Determining the Egg Fertilization Rate of Bemisia tabaci Using a Cytogenetic Technique
Summary
We present a simple cytogenetic technique using 4′,6-diamidino-2-phenylindole (DAPI) to determine the fertilization rate and primary sex ratio of the haplodiploid invasive pest Bemisia tabaci.
Abstract
A few species of sap-sucking whiteflies are some of the most damaging terrestrial pests worldwide because of the crop damage they inflict and plant viruses they vector. Despite numerous studies of the biology of these species in different environments, a key life history parameter, offspring sex ratios, has received little attention, yet is important for predicting population dynamics. The primary sex ratio (sex ratio at oviposition) of Bemisia tabaci has never been reported but can be found by determining the egg fertilization rate of this haplodiploid insect. The technique involves the dechorionation of eggs with bleach, a series of fixation steps, and the application of the general DNA fluorescent stain, DAPI (4′,6-diamidino-2-phenylindole, a DNA-binding fluorescent dye), to bind to female and male pronuclei. Here, we present the technique, and an example of its application, to test whether an endosymbiotic bacterium, Rickettsia sp. nr. bellii, influenced the primary sex ratio of B. tabaci. This method may assist in population studies of whiteflies, or in determining if sex allocation exists with certain environmental stimuli.
Introduction
The study of sex allocation, or the relative investment in male and female offspring, is a cornerstone of behavioral ecology1,2,3. In addition to its power for testing adaptive models of behavior, knowing the sex allocation strategy of an organism may improve models of its population dynamics. In many species, sex allocation is controlled by mothers. To determine sex allocation, it is important to determine the primary sex ratio or the proportion of females at the time of egg deposition. Although the sex ratio at adult emergence may provide clues to sex allocation, differential developmental mortality between male and female juveniles may commonly skew the adult sex ratio substantially. In some species of Hymenoptera, the order of insects that contains ants, bees, and wasps, the primary sex ratio has been determined with cytogenetic assays, staining the embryos to view genetic DNA. Because hymenopterans are haplodiploid, an incipient male egg is haploid and contains only the female pronucleus (n), while incipient female eggs are diploid and contain both male and female pronuclei (2n). Although Aleyrodidae, the sap-feeding family of true bugs (Hemiptera) known as whiteflies, are also haplodiploid, there has not been an established assay to find the primary sex ratio in these insects. This is perhaps surprising given the intensity of study of the few cosmopolitan serious pests in this family and the importance of sex ratios in competitive interactions of whiteflies4,5,6,7,8,9,10 and in population dynamics generally. In haplodiploid insects too, sex ratios are unconstrained by sex determination systems, allowing the possibility of selective fertilization and labile sex ratios that vary with the environment2. Here we present a technique to determine the primary sex ratio of the species complex of whiteflies known collectively as the sweetpotato whitefly, B. tabaci. This one species name encompasses more than 28 species worldwide11and includes some of the most damaging global invasive pests12,13. The application of this technique to determine sex allocation patterns in B. tabaci and other Aleyrodidae will allow a more rigorous investigation of variables, including temperature, host plant, endosymbiotic bacteria, or plant/whitefly pathogens, that may influence whitefly primary sex ratios and whitefly population dynamics.
We are unaware of any comparable egg-staining techniques for B. tabaci. The protocol is convenient in comparison with staining methods used for other insect eggs14 as it omits an overnight fixation step and, therefore, can be completed within 3 h. As one example of an application, an endosymbiotic bacterium, Rickettsia sp. nr. bellii, is associated with female bias in our laboratory lines of B. tabaci Middle East-Asia Minor 1 (MEAM1)15,16. In one B. tabaci MEAM1 laboratory line ("MAC1," collected from the Maricopa Agricultural Center), we test whether Rickettsia-infected (R+) females fertilize more eggs than uninfected (R–) females.
Protocol
NOTE: Ensure that all work is performed at room temperature in a well-ventilated area or under a fume hood. All ‘drops’ in this protocol are defined as 5–20 µL, depending on the operator’s preference.
1. Initial Setup
- Allow female whiteflies to oviposit on clean leaves. Examples for oviposition arenas include clip cages or leaves cut to fit on agar in a Petri dish. Make a coverable hole in the clip cage or Petri dish cover to insert and remove the adults. Alternatively, quickly put a vessel of collected adults on ice and, then, deposit them on the leaf. Limit the time between oviposition and egg fixation to no more than 60 min, to ensure the observation of the sperm transition into the paternal pronucleus in eggs.
NOTE: Eggs not removed from the leaf can be reared to adulthood to record adult sex ratios. - Before or during whitefly oviposition, prepare a microscope slide by cleaning it with soap and water, drying it well, and then stretching a piece of paraffin film over one end, making sure the paraffin film surface does not break (Figure 1).
NOTE: The paraffin film is hydrophobic and semi-opaque, allowing liquids to form drops and eggs to be more easily seen.
2. Dechorionation
- With a glass Pasteur pipette, add drops of bleach solution (0.83% sodium hypochlorite) to the paraffin film.
NOTE: It is easier to keep track of eggs if only 2–3 eggs are in each drop. Alternatively, to manage a large egg number, collect the eggs in drops of 1x phosphate-buffered saline (PBS).
CAUTION: Bleach is corrosive; wear gloves when handling bleach and do not inhale. - Remove the adult whiteflies from the leaf.
- Place the leaf under a microscope to see the eggs clearly and collect the eggs singly and carefully with a thin probe. To make a probe, insert a minuten nadel pin at 45° or at a comfortable working angle into a melted pipette tip (Figure 2).
- Transfer the eggs to bleach or 1x PBS. If using 1x PBS, remove the 1x PBS with a glass Pasteur pipette once all the eggs are collected and, then, add bleach to the eggs.
NOTE: When collecting eggs, slowly lift the egg from its base until the pedicel is removed from the leaf. The pedicel is sticky, and the egg will commonly stick to the probe tip until it is dipped into the bleach.
NOTE: It is recommended to use separate glass Pasteur pipettes for all reagents, and the pipettes may be modified with heat to make the tips narrower and reduce the risk of accidentally aspirating whitefly eggs. To prevent residue and contamination, clean the pipettes with deionized water after every use. - Wait for 10 min. If there is an interest in embryogenesis in eggs older than 1 h, leave the eggs in bleach for up to 15 min.
NOTE: For eggs that are up to 1 h old, 10 min is sufficient.
3. Fixation
NOTE: These steps are taken from a Hymenopteran protocol17.
- Remove the bleach (containing the chorion fragments) with a glass Pasteur pipette and discard it. Add drops of glacial acetic acid with a glass Pasteur pipette and wait 3 min.
CAUTION: Proceed with this step under a fume hood. Glacial acetic acid is corrosive; wear gloves when handling glacial acetic acid and do not inhale, especially in combination with the residual bleach. - Remove the glacial acetic acid with a glass Pasteur pipette and add drops of Clarke’s solution (3:1 of absolute ethanol:glacial acetic acid) with a glass Pasteur pipette. Wait until most of the solution has evaporated (or 10 min max).
- Add drops of 70% ethanol to the eggs with a glass Pasteur pipette and wait until most of the ethanol has evaporated (or 10 min max).
4. Staining
- Remove any residual ethanol with a glass Pasteur pipette and add drops of 1x PBS to the eggs with a glass Pasteur pipette to get the pH close to 7.0. Set the microscope slide in a humidity chamber to prevent desiccation, for example, in an empty pipette tip box with a wet paper towel inside (Figure 3). Wait at least 30 min.
NOTE: This is the best point if a pause is needed, as long as the humidity chamber can prevent desiccation. - Remove the 1x PBS with a glass Pasteur pipette, and add drops of 0.1 μg/mL DAPI, a DNA fluorescent stain, with a glass Pasteur pipette. Set the microscope slide in a dark humidity chamber and wait at least 15 min.
CAUTION: DAPI is an irritant, so handle it with gloves.
5. Washing
- Remove the DAPI with a glass Pasteur pipette.
- Add drops of 1x TBST (5x solution made from 30 g of Tris, 43.8 g of NaCl, 5 mL of polysorbate 20, and 1.0 g of NaN3 [pH 7.5], and brought to 1 L with PCR grade water) to the eggs with a glass Pasteur pipette. Wait 5 min before removing the 1x TBST with a glass Pasteur pipette. Repeat this step 2 times.
6. Mounting
- After the final wash, carefully pipette all the eggs from the paraffin film onto a clean part of the microscope slide. Remove excess 1x TBST; then, add 20 µL of mounting media (80% glycerol and 20% 1x TBST with 2% n-propyl-gallate) and place a clean cover slide on top of the eggs.
- For long-term storage, seal the cover slide with clear nail polish and, then, either store the slide in the dark at 2 °C or immediately view it under a fluorescent microscope.
Representative Results
To test whether Rickettsia affects the fertilization rate of B. tabaci MEAM1 females, we reared Rickettsia-infected (R+) or uninfected (R–) B. tabaci on cowpea plants (Vigna unguiculata) in separate cages at 27 °C, 70% relative humidity, and a 16 h light/8 h dark photoperiod. R+ and R– fourth instar whiteflies were carefully removed from leaves and isolated in 200 µL strip tubes. When adults emerged, they were collected in groups of 50% females and transferred to clean leaves in Petri dishes (n = ~50-100/leaf) for 4 days of mating. Groups of approximately 20 females and several males were then transferred to a clean leaf disk (one for R– adults, one for R+ adults) in 35 mm Petri dishes resting on 1% agar. The Petri dish lid had been cut out and the fine fabric mesh used for containment also prevented excess condensation. After approximately 45 min, all adults were removed, some of the eggs were harvested to determine the fertilization rate, and the eggs that were not collected were reared to adulthood to calculate the adult sex ratio. One cohort for a single day, for both R+ and R– whiteflies, was defined as a block. There were seven blocks for calculating the fertilization rate or primary sex ratio, while there were six blocks for calculating the adult sex ratio, as there were not enough leftover eggs from one block to rear to adulthood. A generalized linear model was used in the statistical package R to determine whether the fertilization rates or adult sex ratios were significantly influenced by Rickettsia infection and/or block. The response variables were the proportion of fertilized eggs/all eggs, or the proportion of female adults/all adults, respectively, while explanatory variables were the block and Rickettsia infection status.
Egg dechorionation followed by DAPI nuclear staining allowed the unambiguous assignment of fertilization (and embryo sex) when observed with a fluorescent microscope (Figure 4). For this experiment, 90 eggs laid by R–B. tabaci MEAM1 females and 82 eggs by R+B. tabaci MEAM1 females were scored. As for eggs reared to adulthood, 60 R– and 95 R+ adults were scored. While a female bias in adult sex ratios has been shown consistently in earlier studies15,18,19, in the current study, adult R+ sex ratios (69% females, median) were female-biased compared to R– females (50% females, median), but the sex ratios in the two treatments were not significantly different (χ2 = 1.02, df = 1, p = 0.31; Figure 5). The primary R– sex ratios (60% fertilized eggs, median) were female-biased compared to R+ sex ratios (44% fertilized eggs, median) but were also not significantly different (χ2 = 0.51, df = 1, p = 0.47), providing no evidence for greater fertilization rates by R+ females (Figure 5). Block also did not have a significant effect on the primary (χ2 = 0.29, df = 1, p = 0.59) or adult (χ2 = 1.20, df = 1, p = 0.27) sex ratio.

Figure 1: Photo of a microscope slide with paraffin film and drops of bleach. Please click here to view a larger version of this figure.

Figure 2: An example of a probe, fashioned with heat, from a pipette tip and a minuten nadeln pin. Please click here to view a larger version of this figure.

Figure 3: An example of a humidity chamber, fashioned from an empty pipette tip box and a wet paper towel. Please click here to view a larger version of this figure.
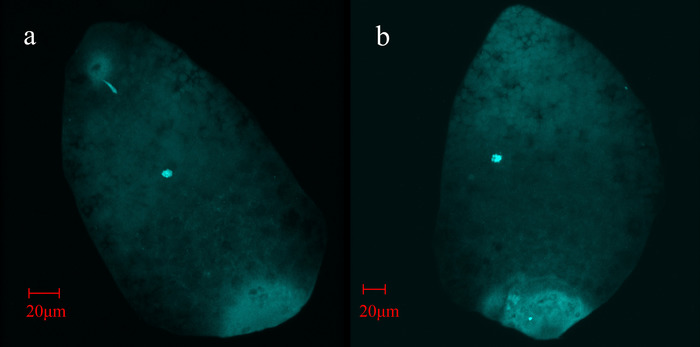
Figure 4: Fluorescent microscope images of B. tabaci eggs. (a) B. tabaci MEAM1 fertilized, incipient female egg. (b) Unfertilized, incipient male egg. The eggs were fixed at less than 1 h after oviposition and stained with DAPI. The base of each egg is fluorescing due to bacterial DNA (Portiera, Hamiltonella, and possibly Rickettsia) in the bacteriome progenitor cell, which is included in the laid egg20,21. In each egg, the female pronucleus is near the center of the egg, and in the female egg only, the sperm is visible as a bright streak near the apex of the egg near what is presumably an autofluorescing micropyle. The images are screenshots taken from a Z-stack generated video, produced by a laser scanning confocal inverted microscope. The scale bars were estimated from previous microscope images. Please click here to view a larger version of this figure.
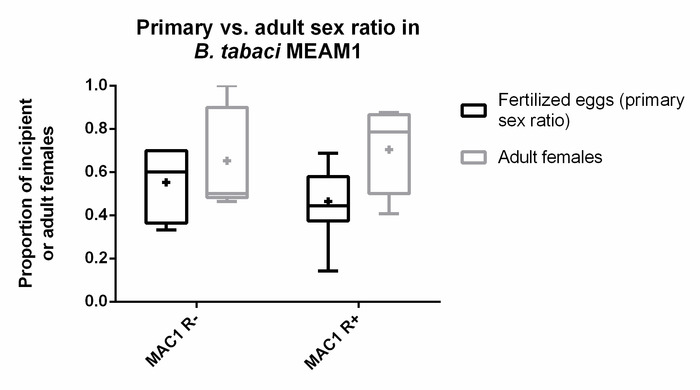
Figure 5: Primary and adult sex ratios of Rickettsia-infected or uninfected B. tabaci. Box and whisker plots of the primary sex ratio (percentage of fertilized eggs, or percentage of female zygotes) in black compared to the adult sex ratio (percentage of adult females) in grey, of the Rickettsia-infected (R+) and uninfected (R–) B. tabaci MEAM1, "MAC1" genetic line. The box and whisker plots show the median as the middle line, the mean as a plus sign, and upper and lower quartiles as the lines that make the ends of the box, and the range is represented in the outer lines extending from the box. For R– eggs scored: n = 90; for R+ eggs scored: n = 82. For R– adults counted: n = 60; for R+ adults counted: n = 95. In a logistical analysis of the primary sex ratio (proportion of fertilized eggs) performed in the statistical package R, no significant effects were found for block (n = 7, χ2 = 0.29, df = 1, p = 0.59) or for Rickettsia infection (χ2 = 0.51, df = 1, p = 0.47). Adult sex ratios, as influenced by block (n = 6) and Rickettsia infection status, were similarly analyzed. Here as well, no significant effects were found for block (χ2 = 1.20, df = 1, p = 0.27) or for Rickettsia infection (χ2 = 1.02, df = 1, p = 0.31). Please click here to view a larger version of this figure.
Discussion
This protocol is the first to capture the fertilization rate or primary sex ratio of B. tabaci. The challenge of this protocol is that it requires researchers to learn how to handle the whitefly eggs quickly, ensuring that not more than 1 h has passed since the eggs were oviposited until they are fixed. During preliminary experiments, eggs that were fixed at 3 h or more postoviposition were too old to observe fertilization, as syngamy had occurred and mitotic divisions were underway. Between 1 to 3 h, the pronuclei took on a rounder shape. While the early presence of two nuclei indicates a fertilized egg, a bit later, the apposition of the two nuclei in preparation for syngamycan appear to be one nucleus, and later again, the two products of the first mitotic division are found in both male and female eggs. Therefore, the distinction between the sexes is not clear at these later time points, and we advise limiting the interval from oviposition to fixation to 1 h as a conservative measure. It is also challenging to learn how to be gentle with the eggs with each transfer of liquid so that they are not accidentally aspirated into the pipette. At the time of viewing the eggs under a fluorescent microscope, a few of the eggs may have broken during the protocol, so those eggs cannot be sexed and counted as the pronuclei and yolk may have escaped. Otherwise, once the operator becomes comfortable with these steps, the protocol can be completed conveniently within 3 h, as it does not require an overnight fixation step. It is also flexible in that it can be modified to stain older eggs, for those researchers interested in capturing development.
An application of this protocol includes research on sex allocation. Although many dozens of studies of whitefly biology have reported adult sex ratios in different environments, adult sex ratios confound sex allocation of the mother with sex-specific developmental mortality of nymphs and make it impossible to determine the cause of any sex ratio pattern. The cytogenetic technique described here allows the possibility of understanding whitefly sex allocation patterns more generally. While the large dispersive populations of B. tabaci and other pests such as the greenhouse whitefly, Trialeurodes vaporariorum, might be predicted to result in 1:1 sex ratios, as often exhibited in laboratory settings22,23, we also predict that reproductive interference, endosymbionts, and potentially host plant quality could influence primary sex ratios. That these same factors could also influence sex-specific mortality patterns during development, systematically skewing sex ratio estimates, underscores the need for a direct measure of the primary sex ratio.
While female bias associated with Rickettsia infection has been consistently found in the genetic line “MAC1”15,18,19, the adult sex ratios were not significantly female biased in the current study. The primary sex ratios observed with the cytogenetics technique were not significantly biased either. We censused the primary and adult sex ratios of B. tabaci females on just 1 day, at the beginning of an oviposition bout, when the females were 4 to 5 days old, so the sex ratios studied may not have represented the sex ratios that we observed over longer periods. Nonetheless, the technique made it possible to determine the fertilization rate or primary sex ratio and, in this instance, showed a correspondence between the primary and adult sex ratios.
Determining fertilization may also be valuable in instances in which researchers might want to determine the type of reproductive isolation among whitefly populations. While it has been a matter of some controversy12,24,25, it is now generally accepted that the name B. tabaci refers to tens of cryptic species, with evidence being provided by both genetic divergence and crossing tests in which few females are produced12,25. In these studies, it would be of interest to know where the isolation occurs. Is sperm transferred, and does heterospecific sperm fertilize eggs, or are attempted matings unsuccessful? Regarding the determination of the primary sex ratio, this technique can determine whether the primary sex ratio is influenced by endosymbionts, selfish genetic elements, or one of many other factors, including conspecifics, competitors, predators, parasitoids, pathogens, the host plant, or abiotic effects.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was funded by an NSF grant (DEB-1020460) to M.S.H. and a USDA AFRI grant (2010-03752) to M.S.H. The authors thank Brennan Zehr for staining whitefly eggs with much skill and Zen. The authors thank Mike Riehle for allowing the use of his fluorescent microscope for imaging. The authors thank Suzanne Kelly and Marco Gebiola for the egg images. The authors thank Suzanne Kelly and Jimmy Conway for helping at crucial moments during the experiments.
Materials
| 1XPBS | Any | ||
| 1XTBST | Any | 5X solution made from 30g Tris, 43.8g NaCl, 5 mL Tween-20 and 1.0g NaN3 pH7.5, and brought to 1L with PCR grade water | |
| Bleach | Clorox | Any household bleach will work as long as it can be diluted to 0.83% Sodium hypochlorite | |
| Clear nail polish | Any | ||
| DAPI dilactate | Santa Cruz Biotechnology | sc300415 | |
| Ethanol | Any | Dilute to 70% EtOH | |
| Fluorescent microscope | Nikon | Nikon Eclipse 50i was used in this experiment, but any fluorescent microscope with 340/380 nm excitation filter and at least 4-10X magnification can be used | |
| Glacial acetic acid | Mallinckrodt | UN2789 | |
| Glycerol | Any | ||
| Microscope | Wild | A Wild M5A microscope was used for this experiment, but any microscope where the operator can clearly see the whitefly eggs can be used | |
| Microscope slide covers | Any | Methods are for 18mm x 18mm sized slide covers. More mounting media will need to be added for larger slide covers. | |
| Microscope slides | Any | ||
| Minuten nadel pins | BioQuip | 1208SA | Minuten nadel pins are optional for fashioning as probes with pipette tips |
| NaCl | Any | ||
| NaN3 | Any | ||
| n-propyl-gallate | Sigma/Santa Cruz Biotechnology | P3130/sc-250794 | |
| Parafilm | Bemis | ||
| Pasteur pipettes | Fisher Scientific | 13-678-20A | Fisherbrand Disposable Borosilicate glass Pasteur pipettes 5.75 in. A Bunsen burner may also be needed if operator would like to lengthen and narrow pipettes |
| PCR grade water | Any | ||
| Pipette tips | Any | Pipette tips are optional for fashioning as probes with minuten nadel pins | |
| Small dropper bulb | Any | Must fit on Pasteur pipette | |
| Tris | Any | ||
| Tween-20 | Any |
Riferimenti
- Charnov, E. L., May, R. M. Sex ratio in spatially structured populations. The Theory of Sex Allocation. , 67-92 (1982).
- Godfray, H. C. J. . Parasitoids: behavioral and evolutionary ecology. , (1994).
- Bourke, A. F. G., Franks, N. R. . Social Evolution in Ants. , (1995).
- Pascal, S., Callejas, C. Intra- and interspecific competition between biotypes B and Q of Bemisia tabaci (Hemiptera: Aleyrodidae) from Spain. Bulletin of Entomological Research. 94 (4), 369-375 (2004).
- Liu, S. -. S., et al. Asymmetric mating interactions drive widespread invasion and displacement in a whitefly. Science. 318 (5857), 1769-1772 (2007).
- Crowder, D. W., Sitvarin, M. I., Carrière, Y. Mate discrimination in invasive whitefly species. Journal of Insect Behavior. 23 (5), 364-380 (2010).
- Crowder, D. W., Sitvarin, M. I., Carrière, Y. Plasticity in mating behaviour drives asymmetric reproductive interference in whiteflies. Animal Behaviour. 79 (3), 579-587 (2010).
- Tsueda, H., Tsuchida, K. Reproductive differences between Q and B whiteflies, Bemisia tabaci, on three host plants and negative interactions in mixed cohorts. Entomologia Experimentalis et Applicata. 141, 197-207 (2011).
- Wang, P., Crowder, D. W., Liu, S. -. S. Roles of mating behavioural interactions and life history traits in the competition between alien and indigenous whiteflies. Bulletin of Entomological Research. 102 (4), 395-405 (2012).
- Sun, D. -. B., Li, J., Liu, Y. -. Q., Crowder, D. W., Liu, S. -. S. Effects of reproductive interference on the competitive displacement between two invasive whiteflies. Bulletin of Entomological Research. 104 (3), 334-346 (2014).
- Liu, S. -. S., Colvin, J., De Barro, P. J. Species concepts as applied to the whitefly Bemisia tabaci systematics: how many species are there?. Journal of Integrative Agriculture. 11 (2), 176-186 (2012).
- Gilbertson, R. L., Batuman, O., Webster, C. G., Adkins, S. Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Annual Review of Virology. 2 (1), 67-93 (2015).
- Giorgini, M., et al. Rickettsia symbionts cause parthenogenetic reproduction in the parasitoid wasp Pingala soemius (Hymenoptera: Eulophidae). Applied and Environmental Microbiology. 76 (8), 2589-2599 (2010).
- Himler, A. G., et al. Rapid spread of a bacterial symbiont in an invasive whitefly is driven by fitness benefits and female bias. Science. 332 (6026), 254-256 (2011).
- Cass, B. N., et al. Dynamics of the endosymbiont Rickettsia in an insect pest. Microbial Ecology. 70 (1), 287-297 (2015).
- Vavre, F., de Jong, J. H., Stouthamer, R. Cytogenetic mechanism and genetic consequences of thelytoky in the wasp Trichogramma cacoeciae. Heredity. 93 (6), 592-596 (2004).
- Cass, B. N., et al. Conditional fitness benefits of the Rickettsia bacterial symbiont in an insect pest. Oecologia. 180 (1), 169-179 (2016).
- Hunter, M. S., Asiimwe, P., Himler, A. G., Kelly, S. E. Host nuclear genotype influences phenotype of a conditional mutualist symbiont. Journal of Evolutionary Biology. 30, 141-149 (2017).
- Gottlieb, Y., et al. Inherited intracellular ecosystem: symbiotic bacteria share bacteriocytes in whiteflies. FASEB Journal. 22 (7), 2591-2599 (2008).
- Luan, J., Sun, X., Fei, Z., Douglas, A. E. Maternal inheritance of a single somatic animal cell displayed by the bacteriocyte in the whitefly Bemisia tabaci. Current Biology. 28 (3), 459-465 (2018).
- Guo, J. -. Y., Cong, L., Wan, F. -. H. Multiple generation effects of high temperature on the development and fecundity of Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) biotype B. Insect Science. 20 (4), 541-549 (2013).
- Cui, X., Wan, F., Xie, M., Liu, T. Effects of heat shock on survival and reproduction of two whitefly species, Trialeurodes vaporariorum and Bemisia tabaci biotype B. Journal of Insect Science. 8 (24), (2008).
- Boykin, L. M. Bemisia tabaci nomenclature: Lessons learned. Pest Management Science. 70 (10), 1454-1459 (2014).
- Qin, L., Pan, L. -. L., Liu, S. -. S. Further insight into reproductive incompatibility between putative cryptic species of the Bemisia tabaci whitefly complex. Insect Science. 23 (2), 215-224 (2016).

