Using Deuterium Oxide as a Non-Invasive, Non-Lethal Tool for Assessing Body Composition and Water Consumption in Mammals
Summary
This article describes the deuterium oxide dilution technique in two mammals, an insectivore and carnivore, to determine total body water, lean body mass, body fat mass, and water consumption.
Abstract
Body condition scoring systems and body condition indices are common techniques used for assessing the health status or fitness of a species. Body condition scoring systems are evaluator dependent and have the potential to be highly subjective. Body condition indices can be confounded by foraging, the effects of body weight, as well as statistical and inferential problems. An alternative to body condition scoring systems and body condition indices is using a stable isotope such as deuterium oxide to determine body composition. The deuterium oxide dilution method is a repeatable, quantitative technique used to estimate body composition in humans, wildlife, and domestic species. Additionally, the deuterium oxide dilution technique can be used to determine the water consumption of an individual animal. Here, we describe the adaption of the deuterium oxide dilution technique for assessing body composition in big brown bats (Eptesicus fuscus) and for assessing water consumption in cats (Felis catis).
Introduction
Body condition scoring systems and body condition indices are common techniques used for assessing the health status or fitness of a species1,2. Many domestic and zoological species have unique body condition scoring (BCS) systems that are used to assess an animal's muscle and superficial fatty tissue3. However, BCS assessment relies upon the evaluator—meaning that BCS is an objective or semiquantitative measurement when assessed by a trained evaluator. In wildlife species, body condition indices are commonly used rather than BCS and are based upon a ratio of body mass to body size or body mass to forearm2. Body condition indicis are often confounded by the effects of foraging and can be confounded by body size as well as statistical and inferential problems4.
An alternative to body condition scoring systems and body condition indices is using a stable isotope to determine body composition. One commonly used stable isotope is deuterium oxide (D2O), a non-radioactive form of water in which the hydrogen atoms are deuterium isotopes. The deuterium oxide dilution method described in this study can be a non-subjective, quantitative, and repeatable technique used to estimate body composition in humans5 and a wide range of species4,6,7. This technique can be advantageous for studying the body composition in wildlife. For example, it can be used to assess longitudinal changes in body composition, such as before and after a management action. However, in some wildlife species deuterium oxide can overestimate the actual water content8. Therefore, when adapting the technique for a species, it is important to validate the method by comparing the deuterium oxide method to carcass analysis for non-endangered species. For threatened and endangered species, a non-destructive method such as dual x-ray absorptiometry (DXA) should be considered as an alternative comparison method to the gold-standard destructive method of complete carcass analysis.
In addition to body composition, the D2O dilution technique can be used to determine the water consumption of an individual animal9. This unique application of D2O can be used to answer not only research questions, but can be useful for assessing the water consumption of individual animal(s) housed in large social settings.
Here, we describe the adaption of the D2O dilution technique for assessing body composition in an insectivore, big brown bats (Eptesicus fuscus), and for assessing water consumption in a carnivore, cats (Felis catis).
Protocol
All experiments described here were approved by the University of Missouri Animal Care and Use Committee and conducted under the Missouri Department of Conservation (MDC) Wildlife Scientific Collection permit (Permit #16409 and #17649).
1. Preparation of sterile, isotonic, salinated D2O stock solution
- Make a 50 mL stock solution of 9.0 g/L salinated D2O.
- Weigh 450 mg of pharmaceutical grade NaCl and transfer all NaCl into a 100 mL, sterilized beaker. Record the exact amount of NaCl to 4 decimal places in the laboratory notebook.
- Using a sterile graduated cylinder, measure 50 g of ≥ 99.8% deuterium oxide and transfer to the sterile beaker containing the NaCl. Record the exact amount of deuterium oxide to 4 decimal places in the laboratory notebook or spreadsheet.
- Filter 10 mL of isosmotic strength NaCl (9.0 g/L) through a non-pyrogenic sterile disk filter with submicron pores (0.2 µm).
- Attach a 20 G needle to the non-pyrogenic sterile disk filter with submicron pores (0.2 µm) fitted with a 10 mL syringe barrel. Insert into the septum of a 100 mL sterile empty vial.
- Attach a vacuum tube to a 22 G needle and insert the needle into the septum of the 100 mL sterile empty vial.
- Pour 10 mL of the stock solution into the syringe barrel. Slowly turn on the vacuum until the D2O stock solution begins to slowly filter into the sterile vial. Continue to pour the D2O stock solution into the syringe barrel until all 50 mL is filtered.
NOTE: The stock solution may need to be diluted or concentrated depending on the dose required. The dose of D2O will vary based upon the species and the sensitivity of the analytical method. For cats, the working solution was used to administer a dose of 0.7 g/kg D2O. The stock solution described above minimizes the amount of NaCl solution introduced subcutaneously to the animal while still allowing accurate measurement of the dose. For small mammals such as bats, this concentration must be diluted to a working solution such as 0.1600 g/mL. This concentration allows the dose of 0.75 g/kg D2O to be accurately measured and administered in approximately 100 µL or less NaCl solution.
2. Preparation of sterile, isotonic, salinated D2O stock working solution for bats
- Weigh a 10 mL empty sterile vial and record weight to nearest 4 decimal places. Tare scale.
- Use a 1.0 mL syringe to transfer 0.65 mL of the D2O stock solution to the tared, 10 mL empty sterile vial. Record weight of D2O to 4 decimal places. Tare scale.
- Calculate the volume of D2O in the 10 mL empty vial. Use the following equation.
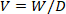
where W is recorded weight and D is the density of 99.8% D2O (1.107 g/mL). - Use the calculated volume and known weight of the D2O to determine the volume of isotonic saline required to make ~0.1600 g/mL working solution.
- Insert into the septum of the 10 mL sterile vial, the 22 G needle (attached to the vacuum tube). Insert into the septum of the 10 mL sterile vial, the 20 G needle (attached to a 0.22 µm syringe filter fitted with a 10 mL syringe barrel).
- Pour the calculated mass/volume of isotonic NaCl into the syringe barrel and turn on the vacuum to allow a slow drip into the sterile 10 mL vial.
- Record the weight of the vial and ensure a ~0.1600 g/mL working solution is created.
3. Determination of body composition of big brown bats (Eptesicus fucsus) with D2O
NOTE: The stock solution of D2O used in the protocol is 0.1598 g/mL. Before collecting blood, ensure that removing up to 200 µL of blood will be ≤ 10% of the total blood volume of the bat and is within the Institutional Animal Care and Use Committee's (IACUC) established guidelines for blood collection. All animals should be fasted or abdomen palpated to ensure an empty stomach. A recent meal could alter the animal's weight resulting in confounded results since calculations for determining body fat rely upon the body mass of the animal.
- Anesthetize a big brown bat.
- Use 5.0% isoflurane for induction. Maintain a stable plane of anesthesia using 0.5%−3.0% isoflurane.
- Determine proper anesthesia depth by testing the pedal withdrawal reflex (pinching the bat's toes). The bat should not respond to the sensation and the respiratory rate should remain slow and stable. Adjust isoflurane as needed to maintain a stable plane of anesthesia.
- Record isoflurane level, heart rate, respiratory rate, and other information as required by IACUC.
- Weigh the big brown bat and record the weight to 4 decimal places.
- Clean the uropatagium (tail membrane) over the interfemoral vein with an alcohol prep pad and allow to dry. Apply a thin layer of petroleum jelly over the interfemoral vein.
- Use a 29 G needle to puncture the dorsal portion of the interfemoral vein and collect 100 µL of blood using plastic sodium heparin capillary tubes. Ensure adequate mixing of the whole blood with the sodium heparin by gently rolling each tube after collection and label the tube.
- Using a DXA machine calibrated for small mammals, obtain three DXA scans of the bat10.
- Determine the mass (in g) of D2O to inject by multiplying the bat weight in kg by the D2O dose of 0.75 g/kg. Determine the volume of the calculated D2O dose (V) by dividing the weight of the D2O dose by the concentration of the working solution.
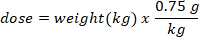
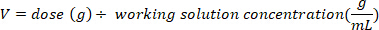
- Use an insulin syringe with a 29 G needle attached to draw up the volume of D2O calculated. Weigh the D2O, insulin syringe, and needle. Record to 4 decimal places.
- Inject the D2O subcutaneously over the dorsal hip region of the anesthetized bat.
- Allow bat to recover from anesthesia and record the time of injection.
- Immediately after injection, weigh the now empty insulin syringe with the 29 G needle attached. Record the weight to 4 decimal places.
- Determine the dose of D2O injected by subtracting the post-injection weight of the insulin syringe from the pre-injection D2O filled insulin syringe. Record to 4 decimal places.
- Within 30 min post blood collection, use a hematocrit centrifuge to spin each capillary tube for 5 min. If the hematocrit centrifuge allows multiple speeds, set to 10,000 x g.
- Use a sharp scissors to cut the plastic capillary tube between the whole blood and plasma. Use a 200 µL pipette to expel the plasma directly into a labelled, 500 µL storage tube.
- After the equilibration period, collect another 100 µL of blood from the interfemoral vein.
NOTE: The equilibration period will vary by species and if the bats go into torpor. For big brown bats, typically 2 h is sufficient for the equilibration period. - Separate plasma into a second labelled, 500 µL microcentrifuge screw top tube by repeating step 3.13. Store samples at -20 °C or colder until analysis.
4. Fourier-transform infrared spectrophotometry analysis
- Set the temperature of a sand bath to 60 °C to facilitate distillation (allow separation of water and D2O from other blood components).
- Pipette 50 µL of each plasma sample and standard onto the inside of a 1.5 mL conical microcentrifuge tube cap. Including standards containing known concentrations of D2O as quality control.
NOTE: Ideally each animal will have three replicates per sample and the average of the three replicates reported. Due to the limited sample volume and the volume of sample required for the FT-IR equipment utilized by the authors, no replicates were performed for the bat samples. If any sample contains less than 50 µL of plasma, pipette the sample amount onto the conical microcentrifuge tube cap and record the volume. - Keep the microcentrifuge cap upside down and screw the 1.5 mL conical microcentrifuge tube onto the cap. Place the inverted (upside-down) tube with the cap in contact with the sand in the sand bath for a minimum of 12 h (overnight).
- After 12 h, remove the cap and replace with a new, clean cap. Pulse the microcentrifuge tube for 10 s in a centrifuge.
- Create the following standards: 0 ppm (0 mg D2O in 1 L distilled water), 293 ppm (293 mg D2O in 1 L distilled water), 585 ppm (585 mg D2O in 1 L distilled water), 878 ppm (878 mg D2O in 1 L distilled water), and 1170 ppm D2O (1170 mg D2O in 1 L distilled water).
NOTE: The values above are suggested for a standard curve. Alternative values such as 250 ppm, 500 ppm, 750 ppm, and so forth can be used. - Install a liquid transmission cell into the Fourier-transform infrared spectrophotometry (FTIR) spectrometer (Table of Materials). Fill the cell with methanol and connect the injection port. Slowly fill the cell with background water while carefully removing the methanol syringe to reduce the risk of air bubbles. Attach tubing to the output port to allow removal of the samples post-analysis.
- Prepare the FTIR spectrometer software (Table of Materials) for analysis of D2O in water. The parameter settings for the spectrometer software used in this protocol are listed in Table 1.
- Collect a background sample using the diluent, 0.22 µm-filtered, distilled water. This should be the same water used for the standards.
- Inject 40 µL of the 0 ppm D2O and record the spectra. Save the spectra as a comma separated values (CSV) file.
- Continue to inject and save the spectra of all standards to create a standard curve.
- Repeat the background and standard curve every 60−90 min.
- Inject 40 µL of each distilled sample into the liquid transmission cell and save the spectra.
NOTE: Alter the injection volume of the standards and distilled samples based upon the volume of liquid transmission cell. Use a smaller volume liquid transmission cell if the sample volume is below 40 µL or dilute 1:1 with background distilled water. - Determine the concentration of D2O of each sample from the FTIR spectra using a spreadsheet program as described by Jennings et al.11 or the spectral software. When replicates are performed, use the average concentration to calculate the body composition.
5. Calculation of body composition
- Convert the deuterium enrichment (ppm) to atom percent concentration for each sample using the following equation12:
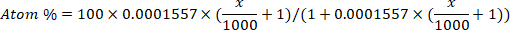
where x is the measured deuterium enrichment (ppm) of the sample and 0.0001557 is the mole fraction of deuterium reported in Vienna Standard Mean Ocean Water (VSMOW)13. - Calculate total body water for each sample using the following equation4,12,14:
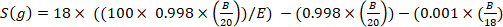 )
)
where E is the measured enrichment (atom%) of deuterium in the sample after background correction, B is the injection mass in g, and 0.998 is the concentration of injected D2O.
NOTE: Deuterium exchange with labile hydrogen causes a 2% overestimation of total body water mass. Total body water should be corrected by reducing the total body water mass estimate by 2% of the body weight. - Estimate the fat-free mass (lean body mass and all other non-fat components) of each bat using the following equation:
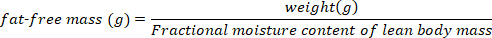
NOTE: Use the conventionally accepted value of 0.732 for the fractional moisture content of lean body mass for healthy, euhydrated, non-lactating bats. The fractional moisture content of fat-free mass can change in lactating big browns based upon the post-partum week15. For other species, use the values published in the literature or determine the fractional moisture content of lean body mass prior to performing calculations of the lean body mass. - Estimate the body fat mass using the following equation:
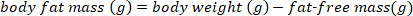
- Convert the body fat mass in g to percent body fat mass using the following equation:
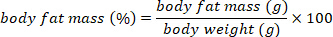
6. Determination of water composition in a carnivore (Felis catus, domestic cat)
- Prepare the stock solution as described in section 1.
- Weigh each cat to the nearest 3 decimal places and record weight. Calculate the dose for each cat as described in step 3.6 using a D2O dose of 0.70 g/kg.
- Prepare each dose as described in steps 3.7−3.8. using a 3 mL or 5 mL syringe with a 22 G needle instead of an insulin syringe.
- Collect 500 µL of whole blood and subsequently administer subcutaneously the 0.7 g/kg D2O. Centrifuge whole blood at 2,000 x g for 15 min and store plasma in 1.5 mL microcentrifuge screw top tubes at -20°C until analysis.
- Collect 500 µL of whole blood 4 h post-injection. Centrifuge whole blood at 2,000 x g for 15 min and store plasma in 1.5 mL microcentrifuge screw top tubes at -20 °C until analysis.
- Collect 500 µL of whole blood 14 days post-injection. Centrifuge whole blood at 2,000 x g for 15 min and store plasma in 1.5 mL microcentrifuge screw top tubes at -20 °C until analysis.
NOTE: The number of days between blood collection can be based upon the experimental needs and the post-injection period in which D2O can be detected above the background levels. Fourteen days was the length of the dietary treatment blocks from Hooper et al.9. - Perform FT-IR analysis according to section 4 and calculate the body composition according to section 5 of this protocol.
- Calculate the water consumption in mL/day using the following equations:
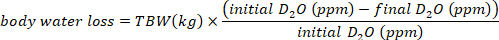
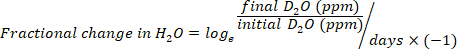
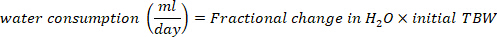
where TBW is total body water, initial D2O and final D2O are the concentrations measured in ppm in the post-injection D2O samples.
Representative Results
The deuterium oxide dilution technique can be used to assess the body composition of a variety of species. To demonstrate the adaptability, we are reporting the first use of the deuterium oxide dilution technique in a North American insectivorous bat species, Eptesicus fuscus, the big brown bat for representative results. A timing plateau was completed by taking pre- and post-D2O injection blood samples as should be done with any species where the equilibration period is unknown. It was determined that two hours post-injection in non-torpid bats was adequate for equilibration. With the equilibration time known, the total body water, lean body mass, and body fat mass for 13 wild-caught big brown bats and 8 captive big brown bats were determined (Table 2). An additional 2 wild-caught big brown bats and 5 captive big brown bats were determined to have a negative body fat mass. A negative body fat mass is calculated due to one or more of the following reasons: not receiving the entire dose of deuterium oxide, becoming torpid during the equilibration phase, having abnormally large fat masses and minimal lean mass, or bats having under 3%−5% body fat as determine by DXA (Table 3).
White-nose syndrome has caused many bat species to decline, so the technique was compared to the body fat measured using DXA. Figure 1 shows the percentage of body fat determined by the D2O dilution technique and DXA (n = 19). The two techniques were well correlated with a Pearson's r = 0.897 (Figure 2) and were not statistically different (one-way analysis of variance (ANOVA), F-value = 0.366, p = 0.549). The body fat showed strong correlations between body fat and body weight (Figure 3). The D2O dilution technique did not consistently over or underestimate the body fat mass.
The deuterium oxide method has been previously validated in cats16. Table 4 shows an example of the total body water, lean body mass, and body fat mass of a single cat9. Hooper et al.9 was the first to report the use of deuterium oxide dilution to measure the water consumption of socially housed animals with the daily water consumption of the cats during each dietary block of the experiment, as shown in Figure 4.
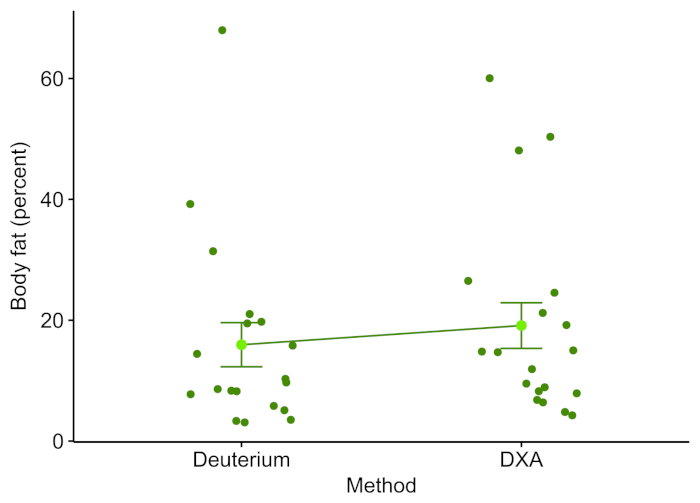
Figure 1: Deuterium oxide and DXA line plot. Each point represents the body fat percentage of an individual bat as determined by DXA or deuterium oxide. The mean is the light green point with error bars indicating the standard error of the mean. Please click here to view a larger version of this figure.
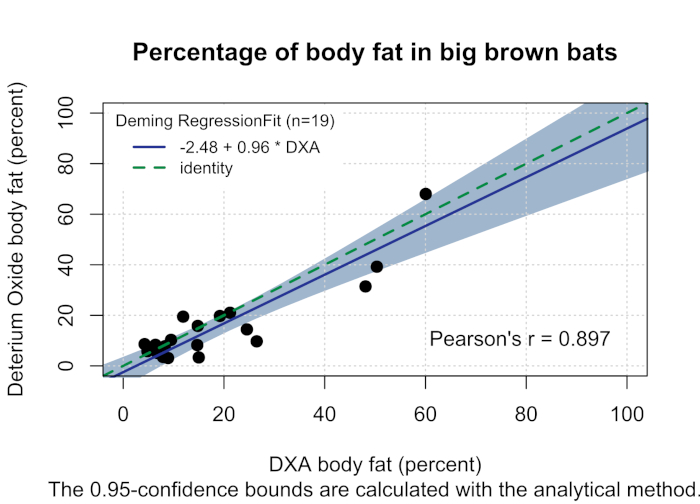
Figure 2: Percentage of body fat in big brown bats. Deming regression (solid blue line, Pearson's r = 0.897) comparing the percentage of body fat determined by DXA (x-axis, the reference method) and the percentage of body fat determined by deuterium oxide (y-axis, the test method) in big brown bats with 95% confidence intervals designated by gray shading. The green dash identity line drawn represents the regression line when the methods are equal. Please click here to view a larger version of this figure.
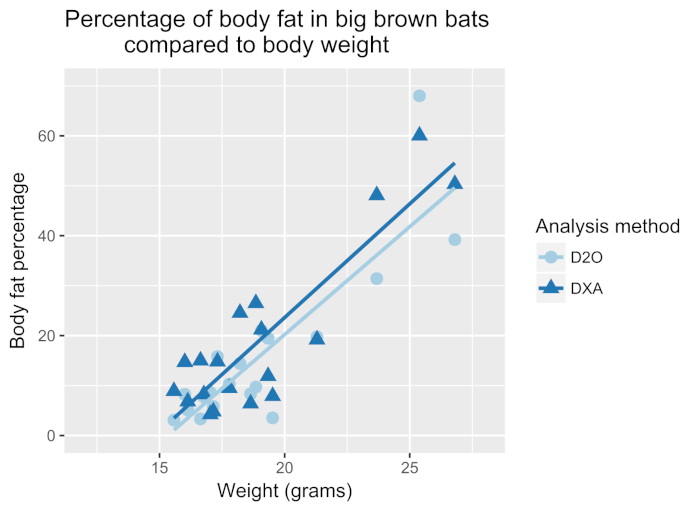
Figure 3: Percentage of body fat in big brown bats compared to body weight. Body weight of each bat plotted against the body fat percentage determined by D2O or DXA. A strong correlation exists between the body weight and body fat as determined by DXA (dark blue line, Pearson's r = 0.88) and D2O (blue line, Pearson's r = 0.86). Please click here to view a larger version of this figure.
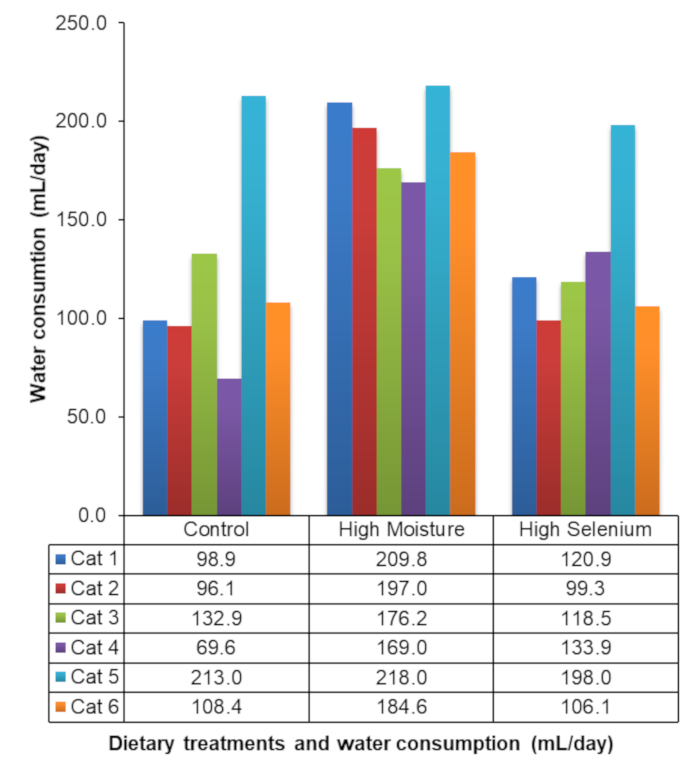
Figure 4: Water consumption of socially housed cats. Representative results of the daily water consumption of socially housed cats during an experiment evaluating the effects of dietary constituents on water consumption. This figure has been modified from Hooper et al.9. Please click here to view a larger version of this figure.
| Parameter | Setting |
| Number of scans | 64 |
| Resolution | 2 |
| Data spacing | 0.946 cm-1 |
| Final format | Absorbance |
| Correction | None |
| Use fixed Y-axis limits in collection window | Min -0.01, Max 0.03 |
| Bench range | Max 6.38, Min -5.02, Loc 1024 |
| Total absorbing peak sensitivity | 50 |
| fringes or channeling sensitivity | 80 |
| Derivative peaks sensativity | 51 |
| Baseline error sensitivity | 50 |
| CO2 levels sensitivity | 19 |
| H2O levels sensitivity | 19 |
| Apodization mode | Happ-Genzel |
| Phase correction | Mertz |
| Filters set based upon | velocity |
| low pass filter | 11,000 |
| high pass filter | 20 |
Table 1: Spectral software settings. Parameter settings used for spectral recording software.
| Animal | Species | Body weight (kg) |
D2O injected (g) |
Total body water (g) |
Lean body mass (g) |
Body fat mass (g) |
Body fat mass (%) |
DXA lean + bmc (g) |
DXA fat (g) |
DXA fat (%) |
| 1 | Eptesicus fuscus | 0.01715 | 0.0740 | 11.80 | 16.15 | 1.00 | 5.80 | 14.65 | 0.75 | 4.80 |
| 2 | Eptesicus fuscus | 0.01950 | 0.0920 | 13.80 | 18.83 | 0.69 | 3.50 | 16.20 | 1.40 | 7.90 |
| 3 | Eptesicus fuscus | 0.01677 | 0.08 | 11.33 | 15.47 | 1.30 | 7.74 | 11.33 | 1.30 | 7.74 |
| 4 | Eptesicus fuscus | 0.02129 | 0.097 | 12.51 | 17.09 | 4.20 | 19.7 | 15.9 | 19.65 | 19.2 |
Table 2: Body composition of big brown bats. The representative results of total body water, lean body mass, and body fat as determined by deuterium oxide dilution in big brown bats are shown in columns 5−8. Representative results of the lean body mass plus bone mineral content and body fat as determined by DXA in the same big brown bats are shown in columns 9−11.
| Animal | Species | Body weight (kg) |
D2O injected (g) |
Total body water (g) |
Lean body mass (g) |
Body fat mass (g) |
Body fat mass (%) |
DXA lean + bmc (g) |
DXA fat (g) |
DXA fat (%) |
Comment |
| 1 | Eptesicus fuscus | 0.0277 | 0.1299 | 34.18 | 46.69 | -19.02 | -68.74 | 9.90 | 26.55 | 62.80 | Equili-bration time insufficient |
| 2 | Eptesicus fuscus | 0.0185 | 0.0810388 | 64.23 | 87.75 | -69.25 | -374.33 | 14.20 | 17.30 | 17.95 | Full dose not injected |
| 3 | Eptesicus fuscus | 0.0164 | 0.0719 | 17.38 | 23.74 | -7.33 | -44.68 | 14.15 | 14.40 | 1.70 | Less than 3% fat |
| 4 | Eptesicus fuscus | 0.0212 | 0.0994 | 54.57 | 74.54 | -53.37 | -252.0 | 16.41 | 19.01 | 13.65 | Bat became torpid (cool to touch) |
Table 3: Body composition of big brown bats. Representative results from bats that did not receive the entire dose of deuterium oxide, became torpid during the equilibration phase, bats with abnormally large fat mass and minimal lean mass, or bats under 3%−5% body fat as determine by DXA. The representative results of total body water, lean body mass, and body fat as determined by deuterium oxide dilution are shown in columns 5−8. Representative results of the lean body mass plus bone mineral content and body fat as determined by DXA are shown in columns 9−11.
| Block | Species | Body weight (kg) |
D2O injected (g) |
Total body water (kg) |
Lean body mass (kg) |
Body fat mass (kg) |
Body fat mass (%) |
Daily water consumption (mL/day) |
Dietary Treatment |
| 1 | Felis Catus | 4.830 | 3.36 | 2.69 | 3.68 | 1.149 | 23.8 | 96.8 | Control |
| 2 | Felis Catus | 4.764 | 3.45 | 2.66 | 3.63 | 1.136 | 23.8 | 217.5 | High Moisture |
| 3 | Felis Catus | 4.727 | 3.25 | 2.50 | 3.41 | 1.314 | 27.8 | 125.1 | High Selenium |
Table 4: Body composition and water consumption in a single feline. Representative results of deuterium oxide dilutional technique for assessing the lean body mass, fat mass, and water consumption of one cat at three different time points during the study conducted by Hooper et al.9.
Discussion
The use of deuterium oxide to determine TBW has been used since the 1940s17 and is used in humans and a variety of domestic and wildlife species4,6,7. Other non-destructive techniques have been developed including bioelectrical impedance analysis (BIA), DXA, and quantitative magnetic resonance (QMR). Each method has advantages and disadvantages that should be considered before selecting a particular methodology for assessing body composition. This protocol selected to use DXA as a comparison method for deuterium oxide to assess body composition, because the equipment is available as a core university resource with minimal cost, minimal time is required per scan (30 s per bat), and it is not sensitive to variables such as body temperature and skin insulation.
When adapting the deuterium oxide dilution technique to a species of interest, a pilot study should be initiated to determine the time required for equilibration18. This can be done by taking a background sample, and a blood sample every 15 minutes post-injection. For small species such as bats, several bats can be bled at the different time intervals instead of a single animal18. The equilibration time can change when animals, such as bats, go into torpor, which explains why some of our animals had a negative percent body fat (Table 3). If a negative percent body fat is obtained, and the deuterium dose had sufficient time to fully equilibrate with the animal's body water, then it is likely the dose was not completely injected. Because the deuterium oxide dilution technique is highly dependent upon the full dose being administered and accurate recording of the amount of deuterium injected, this technique should only be completed by individuals skilled in performing injections. Additionally, anesthetizing or sedating animals can assist with ensuring the entire dose can be administered.
When administering the deuterium oxide, it is important to determine an appropriate concentration to administer to the animal. Using a 0.7 g/kg dose for the cats, the stock solution concentration was appropriate, whereas for the big brown bats a 0.75 g/kg dose required the stock solution of deuterium oxide to be diluted. When diluting the stock solution, an isotonic solution such as 0.9% NaCl should be used. To avoid altering the total body water of small mammals, dilute the dose of deuterium oxide as minimally as possible, just enough to ensure the dose can be measured accurately.
The doses presented here are detectible using FTIR spectrometry. FTIR spectrometry is less expensive and easier to maintain, but not as sensitive as isotope ratio mass spectrometry (IRMS)19,20. FTIR spectrometry can be used to measure deuterium enrichment in plasma and saliva, but it is not recommended to use an FTIR transmission cell to analyze deuterium enrichment in urine19. If urine is the desired sample type, then an attenuated total reflection (ATR) attachment should be used with the FTIR or IRMS should be used to assess deuterium enrichment for calculation of TBW19.
Additionally, the doses used for the cats were adequate to allow detection of deuterium oxide 14 days post-injection. Because the concentration of the deuterium oxide 14 days post-injection was detectable, the water consumption of the cats could be calculated (Figure 4). This innovative use of deuterium oxide can be employed in field studies to measure body water turnover for species with high recapture rates or for animals housed in groups in ex situ or laboratory studies. However, before employing in field studies, researchers must assess if the animal can be captured and held for the duration of the equilibration period. This prolong handling period is one of the disadvantages of the deuterium oxide technique and could be problematic as many endangered species permits limit the duration that a particular animal can be held. Additionally, animals cannot have recently eaten as the washout technique relies upon the measurement of body mass; therefore, a recent meal can confound the results. An additional consideration is whether an animal must be anesthetized or sedated for subcutaneous injection and blood collections or if the animal can be restrained without sedation/anesthesia. It has been suggested that the rate of body water turnover could be a significant indicator for human health21. The increased water consumption in cat 5 (Figure 4) was documented before traditional biochemical marks of renal failure, and concentrations of creatinine and blood urea nitrogen (BUN) were elevated, suggesting that body water turnover could also be an indicator of health in animals.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was supported by MDC Cooperative Agreement (#416), US Forest Service Cooperative Agreement (16-JV-11242311-118), American Academy of Veterinary Nutrition and Waltham/Royal Canin, USA Grant (grant number: 00049049), NIH training grant (grant number: T32OS011126), and the University of Missouri Veterinary Research Scholars Program. The authors thank Shannon Ehlers for pre-reviewing this manuscript. We thank Dr. Robert Backus for providing the D2O standards and allowing use of his laboratory.
Materials
| 0.2 micron non-pyrogenic disk filter | Argos Technologies | FN32S | nylon, 30mm diameter, 0.22um, sterile |
| 1.5 mL conical microcentrifuge tubes | USA Scientific | 1415-9701 | 1.5 ml self-standing microcentrifuge tube, natural with blue cap |
| 10 mL sterile glass vial for injection | Mountainside Medical Equipment | MS-SEV10 | clear, sterile glass injection unit |
| 10 mL syringe | Becton Dickinson | 305219 | sterile 10 mL syringe individually wrapped |
| 100 mL sterile glass vial for injection | Mountainside Medical Equipment | AL-SV10020 | clear, sterile glass injection unit |
| 20 gauge needle | Exel | 26417 | needles hypodermic 20g x 1" plastic hub (yellow) / regular bevel |
| 22 gauge needle | Exel | 26411 | needles hypodermic 22g x 1" plastic hub (black) / regular bevel |
| deuterium oxide | Sigma-Aldrich | 151882-25G | 99.9 atom % D |
| isofluorane | Vetone | 3060 | fluriso isoflurane, USP |
| OMNIC Spectra Software | ThermoFisher Scientific | 833-036200 | FT-IR standard software |
| petroleum jelly | Vaseline | 305212311006 | Vaseline, 100% pure petroleum jelly, original, skin protectant |
| plastic capillary tubes | Innovative Med Tech | 100050 | sodium heparin anticoagulant, 50 μL capacity, 30 mm length |
| Sealed liquid spectrophotometer SL-3 FTIR CAF2 Cell | International Crystal Laboratory | 0005D-875 | 0.05 mm Pathlength |
| sodium chloride | EMD Millipore | 1.37017 | suitable for biopharmaceutical production |
| Thermo Electron Nicolet 380 FT-IR Spectrometer | ThermoFisher Scientific | 269-169400 | discontinued model, newer models available |
Riferimenti
- Schiffmann, C., Clauss, M., Hoby, S., Hatt, J. M. Visual body condition scoring in zoo animals – composite, algorithm and overview approaches. Journal of Zoo Aquarium Research. 5 (1), (2017).
- Peig, J., Green, A. J. New perspectives for estimating body condition from mass/length data: the scaled mass index as an alternative method. Oikos. 118 (12), 1883-1891 (2009).
- Bissell, H. . Body Condition Scoring Resource Center. , (2017).
- McWilliams, S. R., Whitman, M. Non-destructive techniques to assess body composition of birds: a review and validation study. Journal of Ornithology. 154 (3), 597-618 (2013).
- Lukaski, H. C., Johnson, P. E. A simple, inexpensive method of determining total body water using a tracer dose of D2O and infrared absorption of biological fluids. American Journal of Clinical Nutrition. 41 (2), 363-370 (1985).
- Chusyd, D. E., et al. Adiposity and Reproductive Cycling Status in Zoo African Elephants. Obesity (Silver Spring). 26 (1), 103-110 (2018).
- Kanchuk, M. L., Backus, R. C., Calvert, C. C., Morris, J. G., Rogers, Q. R. Neutering Induces Changes in Food Intake Body Weight, Plasma Insulin and Leptin Concentrations in Normal and Lipoprotein Lipase–Deficient Male Cats. The Journal of Nutrition. 132 (6), 1730S-1732S (2002).
- Eichhorn, G., Visser, G. H. Technical Comment: Evaluation of the Deuterium Dilution Method to Estimate Body Composition in the Barnacle Goose: Accuracy and Minimum Equilibration Time. Physiological and Biochemical Zoology. 81 (4), 508-518 (2008).
- Hooper, S. E., Backus, R., Amelon, S. Effects of dietary selenium and moisture on the physical activity and thyroid axis of cats. Journal of Animal Physiolgy and Animal Nutrition (Berl). 102 (2), 495-504 (2018).
- Stevenson, K. T., van Tets, I. G. Dual-Energy X-Ray Absorptiometry (DXA) Can Accurately and Nondestructively Measure the Body Composition of Small, Free-Living Rodents. Physiological and Biochemical Zoology. 81 (3), 373-382 (2008).
- Jennings, G., Bluck, L., Wright, A., Elia, M. The use of infrared spectrophotometry for measuring body water spaces. Clinical Chemistry. 45 (7), 1077-1081 (1999).
- Beuth, J. M. . Body Composition, movemement phenology and habitat use of common eider along the southern new england coast. Master of Science in Biological and Environmental Sciences (MSBES) thesis. , (2013).
- Coplen, T. B., Hopple, J., Peiser, H., Rieder, S. Compilation of minimum and maximum isotope ratios of selected elements in naturally occurring terrestrial materials and reagents. U.S. Geological Survey Water-Resources Investigations Report 01-4222. , (2002).
- Karasov, W. H., Pinshow, B. Changes in lean mass and in organs of nutrient assimilation in a long-distance passerine migrant at a springtime stopover site. Physiological Zoology. 71 (4), 435-448 (1998).
- Hood, W. R., Oftedal, O. T., Kunz, T. H. Variation in body composition of female big brown bats (Eptesicus fuscus.) during lactation. Journal of Comparative Physiology B. 176 (8), 807-819 (2006).
- Backus, R. C., Havel, P. J., Gingerich, R. L., Rogers, Q. R. Relationship between serum leptin immunoreactivity and body fat mass as estimated by use of a novel gas-phase Fourier transform infrared spectroscopy deuterium dilution method in cats. American Journal of Veterinary Research. 61 (7), 796-801 (2000).
- Moore, F. D. Determination of Total Body Water and Solids with Isotopes. Science. 104 (2694), 157-160 (1946).
- Voigt, C., Cruz-Neto, A., Parsons, S., Kunz, T. H. . Ecological and Behavioral Methods in the Study of Bats. , 621-645 (2009).
- International Atomic Energy Agency. . Assessment of Body Composition and Total Energy Expenditure in Humans Using Stable Isotope Techniques. , (2009).
- International Atomic Energy Agency. . Introduction to Body Composition Assessment Using the Deuterium Dilution Technique with Analysis of Saliva Samples by Fourier Transform Infrared Spectrometry. , (2011).
- Shimamoto, H., Komiya, S. The Turnover of Body Water as an Indicator of Health. Journal of Physiological Anthropology and Applied Human Science. 19 (5), 207-212 (2000).

