Isolating Free Carbenes, their Mixed Dimers and Organic Radicals
Summary
We present protocols for the isolation of stable heterocyclic carbenes. The synthesis of a cyclic (alkyl)(amino) carbene (CAAC) and an N-heterocyclic carbene (NHC) is demonstrated using filter cannulas and Schlenk technique. We furthermore present the synthesis of the related oxygen-sensitive, electron-rich mixed “Wanzlick dimer” and the reduced stable organic radical.
Abstract
Protocols for the isolation of the commonly employed cyclic (alkyl)(amino) carbene (CAAC) and N-heterocyclic carbene (NHC) are reported. Furthermore, the synthesis of their mixed CAAC–NHC “Wanzlick” dimer and the synthesis of the related stable organic “olefin” radical are presented. The main goal of this manuscript is to give a detailed and general protocol for the synthetic chemist of any skill level on how to prepare free heterocyclic carbenes by deprotonation using filter cannulas. Due to the air-sensitivity of the synthesized compounds, all experiments are performed under inert atmosphere using either Schlenk technique or a dinitrogen filled glovebox. Controlling Wanzlick’s equilibrium (i.e., the dimerization of free carbenes), is a crucial requirement for the application of free carbenes in coordination chemistry or organic synthesis. Thus, we elaborate on the specific electronic and steric requirements favoring the formation of dimers, heterodimers, or monomers. We will show how proton catalysis allows for the formation of dimers, and how the electronic structure of carbenes and their dimers affects the reactivity with either moisture or air. The structural identity of the reported compounds is discussed based on their NMR spectra.
Introduction
More than half a century ago, Wanzlick reported arguably the first attempts to synthesize N-heterocyclic carbenes1,2,3. However, instead of isolating the free carbenes, he succeeded only in characterizing their dimers. This observation prompted him to suggest an equilibrium between the olefin dimer and the respective free carbenes, which is now commonly referred to as “Wanzlick’s equilibrium” (Figure 1, I.)4,5,6. Later on, it was argued that the dimerization of free carbenes and of course equally the reverse reaction (i.e., the dissociation of the related olefin dimers), is catalyzed by protons7,8,9,10,11,12. It took another 30 years until the first “bottleable” carbene, which did not dimerize at room temperature, was reported by Bertrand13,14. Especially N-heterocyclic carbenes (NHCs; imidazolin-2-ylidenes) became the subject of intensive research after Arduengo had reported a stable crystalline NHC, 1,3-diadamantyl-imidazolin-2-ylidene15. The surprising stability of this carbene was first rationalized by a combination of steric effects due to the bulky adamantyl substituents as well as electronic effects associated with the aromatic N-heterocycle. However, it was shown later in an elegant study by Murphy that even “monomeric” 1,3-dimethyl-imidazolin-2-ylidene16 (i.e., the free carbene derived from N,N-dimethylimidazolium salts) with very small methyl substituents is more stable than its dimer17. Lavallo and Bertrand showed on the contrary, that also the removal of one stabilizing nitrogen atom, as reported by the isolation of a cyclic (alkyl)(amino) carbene (CAAC), can be balanced by introduction of a bulky 2,6-diisopropylphenyl (Dipp) substituent18.
NHCs and CAACs proved extraordinarily fruitful for the coordination chemistry of the d- and p-block elements, transition metal catalysis, or organocatalysis (For thematic issues and books on NHCs, see19,20,21,22,23, for reviews on CAACs, see24,25,26,27,28, for the synthesis of CAACs, see18,29,30,31). The impressive success story of cyclic carbene ligands is mainly due to two reasons32. First, both electronic and steric properties can be readily tuned to fit the requirements of a specific application. Second, the isolation of stable free carbenes offers a convenient method to synthesize metal complexes by straightforward combination with a metal precursor. Accordingly, it is important to understand the factors which control whether a free carbene is stable at or below room temperature or whether it dimerizes to form an olefin. Note that the derived electron rich olefins usually33 do not form complexes upon treatment with a metal precursor, which is at least in part due to their highly reducing character.
Not only are free carbenes key players in synthetic chemistry nowadays. In fact, their electron rich olefin dimers34,35,36 (e.g., tetraazafulvalenes in case of NHCs37 or tetrathiafulvalenes TTF38,39,40 in case of 1,3-dithiol-2-ylidenes; Figure 1, II.), have not only found broad application as reductants41,42,43, but even more so in organic electronics.
TTF is in fact called the “brick-and-mortar” of organic electronics44. This is largely due to the particular electronic properties of electron rich olefins – notably, many of those show three stable redox states upon oxidation, including the open-shell organic radical (For reviews on carbene derived organic radicals, see:45,46,47, for recent contributions in the area of carbene stabilized organic radicals, see:48,49,50,51,52,53,54). Accordingly, TTF allows for the fabrication of conductive/semiconductive material as required for magnetic materials, organic field-effect transistors (OFETs), organic light emitting diodes (OLEDs) and molecular switches or sensors55,56,57,58,59.
Herein, we present convenient protocols for the isolation of two stable carbenes with enormous impact in coordination chemistry and homogeneous catalysis (Figure 2), viz. the cyclic (alkyl)(amino) carbene 1 18, and the dimethylimidazolin-2-ylidene NHC 2 15. We will discuss why both carbenes are stable at room temperature and do not dimerize. We will then elaborate on proton catalysis related to Wanzlick’s equilibrium and the formation of the mixed CAAC–NHC heterodimer 360,61,62. The exciting electronic properties of such triaza-alkenes is connected with the impressive stability of the related organic radical 4 63.
Methodological focus lies on the Schlenk technique using filter cannulas equipped with a glass micro fiber filter for the separation of a supernatant from a precipitate under inert conditions. A dinitrogen filled glovebox is used for weighing in starting material and the storage of air sensitive compounds.
Protocol
CAUTION: Carry out all syntheses in a well-ventilated fume hood. Wear appropriate personal protective equipment (PPE) including a lab coat and safety goggles.
NOTE: The starting materials were synthesized according to the literature: 1-(2,6-diisopropylphenyl)-2,2,4,4-tetramethyl-3,4-dihydro-2H-pyrrol-1-ium tetrafluoroborate (1prot) (For the synthesis of CAACs, see:18,30,31,64,65) and 1,3-dimethyl-4,5-dihydro-1H-imidazol-3-ium iodide (2prot)65. We suggest drying these salts at 120 °C in vacuo overnight in order to ensure the absence of water or halogenated solvents. Silver triflate and potassium hexamethyldisilazide (KHMDS) were obtained by commercial vendor and used as is without further purification. All manipulations were performed using Schlenk techniques or in a dinitrogen filled glovebox (O2 < 0.1 ppm; H2O < 0.1 ppm). Solvents were dried by a two-column, solid-state purification system and stored over activated molecular sieves. Tetrahydrofuran, diethylether, hexanes, pentane, benzene and toluene were deoxygenated by three freeze-pump-thaw cycles. Deuterated benzene was dried over molecular sieves, deoxygenated by three freeze-pump-thaw cycles and stored over a mirror of potassium, deuterated acetonitrile was distilled from calcium hydride and stored over molecular sieves. Glassware was oven-dried at 150 °C for at least 12 h prior to use and brought hot directly into the glovebox (cycling the antechamber at least three times over the course of at least 15 min). Glass micro fiber filters were stored at 150 °C; cannulas were either oven-dried or thoroughly purged with air prior to use in order to ensure the absence of residual organic solvent (water, respectively).
1. Synthesis of cyclic (alkyl)(amino) carbene (Compound 1)
- Transfer a hot, oven-dried 100 mL Schlenk flask equipped with a stir bar and a rubber septum into a dinitrogen filled glovebox.
- Weigh out the iminium salt 1-(2,6-diisopropylphenyl)-2,2,4,4-tetramethyl-3,4-dihydro-2H-pyrrol-1-ium tetrafluoroborate (1prot) (2.00 g, 5.36 mmol, 1.0 eq.) and potassium hexamethyldisilazide (KHMDS) (1.05 g, 5.25 mmol, 0.98 eq.) and combine in the 100 mL Schlenk flask. Cap the flask with a rubber septum.
- Transfer the flask to the Schlenk line. Evacuate and refill all connecting hoses with dinitrogen three times in order to remove any traces of water and air.
- Connect a second oven-dried 100 mL Schlenk flask capped with a rubber septum to the Schlenk line. Evacuate/refill the connecting hose three times.
- Open the solid containing flask to dinitrogen and cool the flask using an isopropanol slush bath (-88 °C) or a dry ice/acetone (-78 °C) cooling bath.
- Add 20 mL of diethylether (dry, degassed) over the course of 3 min along the cold flask wall using a syringe. Stir the suspension for 10 min before allowing the reaction mixture to warm to room temperature.
- Once the mixture reaches room temperature, discontinue stirring and allow the potassium tetrafluoroborate salt to settle.
- Prepare a steel cannula equipped with a glass micro fiber filter, which is fitted to one end of the cannula by polytetrafluoroethylene (PTFE) tape. Wind the PTFE tape around the end of the cannula to obtain an overall diameter of about 0.6 cm (0.25 inch; Figure 3a, b). Then fit the glass micro fiber filter by winding further PTFE tape around (Figure 3c).
- Perforate a septum with a small needle (with a smaller diameter than the cannula) and subsequently push the filter cannula through the tiny hole. Swiftly exchange this septum under a gentle flow of dinitrogen with the septum on the Schlenk flask containing the crude carbene. Purge the cannula for at least 1 min with dinitrogen.
- Perforate the second septum capping the second empty Schlenk flask as well with a small needle and introduce the other end of the steel cannula.
- Additionally, insert a thin needle through the septum of the empty flask and close the Schlenk valve connecting this flask to the Schlenk line. Note that overpressure will be released through the extra needle (Figure 4).
- Lower the filter cannula into the overlying solution to start the filtration of the solution containing the free carbene into the second Schlenk flask using slight dinitrogen overpressure provided by the line. Eventually, also lower the filter cannula into the suspension with the settled salt at the bottom of the flask.
- After quantitative transfer of the carbene, reopen the valve of the second Schlenk flask to the Schlenk line for dinitrogen supply. Remove the small needle as well as the steel cannula and seal the perforated septum of the Schlenk flask with adhesive tape.
Alternatively, replace the perforated septum by a well-greased glass stopper. - Remove the solvent in vacuo to obtain the free carbene 1 quantitatively as a colorless to slightly yellow and greasy solid (1.53 g). Quantitative removal of hexamethyldisilazane
[HN(SiMe3)2] requires typically a vacuum around 1 * 10-3 mbar or gentle heating. Transfer 1 to a glovebox for storage.
2. Synthesis of the N-heterocyclic carbene (Compound 2)
- Transfer a hot, oven-dried 100 mL Schlenk flask, a rubber septum and a stir bar into a dinitrogen filled glovebox.
- Weigh out the imidazolium salt 1,3-dimethyl-4,5-dihydro-1H-imidazol-3-ium iodide 2prot
(2.00 g, 8.93 mmol, 1.0 eq.) and KHMDS (1.75 g, 8.75 mmol, 0.98 eq.). Combine both in the Schlenk flask, add the stir bar and seal the flask with the rubber septum. - Transfer the Schlenk flask to the Schlenk line and evacuate/refill the connecting hose three times. Additionally, connect a second oven-dried 100 mL Schleck flask equipped with a septum to the Schlenk line. Evacuate/refill with dinitrogen three times.
- Add 10 mL of diethylether (dry, degassed) via a syringe to the 2prot / KHMDS mixture and stir for 20 min at room temperature.
- To separate the precipitated salt, use a steel cannula equipped with a glass micro fiber filter to one end and transfer the solution into the second Schlenk flask as described previously (steps 1.8 – 1.13).
- Remove the solvent in vacuo to afford the free carbene 2 as a slightly yellow oil in a yield of 390 mg (45%). Transfer 2 to a glovebox for storage and the next step.
3. Synthesis of the CAAC–NHC salt (Compound 3prot)
- Transfer a hot, oven-dried 100 mL Schlenk flask equipped with a stir bar and a rubber septum into a dinitrogen filled glovebox.
- Weigh out the cyclic iminium salt 1prot (1.50 g, 4.02 mmol, 1.0 eq.) and the free carbene 2
(409 mg, 4.22 mmol, 1.05 eq.). Combine both in the Schlenk flask and seal the flask with a rubber septum. - Transfer the Schlenk flask to a Schlenk line. Evacuate/refill the connecting hoses with dinitrogen three times.
- Add 20 mL of tetrahydrofuran (dry, degassed) via a syringe according to description in steps 1.5 – 1.6. Swiftly replace the perforated septum by a well-greased glass stopper. Stir the reaction mixture for at least 12 h at room temperature.
- Allow the precipitate to settle. Exchange the glass stopper by a rubber septum with a steel cannula equipped with a glass micro fiber filter to one end to transfer the yellow supernatant solution into the second Schlenk flask as described previously (1.8 – 1.12)
- Exchange the glass stopper by a rubber septum and wash the residue with tetrahydrofuran: Add dry tetrahydrofuran (20 mL) via a syringe and stir until you obtain a fine suspension. Remove the supernatant using a filter cannula as described previously (1.8 – 1.12). If the residue is still yellow/orange repeat the washing step with additional 20 mL tetrahydrofuran. Exchange the perforated septum along with the filter cannula by a well-greased glass stopper.
- Dry the residue in vacuo to afford the protonated heterodimer quantitatively as an off-white powder. Transfer 3prot to a glovebox for storage and the next step.
4. Synthesis of the mixed Wanzlick CAAC–NHC dimer (Compound 3)
- Transfer a hot, oven-dried 100 mL Schlenk flask equipped with a stir bar and a rubber septum into a dinitrogen glovebox.
- Weigh out 3prot (1.5 g, 3.19 mmol, 1.0 eq.) and KHMDS (624 mg, 3.13 mmol, 0.98 eq.). Combine both in the Schlenk flask and cap the flask with the rubber septum.
- Connect this Schlenk flask and a second oven-dried empty 100 mL Schlenk flask equipped with a rubber septum to the Schlenk line. Evacuate/refill the connecting hoses with dinitrogen three times.
- Add 10 mL of toluene (dry, degassed) via a syringe to the mixture of 3prot and KHMDS. Stir for 12 h at room temperature, then stop stirring and allow the precipitate to settle.
- Transfer the supernatant solution, containing the dimer 3, into the second Schlenk flask using a filter cannula as described previously (steps 1.8 – 1.13).
- Remove the solvent in vacuo.
- Wash the residue with hexanes to remove residual HN(SiMe3)2: Add 5 mL hexanes (dry, degassed) and stir until you obtain a fine suspension. Remove the supernatant using a filter cannula as described previously (steps 1.8 – 1.13). Exchange the perforated septum along with the filter cannula by a well-greased glass stopper.
- Dry the residue in vacuo to obtain CAAC–NHC heterodimer 3 as a off white powder in a yield of 970 mg (80%). Transfer 3 to a glovebox for storage.
5. Synthesis of the organic radical CAAC–NHC-2 (compound 4)
- Transfer a hot, 20 mL Schlenk flask equipped with a stir bar and a rubber septum into a dinitrogen glovebox.
- Weigh out the silver trifluoromethanesulfonate [Ag(OTf); 134 mg, 0.52 mmol, 1.0 eq.] and compound 3 (200 mg, 0.52 mmol, 1.0 eq.). Combine both in the 20 mL Schlenk flask and cap with a rubber septum.
- Connect this Schlenk flask and a second oven-dried empty 20 mL Schlenk flask equipped with a stir bar and a septum to the Schlenk line. Evacuate/refill the connecting hoses with dinitrogen three times.
- Add 5 mL of tetrahydrofuran (dry, degassed) via syringe to receive a deep maroon mixture.
- Filter the solution into the second Schlenk flask using a filter cannula as described previously (steps 1.8 – 1.13).
- Remove the solvent in vacuo to obtain the stable radical quantitatively as a red powder. Transfer 4 to a glovebox for storage.
Representative Results
Free carbenes react typically readily with water66. Hence, carefully dried glassware and solvents are required67. In the procedure described above, we used cannulas fitted with a glass micro fiber filter in order to separate air sensitive solutions from a precipitate under inert conditions. We used this technique for both the extraction of solids (i.e., the desired product is dissolved) as well as the washing of solid compounds (i.e., the desired product is an insoluble solid).
The choice of the solvent requires additional attention relating to the solubility of starting material and the free carbenes. In this protocol, the cyclic iminium salt 1prot features a tetrafluoroborate anion, which is removed via precipitation as potassium salt upon generation of the free carbene. Therefore, diethylether, toluene, or benzene are appropriate solvents, whereas more polar solvents such as tetrahydrofuran will dissolve a considerable amount of the potassium tetrafluoroborate salt (KBF4). The absence of tetrafluoroborate in the product can be verified by 19F NMR spectroscopy. Note that KHMDS is comparably well soluble in apolar solvents such as benzene and even hexanes, while the cyclic iminium salts are essentially insoluble even in diethylether. From an applied synthetic point of view, it is thus advisable to conduct the reaction with a slight excess of iminium salt in relation to KHMDS in order to avoid potential contamination of the product by residual KHMDS.
Carbene 1 is indefinitely stable at room temperature, carbene 2 at -30 °C, both do not dimerize as evidenced by the signals of the carbene carbon atom in the 13C NMR spectrum at 313.9 ppm18 (Figure 5, top) and 216.9 ppm, respectively (Figure 5, bottom). Note that the low-field shift indicates exceptional strong π-acidity of the CAAC 163,68,69. Equally note that the absence of a signal around 100 ppm in the spectrum for 1, which is typically obtained upon hydrolysis by moist air, substantiates the efficient exclusion of air using the filter cannula technique. The stability of 1 is largely due to the sterically demanding diisopropylphenyl substituent at the nitrogen atom, which prevents dimerization (Figure 6, top). Overall, the combination of electronic structural considerations as well as steric bulk determines the equilibrium for the dimerization of carbenes. Generally, free carbenes with a small highest occupied molecular orbital/lowest occupied molecular orbital (HOMO/LUMO) gap dimerize comparable easily based on electronic considerations. This can be understood by considering the dimerization of a carbene as the interaction of the lone pair (associated with the HOMO and the nucleophilicity of a carbene) with the formally vacant pz orbital (usually associated with the LUMO and the electrophilicity of the carbene; Figure 6, bottom left). Planar NHCs with an unsaturated backbone feature two strongly π-donating amino groups (i.e., the LUMO is comparably high in energy and hence associated with low electrophilicity). As a result, the dimeric olefins become exceedingly electron rich and Wanzlick’s equilibrium is driven towards the free carbene (Figure 6, bottom right)17. The dimethylimidazolin-2-ylidene is thus stable as a monomer due to electronic considerations. Indeed, deprotonation of N,N-dimethylimidazolium iodide generates cleanly the free carbene 2 (vide supra, Figure 2, 2). Accordingly, the 13C NMR spectrum confirms the formation of the free carbene with a signal at 216.9 ppm (Radius and coworkers16 report a signal at 214.6 ppm for an alternative synthetic approach based on deprotonation by sodium hydride in liquid ammonia) without the presence of olefin dimer (Figure 5). Note, 2 is volatile and will therefore be removed under prolonged drying in vacuo, which leads to the comparably low yield of 45%. Nevertheless, note the quantitate removal of HN(SiMe3)2 in vacuo as evidenced by the absence of signals around 0 ppm.
The electrophilic, cationic and cyclic iminium salt (i.e., the protonated CAAC 1prot), reacts readily with the free NHC 2 to form the mixed CAAC–NHC salt 3prot (Figure 2, Figure 3). The free CAAC 1 is of course much more basic than NHC 2 and is therefore expected to give 3prot as well in reaction with the corresponding imidazolium salt derived from 2 (i.e., N,N-dimethylimidazolium salt 2prot). Furthermore, note that the CAAC moiety stabilizes the intermediate 3prot due to the absence of aromaticity as associated with the aromatic imidazolinylidene derivatives. The 1H NMR spectrum shows a characteristic singlet at 5.02 ppm belonging to the proton at the “carbene” position of the CAAC scaffold (Figure 7, top). This shift suggests that the related proton can be removed by strong bases.
Indeed, 3prot is deprotonated by KHMDS in toluene under concomitant precipitation of KBF4 (Figure 2, Figure 3). Note again that the salt 3prot is essentially insoluble in toluene, whereas the deprotonated olefin is well soluble. Accordingly, utilization of a tiny excess of salt 3prot guarantees quantitative conversion of KHMDS. The purity of the CAAC–NHC dimer 3 is verified by 1H and 13C NMR spectroscopy. The 1H NMR spectrum of 3 (Figure 7, middle) reveals a significant upfield shift of the NHC methyl groups to 2.53 ppm and 1.39 ppm in relation to the starting material 3prot (4.26 ppm and 3.55 ppm, respectively). This shift is indicative for the elimination of the positive charge on the NHC nitrogen atom and the formation of the olefin 3.
The 13C NMR spectrum unambiguously proves the formation of an olefinic dimer (Figure 7, bottom) by the absence of the carbene signal. While stable carbenes typically are not dioxygen sensitive, electron-rich olefins react rapidly with dioxygen to form the urea and amide derivatives, respectively70. Thus, careful deoxygenation of all solvents is crucial for this step. Furthermore, note that the slight overpressure while performing the filtration is crucial for the absence of air in the reaction vessel.
The dimers of carbenes are electron rich and hence considerably reducing. Importantly, they can be either one- or two-electron reductants. In case of triaminoolefins, the cationic radicals are exceptionally stable60. Hence, organic radical 4 is conveniently accessible by oxidation with silver trifluoromethansulfonate (Figure 2, Figure 4). The immediate color change from yellow to deep maroon upon addition of the oxidant indicates the formation of a radical compound and 1H NMR spectroscopy confirms the clean formation of a paramagnetic compound due to the absence of any signals. Note that this radical is oxygen sensitive.
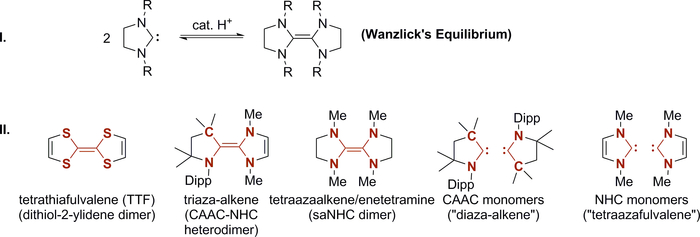
Figure 1. Wanzlick’s equilibrium. Equilibrium between free carbene and its dimer (I.) and corresponding continuum from electron rich olefins to stable carbenes (II.). Dipp: 2,6-diisopropylphenyl. Please click here to view a larger version of this figure.
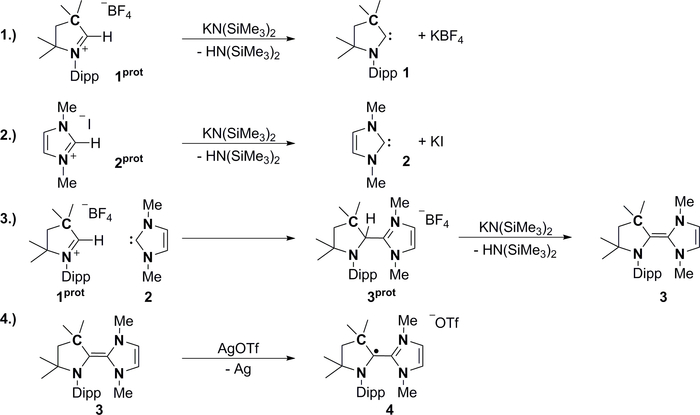
Figure 2. Reaction schemes. Stable carbenes 1 and 2 dimerize only under acid catalysis to the mixed Wanzlick dimer 3, which can be reduced to the corresponding organic radical 4. Please click here to view a larger version of this figure.
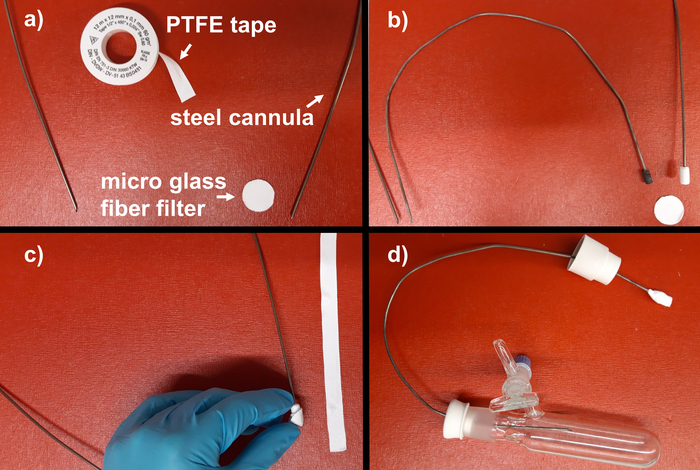
Figure 3. Preparation of a filter cannula. (a) Cannula, glass micro fiber filter, PTFE tape; (b) cannula with PTFE tape wrapped end and cannula with fitted joint; (c) attachment of glass micro fiber filter with PTFE tape; (d) filter cannula attached to Schlenk flask. Please click here to view a larger version of this figure.

Figure 4. Filtration setup. Filtration of a solution containing a free carbene into a second Schlenk flask. Please click here to view a larger version of this figure.
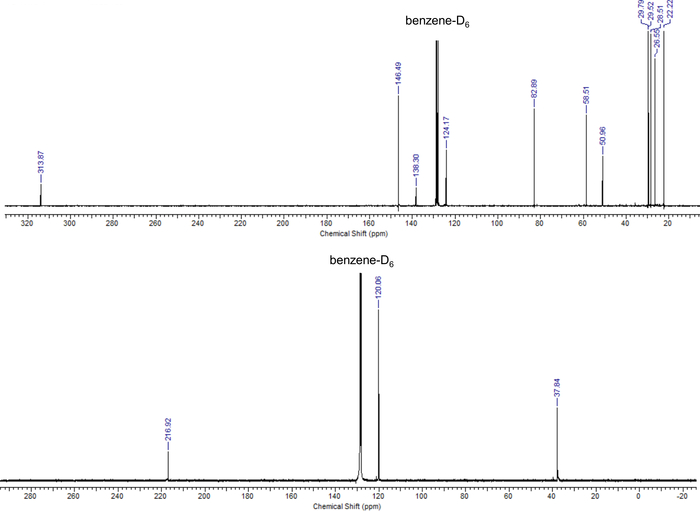
Figure 5. NMR data of free carbenes. 13C NMR spectrum of the free carbenes 1 (67 MHz, top) and 2 (100 MHz, bottom) in benzene-D6. Please click here to view a larger version of this figure.
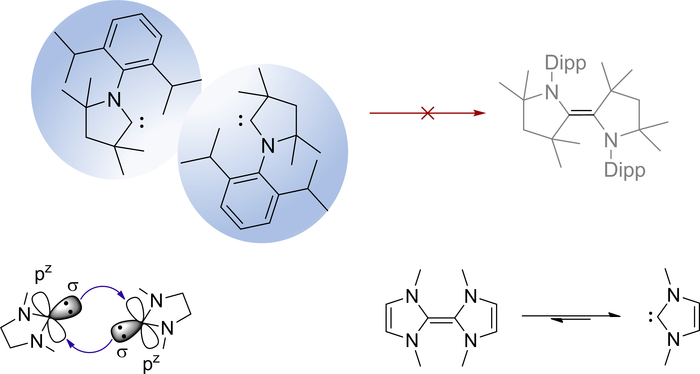
Figure 6. Control of carbene dimerization. Bulky substituents prevent the dimerization of CAACs (top), whereas frontier orbital interactions are responsible for Wanzlick’s equilibrium in case of insufficient steric bulk (bottom). Please click here to view a larger version of this figure.
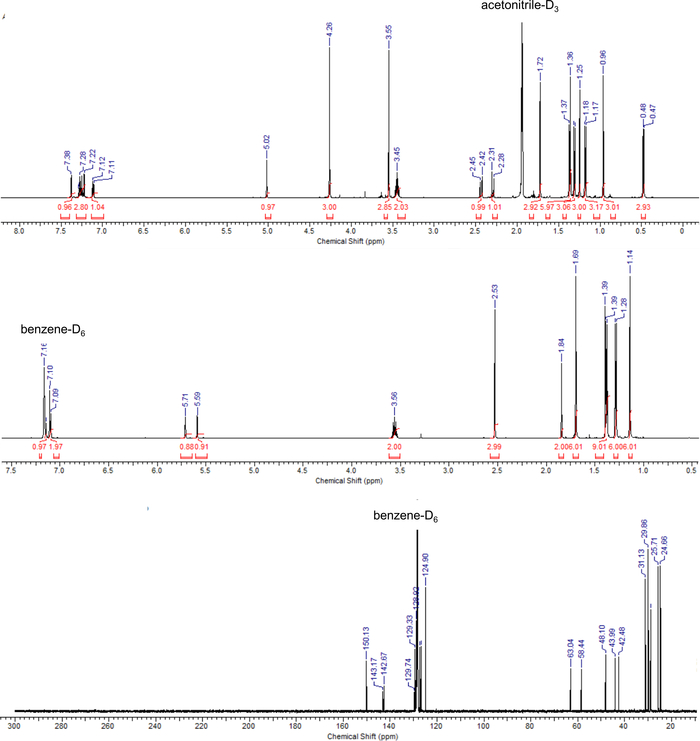
Figure 7. NMR data of Wanzlick dimer. 1H NMR spectrum of 3prot in acetonitrile-D3 (600 MHz, top); 1H NMR spectrum of 3 in benzene-D6 (600 MHz, middle); 13C NMR spectrum of 3 in benzene-D6 (150 MHz, bottom). Please click here to view a larger version of this figure.
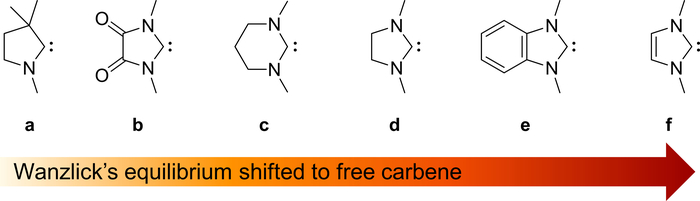
Figure 8. Electronic properties of carbenes. Increasing stability of the free carbenes due to electronic properties. Please click here to view a larger version of this figure.
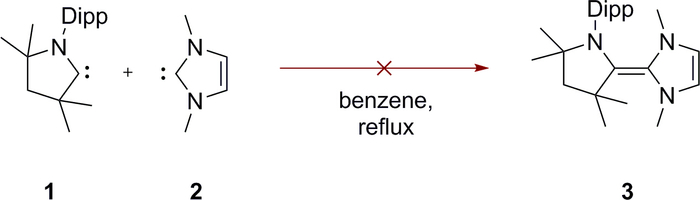
Figure 9. Activation energy for dimerization. Carbenes 1 and 2 do not dimerize to give the triaminoolefin 3. Please click here to view a larger version of this figure.
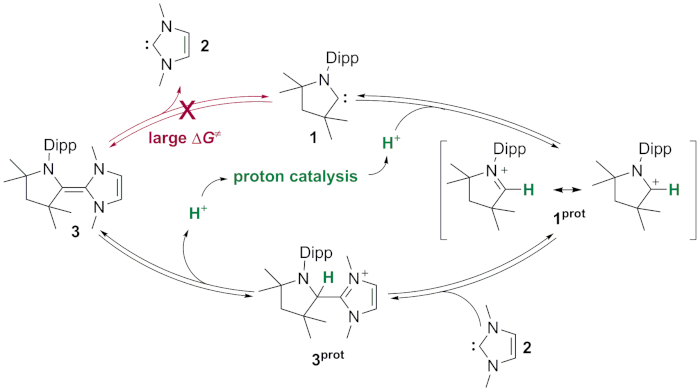
Figure 10. Proton catalysis. Catalytic cycle of acid catalyzed dimerization of stable carbenes 1 and 2. Please click here to view a larger version of this figure.
Discussion
Herein, we present a general and adaptable protocol for the synthesis of stable carbenes (NHC, CAAC) and their electron rich dimer. All steps can readily be upscaled to at least a 25 g scale. Crucial for a successful synthesis are the strict exclusions of moisture (air, respectively) for the synthesis of the carbenes, and of oxygen (air, respectively) for the electron rich olefin. The herein applied filtration cannula technique in combination with a Schlenk line is a very convenient method to separate solutions from precipitates under inert conditions. The filter cannulas presented herein were prepared by winding PTFE tape around the end of a steel cannula (vide supra Figure 3a, b). We recommend to have a machine shop attach a larger joint to the end of the cannula in order to save time and reduce the amount of required PTFE tape (vide supra Figure 3b, small cannula).
The presented procedure relies on slight dinitrogen overpressure provided by the Schlenk line. This approach prevents the introduction of air into the reaction vessel very efficiently. Potential drawbacks are (1) slow filtration rates by clogging of the filter and (2) popping off the septum due to the overpressure. These drawbacks can be addressed by (1) avoiding pushing the filter cannula into the precipitate prior to when really needed (as described above) or by replacement of the cannula by a second prepared filter cannula, once the filtration rate becomes intolerably slow. The off popping of the septum (2) can be straight-forwardly addressed by adjusting a very gentle overpressure.
Alternatively, one might consider to suction filtrate the solution into the other vessel through application of a slight under pressure from the second container; nevertheless, we discourage this latter approach due to a high risk of contamination of the product through undesired introduction of air. We would like to furthermore point out the advantage of using glass micro fiber filters versus filtration techniques over porous materials such as diatomaceous earth (Celite), because it arguably avoids potential contamination of the product due to residual moisture in the microporous material. Note that using (more expensive) argon instead of dinitrogen as inert gas is more efficient for excluding moisture from the reaction due to the higher density in comparison to air. Nevertheless, we have never observed the formation of a considerable amount of byproducts derived from reaction with air, provided the reactions are conducted carefully and on a scale larger than approximately 0.25 mmol.
The stability of the presented carbenes is due to either kinetic protection by bulky substituents (CAAC 1) or thermodynamic stabilization of the free carbene by electronics (NHC 2). The aromatic NHC 2 with an unsaturated backbone features two strongly π-donating and σ-electron withdrawing amino groups, resulting in a large HOMO/LUMO gap. Thus, the carbene is energetically favored over the electron-rich dimer. In CAACs, one π-donating nitrogen atom is substituted by a σ-donating alkyl group which makes them more nucleophilic and more electrophilic than NHCs (i.e., the HOMO/LUMO gap is smaller and dimerization is electronically much more facile). However, the bulky diisopropylphenyl substituent provides kinetic stabilization to prevent dimerization.
Evidently, these two carbenes serve as examples for a general trend observed for NHCs with small methyl substituents (Figure 8). CAACs and diamido-carbenes (DACs) feature stronger π-accepting properties compared to NHCs71,72,73. Accordingly, these carbenes are more electrophilic, feature a much smaller HOMO/LUMO gap, and dimerize swiftly without steric protection (Figure 8a, b). For example, the 5-membered DAC dimerizes even with mesityl N-substituents at room temperature and is only stable at low temperatures. A protocol for the preparation of carbonyl substituted carbenes was reported in JOVE by Hudnall74. The six membered NHC derivative (Figure 8c) dimerizes equally easily, because it lacks stabilization by aromatization and shows furthermore reduced electron density in the pz orbital due to pyramidalization of the amino groups. On the contrary, the five-membered imidazolidin-2-ylidene (Figure 8d) shows reduced pyramidalization of the amino groups and dissociates upon heating to 100 °C6. The planar benzimidazolin-2-ylidene (Figure 8e) is subject to Wanzlick’s equilibrium at room temperature75. Impressively, the imidazolin-2-ylidenes (Figure 8d), which enjoys both stabilization by aromaticity as well as planarity, is even isolable as a free carbene at room temperature15,70.
Note that the isolation of highly reactive free carbenes with a small HOMO/LUMO gap such as CAACs require more attention related to the exclusion of moist air during the synthesis than comparably stable free carbenes like conventional NHCs. Moreover, note that radicals, electron rich olefins, and accordingly carbenes, which are in equilibrium with their dimers, are usually very oxygen sensitive and require therefore the rigorous exclusion of air during the filtration.
Addition of 1 a 2 does not result in the formation of the mixed CAAC–NHC dimer 3, not even after prolonged heating to reflux in benzene-D6 (Figure 9). This is due to the large energy barrier (i.e., activation energy) for the dimerization caused by the repulsion between the two carbene lone pairs. However, acid catalysis facilitates the conversion of free carbenes into their respective dimers. Protonation of one of the two carbenes 1 or 2 leads to the formation of the cyclic iminium salt 1prot (imidazolium salt 2prot, respectively). These salts are evidently much more electrophilic than their neutral carbene congeners. Nucleophilic attack by another carbene now becomes feasible and results in the formation of the protonated dimer 3prot. Subsequent deprotonation generates the carbene dimer 3 (Figure 10). This process corresponds overall to a dimerization catalyzed by a proton (i.e., traces of acid)9,10,76.
In conclusion, isolated free carbenes are convenient building blocks for organic and inorganic synthetic applications. We believe that understanding and controlling Wanzlick’s equilibrium, which is the dimerization of carbenes, is key for understanding the coordination chemistry of heterocyclic carbenes. Therefore, the heterodimerization of CAACs and NHCs is outlined and put in the context of the proton-catalytic dimerization of carbenes and dissociation of dimers. Furthermore, we exemplify the extraordinary properties of carbene dimers by isolation of the organic radical of the triaminoolefin 4. Most importantly, the herein outlined application of filter cannulas is key for the convenient isolation of moisture sensitive free carbenes and oxygen sensitive carbene dimers or radicals.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors thank the Fonds der Chemischen Industrie for a Liebig fellowship and the Hertha and Helmut Schmauser foundation for financial support. Support by K. Meyer is gratefully acknowledged.
Materials
| Equipment | |||
| Glass micro fiber filter, 691, 24 mm. Particle retention 1.6 mm | VWR | 516-0859 | |
| magnetic stir bar | FengTecEx | various | |
| PTFE tape | Sigma-Aldrich | Z148814-1PAK | PTFE tape used in this manuscript was obtained from a local supplier. Tape from Sigma Aldrich should show comparable performance. |
| rubber septum | FengTecEx | RS112440 | Joint size: 24/29 |
| rubber septum | FengTecEx | RS111420 | Joint size: 14/23 |
| rubber septum | FengTecEx | RS111922 | Joint size: 19/26 |
| schlenk flasks | FengTecEx | various | 100 mL |
| steel cannula | FengtecEx | C702024 | Attachment of a steel joint by a machine shop not required, but facilitates preparation of filter cannula |
| syringe cannula | FengtecEx | S380221 | |
| Name | Company | Catalog Number | Comments |
| Reactants | |||
| 1-(2,6-diisopropylphenyl)-2,2,4,4-tetramethyl-3,4-dihydro-2H-pyrrol-1-ium tetrafluoroborate | Synthesized according to: Jazzar, R., Dewhurst, R. D., Bourg, J. B., Donnadieu, B., Canac, Y., Bertrand, G. Intramolecular “Hydroiminiumation” of alkenes: Application to the synthesis of conjugate acids of cyclic alkyl amino carbenes (CAACs). Angewandte Chemie International Edition 46 (16), 2899-2902, (2007). | ||
| 1,3-dimethyl-4,5-dihydro-1H-imidazol-3-ium iodide | Synthesized according to: Benac, B. L., Burgess, E. M., Arduengo, A. J. 1,3-Dimethylimidazole-2-Thione. Organic Synthesis 64, 92, (1986). | ||
| potassium hexamethyldisilazide | Sigma-Aldrich | 324671-100G | CAS 40949-94-8 |
| silver trifluoromethanesulfonate | Sigma-Aldrich | 85325-25G | CAS 2923-28-6 |
| Name | Company | Catalog Number | Comments |
| Solvents | |||
| acetonitrile-D3 | Deutero | 00202-10m | distilled from CaH2, stored over activated molecular sieves |
| benzene-D6 | Deutero | 00303-100ml | dried over activated molecular sieves, stored over potassium |
| diethylether | – | – | dried by two-column, solid-state purification system and degassed by three freeze-pump-thaw cycles, stored over activated molecular sieves |
| hexanes | – | – | dried by two-column, solid-state purification system and degassed by three freeze-pump-thaw cycles, stored over activated molecular sieves |
| tetrahydrofuran | – | – | dried by two-column, solid-state purification system and degassed by three freeze-pump-thaw cycles, stored over activated molecular sieves |
| toluene | – | – | dried by two-column, solid-state purification system and degassed by three freeze-pump-thaw cycles, stored over activated molecular sieves |
Riferimenti
- Wanzlick, H. W., Schikora, E. Ein neuer Zugang zur Carben-Chemie. Angewandte Chemie. 72, 494 (1960).
- Wanzlick, H. W., Kleiner, H. J. Nucleophile Carben-Chemie. Angewandte Chemie International Edition. 73 (14), 493 (1961).
- Wanzlick, H. W., Schikora, E. Ein nucleophiles Carben. Chemische Berichte. 94 (94), 2389-2393 (1961).
- Böhm, V. P. W., Herrmann, W. A. The "Wanzlick Equilibrium". Angewandte Chemie International Edition. 39 (22), 4036-4038 (2000).
- Hahn, F. E., Wittenbecher, L., Le Van, D., Fröhlich, R. Evidence for an Equilibrium between an N-heterocyclic Carbene and Its Dimer in Solution. Angewandte Chemie International Edition. 39 (3), 541-544 (2000).
- Denk, M. K., Hatano, K., Ma, M. Nucleophilic Carbenes and the Wanzlick Equilibrium: A Reinvestigation. Tetrahedron Letters. 40 (11), 2057-2060 (1999).
- Liu, Y., Lemal, D. M. Concerning the Wanzlick equilibrium. Tetrahedron Letters. 41, 599-602 (2000).
- Arduengo, A. J., Goerlich, J. R., Marshall, W. J. A Stable Thiazol-2-ylidene and its Dimer. Liebigs Annalen der Chemie. , 365-374 (1997).
- Alder, R. W., Blake, M. E., Chaker, L., Harvey, J. N., Paolini, F., Schutz, J. When and how do diaminocarbenes dimerize?. Angewandte Chemie International Edition. 43 (44), 5896-5911 (2004).
- Chen, Y. -. T., Jordan, F. Reactivity of the Thiazolium C2 Ylide in Aprotic Solvents: Novel Experimental Evidence for Addition Rather Than Insertion Reactivity. Journal of Organic Chemistry. 56 (17), 5029-5038 (1991).
- Lemal, D. M., Lovald, R. A., Kawano, K. I. Tetraaminoethylenes. The Question of Dissociation. Journal of the American Chemical Society. 86 (12), 2518-2519 (1964).
- Alder, R. W., Chaker, L., Paolini, F. P. V. Bis(diethylamino)carbene and the mechanism of dimerisation for simple diaminocarbenes. Chemical Communications. 19 (19), 2172-2173 (2004).
- Baceiredo, A., Bertrand, G., Sicard, G. Synthesis of the First α-Diazo Phosphines. Phosphorus-Carbon Multiple-Bond Character of Phosphinocarbenes. Journal of the American Chemical Society. 107 (16), 4781-4783 (1985).
- Igau, A., Gruetzmacher, H., Baceiredo, A., Bertrand, G. Analogous alpha,alpha’ Bis-Carbenoid Triply Bonded Species: Synthesis of a Stable lambda3-Phosphinocarbene-lambda5-Phosphaacetylene. Journal of the American Chemical Society. 110 (19), 6463-6466 (1988).
- Arduengo, A. J., Harlow, R. L., Kline, M. A Stable Crystalline Carbene. Journal of the American Chemical Society. 113 (1), 363-365 (1991).
- Schaub, T., Backes, M., Radius, U. Nickel (0) Complexes of N-Alkyl-Substituted N-Heterocyclic Carbenes and Their Use in the Catalytic Carbon−Carbon Bond Activation of Biphenylene. Organometallics. 25, 4196-4206 (2006).
- Jolly, P. I., Zhou, S., Thomson, D. W., Garnier, J., Parkinson, J. A., Tuttle, T., Murphy, J. A. Imidazole-derived carbenes and their elusive tetraazafulvalene dimers. Chemical Science. 3 (5), 1675-1679 (2012).
- Lavallo, V., Canac, Y., Prasang, C., Donnadieu, B., Bertrand, G. Stable Cyclic (Alkyl)(Amino)Carbenes as Rigid or Flexible, Bulky, Electron-Rich Ligands for Transition-Metal Catalysts: A Quaternary Carbon Atom Makes the Difference. Angewandte Chemie International Edition. 44 (35), 5705-5709 (2005).
- Hahn, F. E. Introduction: Carbene Chemistry. Chemical Reviews. 118 (19), 9455-9456 (2018).
- Rovis, T., Nolan, S. P. Stable carbenes: from "laboratory curiosities" to catalysis mainstays. Synlett. 24 (10), 1188-1189 (2013).
- Arduengo, A. J., Bertrand, G. Carbenes introduction. Chemical Reviews. 109 (8), 3209-3210 (2009).
- Diez Gonzalez, S. . N-Heterocyclic Carbenes: From Laboratory Curiosities to Efficient Synthetic Tools. , (2010).
- Nolan, S. P. . N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis. , (2014).
- Soleilhavoup, M., Bertrand, G. Cyclic (alkyl)(amino) carbenes (CAACs): Stable carbenes on the rise. Accounts of Chemical Research. 48 (2), 256-266 (2015).
- Roy, S., Mondal, K. C., Roesky, H. W. Cyclic alkyl (amino) carbene stabilized complexes with low coordinate metals of enduring nature. Accounts of Chemical Research. 49 (3), 357-369 (2016).
- Melaimi, M., Soleilhavoup, M., Bertrand, G. Stable cyclic carbenes and related species beyond diaminocarbenes. Angewandte Chemie International Edition. 49 (47), 8810-8849 (2010).
- Melaimi, M., Jazzar, R., Soleilhavoup, M., Bertrand, G. Cyclic (Alkyl)(amino) Carbenes (CAACs): recent developments. Angewandte Chemie International Edition. 56 (34), 10046-10068 (2017).
- Paul, U. S. D., Radius, U. What Wanzlick Did Not Dare To Dream: Cyclic (Alkyl)(amino) carbenes (CAACs) as New Key Players in Transition‐Metal Chemistry. European Journal of Inorganic Chemistry. 2017 (28), 3362-3375 (2017).
- Jazzar, R., Bourg, J. B., Dewhurst, R. D., Donnadieu, B., Bertrand, G. Intramolecular "Hydroiminiumation and-amidiniumation" of alkenes: A convenient, flexible, and scalable route to cyclic iminium and imidazolinium salts. Journal of Organic Chemistry. 72, 3492-3499 (2007).
- Zeng, X., Frey, G. D., Kinjo, R., Donnadieu, B., Bertrand, G. Synthesis of a Simplified Version of Stable Bulky and Rigid Cyclic (Alkyl)(Amino)Carbenes (CAACs), and Catalytic Activity of the Ensuing Gold(I) Complex in the Three-Component Preparation of 1,2-Dihydroquinoline Derivatives. Journal of the American Chemical Society. 131 (24), 8690-8696 (2009).
- Chu, J., Munz, D., Jazzar, R., Melaimi, M., Bertrand, G. Synthesis of hemilabile cyclic (alkyl)(amino) carbenes (CAACs) and applications in organometallic chemistry. Journal of the American Chemical Society. 138 (25), 7884-7887 (2016).
- Munz, D. Pushing Electrons—Which Carbene Ligand for Which Application?. Organometallics. 37 (3), 275-289 (2018).
- Cardin, D. J., Cetinkaya, B., Lappert, M. F., Manojlovic-Muir, L. J., Muir, K. W. An electron-rich olefin as a source of co-ordinated carbene; synthesis of trans-PtCl2[C(NPhCH2)2]PEt3. Chemical Communications. 8 (8), 400-401 (1971).
- Hocker, J., Merten, R. Reactions of Electron-Rich Olefins with Proton-Active Compounds. Angewandte Chemie International Edition. 11 (11), 964-973 (1972).
- Hoffmann, R. W. Reactions of Electron-Rich Olefins. Angewandte Chemie International Edition. 7 (10), 754-765 (1968).
- Deuchert, K., Hünig, S. Multistage Organic Redox Systems—A General Structural Principle. Angewandte Chemie International Edition. 17 (12), 875-886 (1978).
- Taton, T. A., Chen, P. A Stable Tetraazafulvalene. Angewandte Chemie International Edition. 35 (9), 1011-1013 (1996).
- Wudl, F., Wobschall, D., Hufnagel, E. J. Electrical conductivity by the bis(1,3-dithiole)-bis(1,3-dithiolium) system. Angewandte Chemie International Edition. 94 (2), 670-672 (1972).
- Wudl, F., Smith, G. M., Hufnagel, E. J. Bis-1,3-dithiolium Chloride: an Unusually Stable Organic Radical Cation. Chemical Communications. (21), 1453-1454 (1970).
- Ferraris, J., Cowan, D. O., Walatka, V., Perlstein, J. H. Electron transfer in a new highly conducting donor-acceptor complex. Angewandte Chemie International Edition. 95 (3), 948-949 (1973).
- Broggi, J., Terme, T., Vanelle, P. Organic electron donors as powerful single-electron reducing agents in organic synthesis. Angewandte Chemie International Edition. 53 (2), 384-413 (2014).
- Murphy, J. A. Discovery and Development of Organic Super-Electron-Donors. Journal of Organic Chemistry. 79 (9), 3731-3746 (2014).
- Garnier, J., et al. Hybrid super electron donors – preparation and reactivity. Beilstein. Journal of Organic Chemistry. 8, 994-1002 (2012).
- Bendikov, M., Wudl, F., Perepichka, D. F. Tetrathiafulvalenes, Oligoacenenes, and Their Buckminsterfullerene Derivatives: The Brick and Mortar of Organic Electronics. Chemical Reviews. 104 (11), 4891-4946 (2004).
- Martin, C. D., Soleilhavoup, M., Bertrand, G. Carbene-stabilized main group radicals and radical ions. Chemical Science. 4, 3020 (2013).
- Mondal, K. C., Roy, S., Roesky, H. W. Silicon based radicals, radical ions, diradicals and diradicaloids. Chemical Society Reviews. 45, 1080-1111 (2016).
- Kim, Y., Lee, E. Stable Organic Radicals Derived from N-Heterocyclic Carbenes Chemistry. Chemistry: A European Journal. 24 (72), 19110-19121 (2018).
- Messelberger, J., Grünwald, A., Pinter, P., Hansmann, M. M., Munz, D. Carbene derived diradicaloids – building blocks for singlet fission?. Chemical Science. 9, 6107-6117 (2018).
- Hansmann, M. M., Melaimi, M., Munz, D., Bertrand, G. Modular Approach to Kekulé Diradicaloids Derived from Cyclic (Alkyl)(amino)carbenes. Journal of the American Chemical Society. 140 (7), 2546-2554 (2018).
- Hansmann, M. M., Melaimi, M., Bertrand, G. Organic Mixed Valence Compounds Derived from Cyclic (Alkyl)(amino)carbenes. Journal of the American Chemical Society. 140 (6), 2206-2213 (2018).
- Rottschäfer, D., Neumann, B., Stammler, H. -. G., van Gastel, M., Andrada, D. M., Ghadwal, R. S. Crystalline Radicals Derived from Classical N‐Heterocyclic Carbenes. Angewandte Chemie. 130 (7), 4765-4768 (2018).
- Rottschäfer, D., Neumann, B., Stammler, H. -. G., Andrada, D. M., Ghadwal, R. S. Kekulé diradicaloids derived from a classical N-heterocyclic carbene. Chemical Science. 9 (22), 4970-4976 (2018).
- Rottschäfer, D., Ho, N. K. T., Neumann, B., Stammler, H. -. G., van Gastel, M., Andrada, D. M., Ghadwal, R. S. N‐Heterocyclic Carbene Analogues of Thiele and Chichibabin Hydrocarbons. Angewandte Chemie International Edition. 57 (20), 5838-5842 (2018).
- Barry, B. M., Soper, R. G., Hurmalainen, J., Mansikkamaki, A., Robertson, K. N., McClennan, W. L., Veinot, A. J., Roemmele, T. L., Werner-Zwanziger, U., Boere, R. T., Tuononen, H. M., Clyburne, J. A. C., Masuda, J. D., Barry, B. M. Mono- and Bis(imidazolidinium ethynyl) Cations and Reduction of the Latter To Give an Extended Bis-1,4-([3]Cumulene)-p-carboquinoid System. Angewandte Chemie International Edition. 57 (3), 749-754 (2018).
- Nielsen, M. B., Lomholt, C., Becher, J. Tetrathiafulvalenes as building blocks in supramolecular chemistry II. Chemical Society Reviews. 29 (3), 153-164 (2000).
- Bergkamp, J. J., Decurtins, S., Liu, S. -. X. Current advances in fused tetrathiafulvalene donor-acceptor systems. Chemical Society Reviews. 44 (4), 863-874 (2015).
- Kirtley, J. R., Mannhart, J. Organic electronics: When TTF met TCNQ. Nature Materials. 7 (7), 520-521 (2008).
- Lorcy, D., Bellec, N., Fourmigué, M., Avarvari, N. Tetrathiafulvalene-based group XV ligands: Synthesis, coordination chemistry and radical cation salts. Coordination Chemistry Reviews. 253 (9-10), 1398-1438 (2009).
- Goetz, K. P., Vermeulen, D., Payne, M. E., Kloc, C., McNeil, L. E., Jurchescu, O. D. Charge-transfer complexes: new perspectives on an old class of compounds. Journal of Materials Chemistry. 2 (17), 3065-3076 (2014).
- Munz, D., Chu, J., Melaimi, M., Bertrand, G. NHC-CAAC Heterodimers with Three Stable Oxidation States. Angewandte Chemie International Edition. 55 (41), 12886-12890 (2016).
- Mandal, D., et al. Stepwise Reversible Oxidation of N-Peralkyl-Substituted NHC-CAAC Derived Triazaalkenes: Isolation of Radical Cations and Dications. Organic Letters. 19 (20), 5605-5608 (2017).
- Antoni, P. W., Hansmann, M. M. Pyrylenes: A New Class of Tunable, Redox-Switchable, Photoexcitable Pyrylium-Carbene Hybrids with Three Stable Redox-States. Journal of the American Chemical Society. 140 (44), 14823-14835 (2018).
- Back, O., Henry-Ellinger, M., Martin, C. D., Martin, D., Bertrand, G. (PNMR)-P-31 Chemical Shifts of Carbene-Phosphinidene Adducts as an Indicator of the pi-Accepting Properties of Carbenes. Angewandte Chemie International Edition. 52 (10), 2939-2943 (2013).
- Jazzar, R., Dewhurst, R. D., Bourg, J. B., Donnadieu, B., Canac, Y., Bertrand, G. Intramolecular "Hydroiminiumation" of alkenes: Application to the synthesis of conjugate acids of cyclic alkyl amino carbenes (CAACs). Angewandte Chemie International Edition. 46 (16), 2899-2902 (2007).
- Benac, B. L., Burgess, E. M., Arduengo, A. J. 1,3-Dimethylimidazole-2-Thione. Organic Syntheses. 64, 92 (1986).
- Arduengo, A. J., Davidson, F., Dias, H. V. R., Goerlich, J. R., Khasnis, D., Marshall, W. J., Prakasha, T. K. An air stable carbene and mixed carbene "dimers". Journal of the American Chemical Society. 119, 12742-12749 (1997).
- Frey, G. D., Lavallo, V., Donnadieu, B., Schoeller, W. W., Bertrand, G. Facile Splitting of Hydrogen and Ammonia by Nucleophilic Activation at a Single Carbon Center. Science. 316 (5827), 439-441 (2007).
- Verlinden, K., Buhl, H., Frank, W., Ganter, C. Determining the Ligand Properties of N-Heterocyclic Carbenes from 77Se NMR Parameters. European Journal of Inorganic Chemistry. 2015 (14), 2416-2425 (2015).
- Vummaleti, S. V. C., et al. What can NMR spectroscopy of selenoureas and phosphinidenes teach us about the [small pi]-accepting abilities of N-heterocyclic carbenes?. Chemical Science. 6 (3), 1895-1904 (2015).
- Hahn, F. E., Jahnke, M. C. Heterocyclic Carbenes: Synthesis and Coordination Chemistry. Angewandte Chemie International Edition. 47 (17), 3122-3172 (2008).
- Braun, M., Frank, W., Reiss, G. J., Ganter, C. An N-Heterocyclic Carbene Ligand with an Oxalamide Backbone. Organometallics. 29 (20), 4418-4420 (2010).
- Moerdyk, J. P., Schilter, D., Bielawski, C. W. N,N’-Diamidocarbenes: Isolable Divalent Carbons with Bona Fide Carbene Reactivity. Accounts of Chemical Research. 49 (8), 1458-1468 (2016).
- Mandal, D., et al. Stepwise Reversible Oxidation of N-Peralkyl-Substituted NHC-CAAC Derived Triazaalkenes: Isolation of Radical Cations and Dications. Organic Letters. 19 (20), 5605-5608 (2017).
- Torres, A. J., Dorsey, C. L., Hudnall, T. W. Preparation and Use of Carbonyl-decorated Carbenes in the Activation of White Phosphorus. Journal of Visualized Experiments. (92), e52149 (2014).
- Hahn, F. E., Wittenbecher, L., Van Le, D., Fröhlich, R. Evidence for an Equilibrium Between an N-heterocyclic Carbene and Its Dimer in Solution. Angewandte Chemie International Edition. 3 (39), 5441-5544 (2000).
- Weinstein, C. M., Martin, C. D., Liu, L., Bertrand, G. Cross-Coupling Reactions Between Stable Carbenes. Angewandte Chemie International Edition. 53 (25), 6550-6553 (2014).

