Infecting Mice with Malassezia spp. to Study the Fungus-Host Interaction
Summary
This protocol outlines the establishment of a mouse model for studying Malassezia-host interactions in the skin. It describes the cultivation of Malassezia in vitro, the infection of the murine skin with Malassezia, and the subsequent analysis of the inflammation and the fungal burden in the skin tissue.
Abstract
Animal models are crucial for infectious disease research. They provide an important basis for analyzing the full spectrum of interactions that occur between microbes and their host in vivo in a tissue-specific manner. Pathogenic fungi are increasingly recognized as a serious threat for humans and exploiting such infection models have greatly improved our understanding of fungal pathogenicity. Species of the genus Malassezia are the most abundant fungi of the human skin microbiota and they are also associated with the development of severe inflammatory skin disorders such as seborrheic dermatitis and atopic dermatitis. However, a causative link between Malassezia and disease pathogenesis remains unknown, a fact that can be attributed to the poor knowledge of the complex crosstalk of Malassezia with the skin immune system. This protocol describes the establishment of an experimental mouse model that allows studying the interaction of Malassezia with the mammalian skin in vivo. It outlines the method for cultivating Malassezia spp. under laboratory conditions, how to infect the murine skin with Malassezia spp. and how to assess the outcome of infection by means of the skin inflammation and fungal burden analyses. The model described here works in fully immunocompetent animals and does not rely on immune suppressive or antibiotic pretreatment of the animals. It is furthermore adaptable to virtually all genetically modified mouse strains and can be combined with other skin disease models. These features make this infection model a very powerful tool for studying in detail the innate and adaptive immune response of the host against Malassezia in the skin in vivo.
Introduction
The skin is populated by many different microbes. The constant exposure of the skin to the microbiota contributes to shaping and educating the immune system of the host. Fungi are increasingly recognized as a vital part of the microbiota and they fulfill an important role for the host physiology and immunity, similar to bacteria and viruses1. Species of the genus Malassezia are by far the most abundant fungi colonizing the skin of warm-blooded vertebrates and they make up for more than 90% of the human skin mycobiome2,3. Eighteen different species of Malassezia have so far been identified from the human and animal skin4.
Various pathologies of the skin are thought to arise, at least partially, as a result of a dysbalanced microbiota composition. Dysbiosis may lead to the overgrowth of species with pathogenic potential resulting in opportunistic infections and disease5. Consistently, there is an increasing evidence that Malassezia, besides its commensal lifestyle, contributes to the development of various skin pathologies, ranging from dandruff and pityriarsis versicolor to more severe inflammatory disorders such as seborrheic dermatitis and atopic dermatitis4,6. While a causative link between Malassezia and pityriarsis versicolor has been established, the pathophysiological role of the fungus in more severe skin pathologies remains largely unknown.
Determining the role of Malassezia in skin homeostasis and disease calls for more in-depth knowledge about the interaction of the fungus with the skin and the cutaneous immune system. Of note, research on Malassezia is, compared to other human fungal pathogens (e.g., Candida albicans or Aspergillus fumigatus), still in the fledgling stage. This can be attributed to the difficulty in the cultivation of Malassezia under laboratory conditions and the lack of appropriate experimental models for studying the fungus in contact with the host in vivo. Previous experiments with isolated cells in culture indicated a broad range of direct and indirect interactions between Malassezia and various immune and non-immune cells7. However, these in vitro experiments only partially recapitulate the situation of the complex skin environment in vivo where numerous cellular and molecular events occur concomitantly between the fungus and various cell types.
Herein, we outline the protocol for an experimental model of Malassezia skin infection in mice, which we recently established, to study the fungus-host interaction in vivo7. This includes procedures for (1) the successful cultivation of Malassezia in vitro, (2) the epicutaneous application of Malassezia onto the murine ear skin, and (3) the technical details of how to analyze Malassezia-induced skin inflammation and the fungal burden of infected skin. Importantly, this model does not rely on immunosuppression (e.g., by corticosteroids) or antibiotic treatment of mice prior to infection, as it is practiced in other mouse models of fungal infection8,9. In turn, it allows studying the full spectrum of the innate and adaptive immune response against Malassezia in the normal skin. Of note, inbred wild type mice kept under specific pathogen-free (SPF) conditions are not naturally colonized with Malassezia and, therefore, their exposure to the fungus does not result in persistent colonization but is cleared from the host within approximately 1.5 weeks. However, the model allows for studying the mechanisms of antifungal host response initiation and regulation which, in turn, is the basis of how immune memory is generated. The model is versatile in that it can easily be applied to a wide variety of genetically modified mouse strains and it can be combined with other existing skin disease models, such as models of barrier deficiency, to study the impact of Malassezia under pathological and inflammatory skin conditions7. Therefore, the described model of experimental Malassezia skin infection in mice provides a high degree of flexibility to investigate the interaction of the fungus with the skin immune system in the context of homeostasis and disease.
This protocol describes the experimental skin infection of mice with Malassezia spp. Due to its pathogenic potential, Malassezia spp. are classified as BSL2 pathogens in some countries, including Switzerland. Please check the local guidelines and follow the regulations by the local authorities. BSL2-classified organisms should be handled by trained personnel under a BSL2-certified biosafety cabinet (BSC). Biological waste contaminated with BSL2-classified organisms, as well as, carcasses from mice infected with such organisms should be autoclaved prior to disposal. For experiments with mice, all efforts should be made to minimize suffering and ensure the highest ethical and humane standards according to the 3R principles (replace, refine, reduce)10. The experiments described in this protocol were carried out with M. pachydermatis (ATCC 14522), M. furfur (ATCC 14521) and M. sympodialis (ATCC 42132)7.
Protocol
All procedures described in this protocol were carried out in accordance with the ordinance on handling organisms in contained systems of the Federal Office for the Environment, Switzerland (www.bafu.admin.ch). The mouse experiments were conducted in strict accordance with the guidelines of the Swiss Animals Protection Law and were performed under the protocols approved by the Veterinary Office of the Canton Zurich, Switzerland (license number 168/2018).
1. Cultivation of Malassezia under laboratory conditions
NOTE: Store all the reagents and media used for this protocol at room temperature (RT, 20 – 25 °C) unless stated otherwise, as the lower temperature can inhibit fungal growth.
- Prepare the liquid modified Dixon (mDixon) medium for Malassezia growth. To prepare 500 mL of liquid mDixon medium, dissolve 18 g Malt extract, 10 g desiccated Ox-bile, 5 mL Tween-40, 3 g Peptone, 1 mL Glycerol and 1 mL Oleic Acid in 500 mL distilled H2O (dH2O). Adjust the medium to pH 6 with HCl and autoclave. Store the medium at RT.
- Prepare mDixon agar plates by adding 7.5 g agar to 500 mL mDixon medium prior to autoclaving. Slowly cool down the mDixon agar after autoclaving using a steering bar and a magnetic heating plate to avoid partial solidification of the medium while cooling down.
- Once the agar has cooled down to 50 – 60 °C, dispense the liquid into Petri dishes in a laminar flow hood and let them dry at RT overnight.
NOTE: The agar plates can be stored at 4 °C for several weeks when wrapped and kept upside down to avoid evaporation. - Obtain Malassezia isolates and revive lyophilized stocks of Malassezia according to the instructions obtained by the provider.
- Inoculate 10 mL of liquid mDixon medium in a sterile 100 mL Erlenmeyer flask with the revived Malassezia suspension according to the instructions obtained by the provider. Incubate the culture in a shaking incubator at 30 °C and 180 rpm.
- Inspect the growth of the Malassezia culture regularly by checking for the appearance of cream color and turbidity. Growth kinetics depend on the species and strain of Malassezia and may be particularly slow when Malassezia is freshly revived from a lyophilized stock. (Figure 1A).
- Prepare glycerol stocks by mixing 3 parts of the densely grown Malassezia culture in mDixon medium with 1 part of sterile 99% glycerol. Aliquot the Malassezia/glycerol mixture into sterile screw-cap tubes and store at -80 °C.
- For in vitro propagation, plate Malassezia from the liquid culture in mDixon or from the frozen glycerol stock onto a mDixon agar plate (brought to RT from 4 ˚C) using an inoculation loop.
NOTE: Transfer mDixon agar plates to RT from 4 °C prior to the use, since cold mDixon agar inhibits fungal growth. - Incubate the agar plate(s) with Malassezia upside down in an (non-shaking) incubator at 30 °C. Inspect the growth of Malassezia colonies regularly.
NOTE: Malassezia colonies on mDixon agar plates appear within 3 – 5 days and are cream-colored dull, smooth with convex elevation (Figure 1B). - Store Malassezia colonies on mDixon agar plates at RT for ~ 2 weeks. Thereafter, prepare a new mDixon agar plate by streaking Malassezia from the frozen glycerol stock as described in 1.7.
2. Preparation of the inoculum for experimental Malassezia infection of mice
- Inoculate 10 mL of liquid mDixon medium in a sterile 100 mL Erlenmeyer flask with 3 – 5 individual Malassezia colonies from a mDixon agar plate (see step 1, Figure 1B).
- Incubate the Malassezia culture for ~ 48 to 96 h at 30 °C and 180 rpm until the culture is cream-colored and turbid (Figure 1A).
NOTE: The time necessary for Malassezia growth depends on the Malassezia species and strain and the amount of fungus used for inoculation. - Transfer 2 mL of the Malassezia culture into a sterile 2 mL microcentrifuge tube and centrifuge for 1 min at 10, 000 x g.
- Discard the supernatant and wash the pellet by suspending it in 1 mL of phosphate-buffered salt solution (PBS). Centrifuge again for 1 min at 10, 000 x g.
- After the washing, suspend the pellet in 1 mL of PBS by vigorous pipetting and measure the optical density of the solution at 600 nm (ODA600) using a spectrometer. Dilute the Malassezia suspension 20 – 50 x with PBS for the OD measurement to assure that the reading is between 0.1 and 1.
NOTE: The density of a 3-day culture of Malassezia generally varies between 15 and 30 ODA600, depending on the Malassezia species and strain and on the number of yeast cells used for inoculation of the culture (step 2.1). Malassezia tends to form aggregates, therefore, vigorous pipetting is necessary to ensure homogeneity of the suspension. - Aliquot a volume of the Malassezia suspension in PBS that corresponds to a density of 4 ODA600 into a sterile 2 mL tube. Prepare 1 tube per animal to be infected.
- Centrifuge the tubes containing Malassezia for 1 min at 10, 000 x g.
- Discard the supernatant and suspend the Malassezia pellet in 200 µL of native olive oil (corresponding to 2 ODA600 yeast cells/100 µl olive oil).
NOTE: Olive oil was found to be a good vehicle for epicutaneous infection with Malassezia, as Malassezia is a lipophilic and lipid-dependent yeast. Olive oil is better absorbed by the skin than PBS. However, be aware that it is not easy to suspend Malassezia in olive oil. Improve the Malassezia/olive oil suspension by vortexing. Keep the suspension at RT until it is used for infection. - Prepare tubes with olive oil alone for mock infection of control animals.
3. Infecting mice with Malassezia
- Order female C57BL/6 mice at an age of 6 – 8 weeks and allow them to acclimatize in the experimental animal facility for at least one week. Calculate for 3 – 5 mice per group, including an uninfected control group.
- Prepare sterile anesthetic cocktail containing 1.3 mg/mL Xylazine and 6.5 mg/mL Ketamine in PBS. 5 mL of the anesthetic cocktail is enough to anesthetize 20 animals. Adjust the volume of the cocktail according to the number of animals to be anesthetized.
- Anesthetize animals by injecting 10 µL/g bodyweight of anesthetic cocktail intraperitoneally (corresponding to 65 mg Ketamine and 13 mg Xylazine per kg body weight) and place the anesthetized animals onto a heating pad at 37 °C.
NOTE: At the indicated dose, animals usually remain anesthetized for ~ 30 – 60 min.
- Anesthetize animals by injecting 10 µL/g bodyweight of anesthetic cocktail intraperitoneally (corresponding to 65 mg Ketamine and 13 mg Xylazine per kg body weight) and place the anesthetized animals onto a heating pad at 37 °C.
- Check the reflexes by pinching the rear foot with forceps to assure that the animals are fully anesthetized.
- Apply an eye cream onto the eyes to prevent dehydration during anesthesia.
- Optionally, measure the ear thickness of both ears using a caliper (0 – 5 mm range). Measure two different areas of each ear and calculate the average ear thickness per ear.
NOTE: Measuring ear thickness is optional and depends on the research question. However, if the ear thickness is used as a readout for skin inflammation, it is necessary to measure the baseline ear thickness prior to infection. (see Step 4). - Optionally, disrupt the epidermal barrier of the dorsal ear skin by mild tape stripping: manually apply a small piece of tape to the skin and remove it again. Repeat for 5 consecutive rounds using a fresh piece of tape for each round.
NOTE: Malassezia induces a more pronounced skin inflammation in barrier-disrupted skin compared to unperturbed skin (Figure 2A)7. - Topically apply 100 µL (2 ODA600) of the Malassezia/olive oil suspension onto the dorsal side of each ear using a sterile pipette. Include a control group of animals that are treated with olive oil only (vehicle-treated control group).
NOTE: Vortex the Malassezia/olive oil suspension vigorously to ensure a homogenous Malassezia suspension immediately prior to the application. - Leave the anesthetized animals on the heating pad to avoid hypothermia until they show signs of recovery (whisker movement, increased breathing rate, etc.).
- Inject 200 µL of sterile and pre-warmed 2% glucose solution subcutaneously into the nuchal fold to support their metabolism and rehydration.
NOTE: To prepare a sterile 2% glucose solution, dissolve 1 mg glucose in 50 mL PBS and filter it using a 0.2 µm filter. The solution can be stored at 4 °C. - Transfer the animals back to their cage.
4. Analysis of Malassezia-induced skin inflammation
NOTE: This procedure describes the analysis of Malassezia-induced ear swelling during infection which serves as a parameter of skin inflammation. A prerequisite for analyzing the fungus-induced ear swelling is to measure the baseline ear thickness prior to tape-stripping and/or infection (Step 3.5).
- Prepare isoflurane chamber for short term anesthesia of Malassezia-infected and control animals.
- Transfer one animal at a time to the chamber and wait for the animal to be fully anesthetized.
NOTE: Signs of proper anesthesia include complete body relaxation as well as slow and heavy (flank) breathing. Carefully monitor anesthesia as extended exposure to isoflurane can be fatal. - Remove the animal from the chamber and place it onto a tissue.
- Measure the thickness of the ear(s) using a caliper (range 0 – 5 mm). Measure two different areas of each ear and calculate the average thickness per ear (see step 3.5).
- Transfer the animal back to the cage.
NOTE: Isoflurane anesthesia is very short-lived, and the animals recover within ~ 30 s after removal from the isoflurane chamber. - Calculate the increase in ear thickness by subtracting the average baseline ear thickness, measured prior to the tape stripping and/or infection, from the average ear thickness measured at each time point after infection.
- Plot the calculated values as the increase in ear thickness or, alternatively, as the total ear thickness over time for each animal or group of animals (Figure 2B).
5. Analysis of fungal burden in the infected skin
- Prepare a sterile 2 mL microcentrifuge tube for each ear to be harvested, containing 0.5 mL of sterile 0.05% NP40 in dH2O and an autoclaved steel ball (5 mm diameter).
- Weigh the tubes using a precision balance and write down the precise weight.
- Euthanize the mice by CO2 asphyxiation.
- Remove the ear(s) at the base and transfer into the tube containing 0.5 mL of sterile 0.05% NP40 in dH2O, as described in steps 5.1 – 5.2.
- Weigh the tube containing the ear tissue and calculate the actual weight of each sample by subtracting the weight of the tube without the organ from the weight of the tube with the organ.
- Homogenize the ear tissue for 6 min at 25 Hz using a tissue homogenizer. Ensure that the tissue is well homogenized.
- Plate 100 µL of each sample (corresponding to 1/5 of each homogenate, dilution factor = 5) onto mDixon agar plates and incubate the plates upside down in a 30°C incubator.
NOTE: The amount of homogenate plated should be adjusted according to the fungal load to be expected. Make sure to plate sufficient homogenate to obtain at least 10 and no more than 250 colonies per plate to allow easy enumeration. Optionally, plate multiple plates per sample with different dilutions of homogenate. - Inspect the growth of Malassezia colonies regularly.
NOTE: Colonies usually become visible after 2 – 3 days. The time necessary for Malassezia colonies to grow depends on the species and strain of Malassezia. - Count the colonies per plate.
- Calculate the number of CFU/g tissue by using the following formula:
CFU/g tissue = (number of colonies/plate) x (dilution factor) / (weight of the skin sample in g).
NOTE: The approximate minimal detection limit can be assessed using the following formula: minimal detection limit = (1 colony/plate) x (dilution factor) / (average weight of all skin samples in g). - Fungal loads are usually plotted on a logarithmic scale (Figure 2C).
Representative Results
In vitro cultivation of Malassezia
Compared to other more commonly used fungal model pathogens such as C. albicans or A. fumigatus, Malassezia is more difficult to culture in vitro. This can be attributed to the fact that Malassezia relies on exogenous lipid sources for its nutritive requirements, due to its inability to synthesize fatty acids11. The mDixon medium is suitable for culturing several Malassezia species including M. pachydermatis, M. furfur, M. sympodialis, M. slooffiae, M. globosa and M. yamatoensis in vitro7,12. Figure 1 shows representative images for the growth of M. sympodialis in liquid mDixon medium and on mDixon agar as described in step 1 and step 2.
Analysis of skin inflammation and fungal burden of Malassezia skin inflammation
Exposure of Malassezia to the mouse ear skin that was barrier disrupted by tape-stripping prior to infection results in exacerbated inflammation of the skin, characterized by epidermal and dermal hyperplasia and development of edema7. Step 4 and 5 outline the methods for analyzing Malassezia-induced ear swelling and the fungal burden of the skin. Both parameters represent key readouts for monitoring the course of the infection. Figure 2A illustrates the increase in ear thickness that can be observed after M. furfur exposure to skin that was barrier-disrupted compared to unperturbed skin of WT C57BL/6 mice. Figure 2B shows a representative summary graph of the increase in ear thickness over time. Figure 2C displays the fungal burden in the ear skin on day 2 after infection with M. pachydermatis.
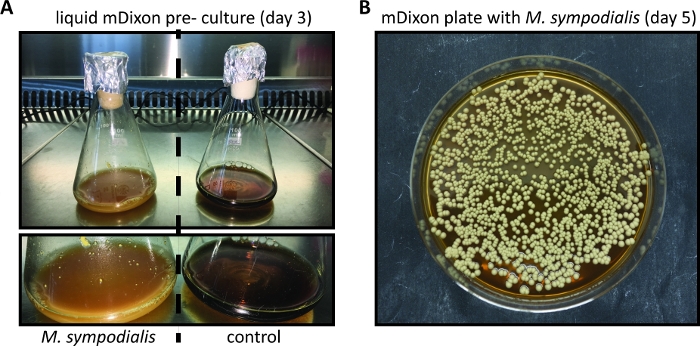
Figure 1: In vitro cultivation of Malassezia.
(A) M. sympodialis strain ATCC 42132 grown for 3 days at 30 °C and 180 rpm in liquid mDixon medium (left) next to a control Erlenmeyer flask containing mDixon medium that was not inoculated (right). (B) Colonies of M. sympodialis strain ATCC 42132 on mDixon agar after 5 days of incubation at 30 °C. Please click here to view a larger version of this figure.
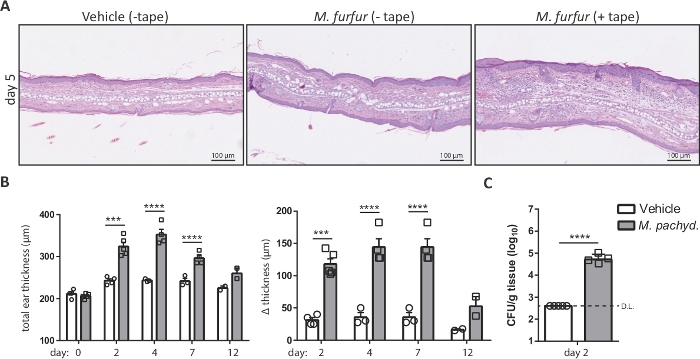
Figure 2: Analysis of Malassezia skin infection on the basis of ear thickness and fungal burden.
(A) Histology of ear sections obtained from C57BL/6 mice that were treated with olive oil (vehicle, left) or infected with M. furfur strain JPLK23 for 5 days (middle and right). On the right, the ear skin was tape-stripped prior to infection. Sections were stained with hematoxylin and eosin (H&E). (B) Summary graphs showing the increase in ear thickness over time for C57BL/6 mice that were exposed to Malassezia or left uninfected as controls. The absolute thickness of M. pachydermatis strain ATCC 14522-exposed or vehicle-treated ear skin at each time point is displayed on the left; the increase in ear thickness at the indicated time points relative to the baseline on day 0 is shown on the right. (C) Fungal burden in the skin of C57BL/6 mice that were infected with M. pachydermatis strain ATCC 14522 or treated with olive oil as a control (vehicle). In both cases, the skin was tape-stripped. Each symbol in the summary graphs B and C represents one animal. The statistical significance of the differences between groups was calculated using one-way ANOVA (B) or Student's t-test (C). ***p <0.001, ****p <0.0001, D.L.: Detection Limit Please click here to view a larger version of this figure.
Discussion
This protocol describes the infection of the skin of the commonly used inbred mouse strain C57BL/6 by Malassezia spp. Adapting this protocol to other mouse strains with a different genetic background (e.g., Balb/c) or to genetically modified mouse strains may need adjustment of the infection dose, the time point(s) of analysis, etc. To ensure reproducibility, groups of mice should always be of the same age and sex. The source of mice should be kept stable, as even slight changes in the genetic background and differences in the microbiota, which exist between vendors and may exist even between different units of a single breeding facility, can have an unpredictable impact on the course of infection. When setting up the Malassezia infection model described in this protocol, it is advised to perform a pilot study to carefully monitor the course of infection, including the extent of colonization, the kinetics of fungal clearance and the degree of inflammation and pathology that might be induced (e.g., if the ear skin is barrier-disrupted prior to infection) to determine the optimal assay conditions.
To ensure reproducibility and to reliably detect differences between experimental groups, the number of animals used per group must be calculated based on the statistical analysis. The sample size is calculated based on effect size, error rate and power, which consider biological and experimental variations (e.g., due to variation in the immune system). For ethical reasons avoid using unnecessarily high numbers of animals. Regarding Malassezia skin infection, treating only one ear with the fungus and using the other ear as a control within the same mouse, is not advised because mice may spread the fungus to both ears when grooming. However, using ½ ear for different methodological read outs such as determination of fungal burden, isolation of immune cells or histological analysis is often enough and results in a significant reduction in animal numbers used for experiments.
18 different species of Malassezia have been described up to date. Inter- and intraspecies variations within the genus Malassezia can affect the interaction with the host, as we have also learned from studies on other human pathogenic fungi13. Different Malassezia species and strains differ in their origin (e.g., M. pachydermatis is the most frequent species isolated from animals, while M. restricta, M. globosa and M. sympodialis are the most prominent members of the fungal skin microbiome in humans with variable distribution of these species between different skin areas). Some species have been associated with commensalism, while others are thought to be more pathogenic, although detailed evidence remains relatively weak. Importantly, some species and strains are inherently more difficult to grow than others. Thus, the decision of which species/strain to use for the infection must be based on the research question.
Experimental infection of the murine skin with some microbial organisms such as Candida albicans or Staphylococcus aureus require the disruption of the epidermal barrier prior to infection, e.g., with sand paper14,15,16. In contrast, the model of Malassezia infection described here is equally efficient with and without barrier disruption7. The degree of inflammation induced by the fungus is massively enhanced if the skin is tape stripped prior to infection7. Therefore, whether the skin should be manipulated before the application of Malassezia depends on the research question. Various models of chronic and acute skin inflammation (e.g., models for delayed type hypersensitivity (DTH) and contact hypersensitivity (CHS)) and models of barrier deficiency exist that may be of interest for investigating the contribution of commensal yeast to skin pathologies.
Inbred mice maintained under specific pathogen free (SPF) conditions are (to our knowledge) not naturally colonized with Malassezia. Therefore, the experimental application of Malassezia to the mouse ear skin represents a primary exposure to the fungus that induces an acute response in the host, which in turn leads to fungal clearance within 1 – 2 weeks7. While the model described in this protocol therefore only partially reflects the situation in immunocompetent humans or other host organisms that are permanently colonized with Malassezia, the experimental infection allows an ample window of opportunity to study antifungal immunity and the cellular and molecular mechanisms that underlie this response. It also allows investigating variations in the response to different Malassezia species and strains under different experimental conditions (e.g., with and without barrier disruption of the skin).
The study of Malassezia – host interactions have been limited in the past to in vitro experiments with isolated cell types in cultures (e.g., keratinocyte cell lines, PBMCs). Although these studies have shed some light on fungal and host determinants that shape the interplay between Malassezia and the host17, they do not allow to gain a comprehensive understanding of the fungus – host interaction in the complex environment of the skin, which involves multiple cell types that are in constant communication, such as keratinocytes, fibroblasts and tissue-resident immune cells, but also leukocyte populations that infiltrate the tissue only upon microbial encounter of the skin. This multicellular network cannot be fully reproduced in the in vitro models, even with most advanced organoid systems. Thus, the experimental infection of mice still represents the gold standard in immunology and infectious disease research, and the availability of the model described here represents a breakthrough in the field of Malassezia research. Importantly, this model relies on the epicutaneous application of Malassezia on the otherwise unperturbed mouse ear skin, and it does not implicate inoculation of the fungus by injection into the tissue, e.g., subcutaneously or intraperitoneally, as previous studies reported18, both of which are more distant from the situation in naturally colonized hosts.
The possibility to combine the model of Malassezia infection described in this protocol with other available mouse models greatly increases the scope and flexibility of the application. The latter include various models of specific skin disorders, such as the model of barrier deficiency that mimics important features of atopic dermatitis, a disease associated with Malassezia in both humans and dogs. Moreover, epicutaneous infection of the skin with Malassezia can easily be applied to mice with genetic defects in host genes of interest, or mice in which a cell type of interest are genetically deleted or can be pharmacologically depleted (e.g., by means of diphtheria toxin administration in diphtheria toxin receptor-expressing mice). Such models represent an inevitable tool for dissecting the host response to commensal and pathogenic microbes, including Malassezia, and to assess the role of these genes and cell type in the fungus-host interaction. The analyses of the Malassezia-host skin interaction can be expanded far beyond of what is described in this protocol. These include analyses by histology (e.g., to determine the degree of skin pathology or the epidermal thickening induced by the fungus), by immunohistochemistry or immunofluorescent staining of tissue sections using antibodies directed against cell type specific markers or other molecules of interest. It may also involve the isolation of cells (e.g., tissue resident or tissue-infiltrating leukocyte subsets) from the infected skin tissue to study the polarization, regulation, and dynamics of the immune response to Malassezia in great depth.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the University of Zürich, Switzerland.
Materials
| Agar | Sigma-Aldrich | A1296-1KG | |
| Attane Isoflurane | Piramal Healthcare | – | |
| Biosaftey cabinet (BSC) Faster Ultra Safe | DASIT GROUP | TEC 5594 | BSL2 certified |
| Centrifuge | Eppendorf | 5415D | compatible with 2ml Eppendorf tubes |
| Dessicated Ox-bile | Sigma-Aldrich | 70168-100G | |
| Eppendorf Tubes (2 ml) | Eppendorf | 0030 120.094 | |
| Glucose | Sigma-Aldrich | 49159-5KG | |
| Gylcerol (99 %) | Honeywell | 10314830 | |
| Heating pad | Eickenmeyer | 648048 | |
| Incubator Hereaus B20 | Heraeus | 412047753 | BSL2 certified |
| Ketasol (100 mg) | Graeub AG | 6680416 | |
| Magentic heating plate MR Hei-Standard | Heidolph Instruments | 442-1355 | |
| Malassezia spp. | ATCC | 14522, 14521, 42132 | |
| Malt extract | Sigma-Aldrich | 70167-500G | |
| Multiply Biosphere Tubes (200 µl) | Sarstedt AG | 7084211 | Safelock |
| Native olive oil | – | – | commerc. available |
| Nonidet P40 | Axon Lab | A1694,0250 | |
| Oditest measurment devise | Kroeplin | S0247 | range 0-5 mm |
| Oleic Acid | Sigma-Aldrich | 75090-5ML | |
| Peptone | Oxoid | LP0037 | |
| Petri dishes | Sarstedt AG | 82.1473 | |
| Phosphat buffered salt solution (PBS, 1x) | Amimed/Bioconcept | 3-05F39 | |
| Rompun (2 %) | Bayer | KP0BFHR | |
| Shaking incubator Infors Minitron | Infors | – | BSL2 certified |
| Spectrometer | Jenway | 20308 | optical density measurement at 600nm |
| Spectrometer Cuvettes | Greiner Bio-One | 613101 | |
| Stainless Steel balls (5mm) | ABF | KU.5G80 1.3541 | |
| Syringes 1 ml Sub-Q | BD Bioscience | 305501 | |
| Tissue Lyzer II | Quiagen | 85300 | |
| Transpore Hypoallergic Tape | 3M | 1527-1 | |
| Tween 40 | Sigma-Aldrich | P1504-100ML | |
| Vitamin A Retinoli Palmitas Eye Cream | BAUSCH & LOMB | commerc. available |
Riferimenti
- Iliev, I. D., Leonardi, I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nature Reviews Immunology. 17 (10), 635-646 (2017).
- Findley, K., et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 498 (7454), 367-370 (2013).
- Gemmer, C. M., DeAngelis, Y. M., Theelen, B., Boekhout, T., Dawson, T. L. Fast, noninvasive method for molecular detection and differentiation of Malassezia yeast species on human skin and application of the method to dandruff microbiology. Journal of Clinical Microbiology. 40 (9), 3350-3357 (2002).
- Theelen, B., et al. Malassezia ecology, pathophysiology, and treatment. Medical Mycology. 56, 10-25 (2018).
- Williams, M. R., Gallo, R. L. The role of the skin microbiome in atopic dermatitis. Current Allergy and Asthma Reports. 15 (11), 65 (2015).
- . . Malassezia and the Skin. , (2010).
- Sparber, F., et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe. 25 (3), 389-403 (2019).
- Koh, A. Y. Murine models of Candida gastrointestinal colonization and dissemination. Eukaryotic Cell. 12 (11), 1416-1422 (2013).
- Solis, N. V., Filler, S. G. Mouse model of oropharyngeal candidiasis. Nature Protocols. 7 (4), 637-642 (2012).
- Russel, W. M. S., Burch, R. L. . The Principles of Humane Experimental Technique. , (1959).
- Wu, G., et al. Genus-Wide Comparative Genomics of Malassezia Delineates Its Phylogeny, Physiology, and Niche Adaptation on Human Skin. PLoS Genetics. 11 (11), 1005614 (2015).
- Leong, C., Buttafuoco, A., Glatz, M., Bosshard, P. P. Antifungal Susceptibility Testing of Malassezia spp. with an Optimized Colorimetric Broth Microdilution Method. Journal of Clinical Microbiology. 55 (6), 1883-1893 (2017).
- Schonherr, F. A., et al. The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunology. 10 (5), 1335-1350 (2017).
- Igyarto, B. Z., et al. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 35 (2), 260-272 (2011).
- Liu, H., et al. Staphylococcus aureus Epicutaneous Exposure Drives Skin Inflammation via IL-36-Mediated T Cell Responses. Cell Host Microbe. 22 (5), 653-666 (2017).
- Nakagawa, S., et al. Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 22 (5), 667-677 (2017).
- Sparber, F., LeibundGut-Landmann, S. Host Responses to Malassezia spp. in the Mammalian Skin. Frontiers in Immunology. 8, 1614 (2017).
- Yamasaki, S., et al. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proceedings of the National Academy of Sciences of the United States of America. 106 (6), 1897-1902 (2009).

