Sigma-Aldrich | 254223-10G | |
| Glucose | Thermo Fisher Scientific | BP350-1 | |
| L-glutamine Crystalline Powder | Fisher Scientific | BP379-100 | |
| BacLight live/dead staining | Invitrogen | L7012 | |
| MS11 Neisseria gonorrhoeae strain | kindly provided by Dr. Herman Schneider, Walter Reed Army Institute for Research | ||
| Potassium phosphate dibasic (K2HPO4) | Fisher Scientific | P290-500 | |
| Potassium phosphate monobasic (KH2PO4) | Fisher Scientific | BP329-1 | |
| Proteose Peptone | BD Biosciences | 211693 | |
| Sodium chloride (NaCl) | Fisher Scientific | S671-10 | |
| Soluble Starch | Sigma-Aldrich | S9765 | |
| Thiamine pyrophosphate | Sigma-Aldrich | C8754-5G | |
| Equipment | |||
| Petri Dishes | VWR | 25384-302 | |
| 8-well coverslip-bottom chamber | Thermo Fisher Scientific | 155411 | |
| 96-well tissue culture plates | Corning, Falcon | 3370 | |
| Biosafety Cabinet (NU-425-600 Class II, A2 Laminar Flow Biohazard Hood) | Nuaire | 32776 | |
| CO2 Incubator | Fisher Scientific | Model 3530 | |
| Confocal microscope equipped with live imaging chamber | Leica | SP5X | |
| Corning 96 Well Black Polystyrene Microplate | Corning | 3904 | |
| Glomax Illuminator | Promega | E6521 | |
| Pipette tips (0.1-10 µL) | Thermo Fisher Scientific | 02-717-133 | |
| Pipette tips (1000 µL) | VWR | 83007-382 | |
| Pipette tips (200 µL) | VWR | 53509-007 | |
| Spectrophotometer Ultrospec 2000 UV | Pharmacia Biotech | 80-2106-00 | |
| Sterile 15 ml conical tubes | VWR | 21008-216 | |
| Sterile Microcentrifuge Tubes (1.7 mL) | Sorenson BioScience | 16070 | |
| Sterile polyester-tipped applicators | Fisher Scientific | 23-400-122 | |
| Sonicator | Kontes | Equivelent to 9110001 |
Riferimenti
- den Heijer, C. D., et al. A comprehensive overview of urogenital, anorectal and oropharyngeal Neisseria gonorrhoeae testing and diagnoses among different STI care providers: a cross-sectional study. BMC Infectious Diseases. 17 (1), (2017).
- Hein, K., Marks, A., Cohen, M. I. Asymptomatic gonorrhea: prevalence in a population of urban adolescents. The Journal of Pediatrics. 90 (4), 634-635 (1977).
- Hananta, I. P., et al. Gonorrhea in Indonesia: High Prevalence of Asymptomatic Urogenital Gonorrhea but No Circulating Extended Spectrum Cephalosporins-Resistant Neisseria gonorrhoeae Strains in Jakarta, Yogyakarta, and Denpasar, Indonesia. Sexually Transmitted Diseases. 43 (10), 608-616 (2016).
- Chlamydia, W. H. O. Chlamydia trachomatis, Neisseria gonorrhoeae, syphilis and Trichomonas vaginalis. Methods and results used by WHO to generate 2005 estimates. World Health Organisation, 2011. Prevalence and incidence of selected sexually transmitted infections. World Health Organisation. , (2011).
- Mayor, M. T., Roett, M. A., Uduhiri, K. A. Diagnosis and management of gonococcal infections. American Family Physician. 86 (10), 931-938 (2012).
- Alirol, E., et al. Multidrug-resistant gonorrhea: A research and development roadmap to discover new medicines. PLOS Medicine. 14 (7), (2017).
- Workowski, K. A., Bolan, G. A. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recommendations and Reports. 64, 1-137 (2015).
- Lahra, M. M., et al. Cooperative Recognition of Internationally Disseminated Ceftriaxone-Resistant Neisseria gonorrhoeae Strain. Emerging Infectious Diseases. 24 (4), (2018).
- Wi, T., et al. Antimicrobial resistance in Neisseria gonorrhoeae: Global surveillance and a call for international collaborative action. PLOS Medicine. 14 (7), 1002344 (2017).
- Singh, S., Singh, S. K., Chowdhury, I., Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiology Journal. 11, 53-62 (2017).
- Greiner, L. L., et al. Biofilm Formation by Neisseria gonorrhoeae. Infection and Immunity. 73 (4), 1964-1970 (2005).
- Zollner, R., Oldewurtel, E. R., Kouzel, N., Maier, B. Phase and antigenic variation govern competition dynamics through positioning in bacterial colonies. Scientific Reports. 7 (1), (2017).
- Stein, D. C., et al. Expression of Opacity Proteins Interferes with the Transmigration of Neisseria gonorrhoeae. across Polarized Epithelial Cells. PLoS One. 10 (8), 0134342 (2015).
- LeVan, A., et al. Construction and characterization of a derivative of Neisseria gonorrhoeae strain MS11 devoid of all opa genes. Journal of Bacteriology. 194 (23), 6468-6478 (2012).
- Merritt, J. H., Kadouri, D. E., O’Toole, G. A. Growing and analyzing static biofilms. Curr Protoc Microbiol. , (2005).
- Peeters, E., Nelis, H. J., Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. Journal of Microbiological Methods. 72 (2), 157-165 (2008).
- White, L. A., Kellogg, D. S. Neisseria Gonorrhoeae Identification in Direct Smears by a Fluorescent Antibody-Counterstain Method. Journal of Applied Microbiology. 13, 171-174 (1965).
- Swanson, J., Kraus, S. J., Gotschlich, E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. Journal of Experimental Medicine. 134 (4), 886-906 (1971).
- Herten, M., et al. Rapid in Vitro Quantification of S. aureus Biofilms on Vascular Graft Surfaces. Frontiers in Microbiology. 8, 2333 (2017).
- Gracia, E., et al. In vitro development of Staphylococcus aureus biofilms using slime-producing variants and ATP-bioluminescence for automated bacterial quantification. Luminescence. 14 (1), 23-31 (1999).
- Webb, J. S., et al. Cell death in Pseudomonas aeruginosa biofilm development. Journal of Bacteriology. 185 (15), 4585-4592 (2003).
- Jurcisek, J. A., Dickson, A. C., Bruggeman, M. E., Bakaletz, L. O. In vitro biofilm formation in an 8-well chamber slide. Journal of visualized experiments. (47), (2011).
- Wang, L. C., Litwin, M., Sahiholnasab, Z., Song, W., Stein, D. C. Neisseria gonorrhoeae Aggregation Reduces Its Ceftriaxone Susceptibility. Antibiotics (Basel). 7 (2), (2018).
- Lebeaux, D., Ghigo, J. M., Beloin, C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology and Molecular Biology Reviews. 78 (3), 510-543 (2014).
- Hall-Stoodley, L., et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunology and Medical Microbiology. 65 (2), 127-145 (2012).

