Development of a Larval Zebrafish Infection Model for Clostridioides difficile
Summary
Presented here is a safe and effective method to infect zebrafish larvae with fluorescently labeled anaerobic C. difficile by microinjection and noninvasive microgavage.
Abstract
Clostridioides difficile infection (CDI) is considered to be one of the most common healthcare-associated gastrointestinal infections in the United States. The innate immune response against C. difficile has been described, but the exact roles of neutrophils and macrophages in CDI are less understood. In the current study, Danio rerio (zebrafish) larvae are used to establish a C. difficile infection model for imaging the behavior and cooperation of these innate immune cells in vivo. To monitor C. difficile, a labeling protocol using a fluorescent dye has been established. A localized infection is achieved by microinjecting labeled C. difficile, which actively grows in the zebrafish intestinal tract and mimics the intestinal epithelial damage in CDI. However, this direct infection protocol is invasive and causes microscopic wounds, which can affect experimental results. Hence, a more noninvasive microgavage protocol is described here. The method involves delivery of C. difficile cells directly into the intestine of zebrafish larvae by intubation through the open mouth. This infection method closely mimics the natural infection route of C. difficile.
Introduction
C. difficile is a gram-positive, spore-forming, anaerobic, and toxin-producing bacillus that is the leading cause of severe infections in the gastrointestinal tract1. Typical symptoms of CDI include diarrhea, abdominal pain, and fatal pseudomembranous colitis, and it can sometimes lead to death1,2. Evidence has shown that host immune responses play a critical role in both the progression and outcome of this disease3. In addition to the immune response, the indigenous gut microbiota is crucial for the onset and pathogenesis of CDI4. In the past decade, both the number of cases and the mortality rate of CDI have increased significantly due to the emergence of a hypervirulent strain of C. difficile (BI/NAP1/027)5,6. A better understanding of underlying immune mechanisms and the role of microbiota during CDI will help lead to new therapeutic developments and advances, enabling better control of this epidemic.
Several animal models, such as the hamster and mouse, have been developed to provide insight into the immune defense against C. difficile7,8. However, the role of innate immune cells is still poorly understood, particularly since innate immune cell behavior is mainly derived from histological analysis or cultured cells in vitro. Therefore, establishing a transparent zebrafish model to reveal the innate immune response to C. difficile inside of a living vertebrate organism will facilitate such studies. Zebrafish larvae have a functional innate immune system, but they lack the adaptive immune system until 4–6 weeks after fertilization9. This unique feature makes zebrafish larvae an excellent model to study the isolated response and function of innate immune cells in CDI.
This report describes new methods using zebrafish larvae to study the interactions between C. difficile and innate immune cells, such as macrophages and neutrophils. First, a localized microinjection protocol that includes C. difficile inoculum and staining is presented. Using in vivo confocal time-lapse imaging, the response of neutrophils and macrophages towards the infection site is recorded, and the phagocytosis of bacteria by neutrophils and macrophages is observed. However, it has been reported that the injection itself causes tissue damage and leads to the recruitment of leukocytes independent of the bacteria10. Therefore, a noninvasive microgavage protocol to deliver C. difficile into the intestine of zebrafish larvae is subsequently described. Previous studies have demonstrated that indigenous gastrointestinal microbiota protect a host against the colonization of C. difficile11. Therefore, gnotobiotic zebrafish larvae are also used to predispose the zebrafish that are infected 12. Afterwards, intestinal dissection is performed to recover viable C. difficile and validate the duration of their presence in zebrafish intestinal tracts.
Protocol
All animal work described here was performed in accordance with legal regulations (EU-Directive 2010/63, license AZ 325.1.53/56.1-TUBS and license AZ 33.9-42502-04-14/1418).
1. Preparation of Low Melting Agarose, Gel Plate, and Microinjection/Microgavage Needles
- Dissolve 0.08 g of low melting agarose (Table of Materials, agarose A2576) in 10 mL of 30% Danieau's medium (0.12 mM MgSO4, 0.18 mM Ca [NO3]2), 0.21 mM KCl, 1.5 mM HEPES (pH = 7.2), and 17.4 mM NaCl, stored at room temperature (RT) to obtain a 0.8% solution.
NOTE: Higher or lower concentrations of agarose can be used. However, the required time to solidify varies for different brands of agarose, even at the same concentration. - Prepare microinjection and microgavage needles from glass capillaries (Table of Materials).
- Use a micropipette puller with the following settings (note that units are specific to the puller used here; see Table of Materials): microinjection needles (air pressure = 500; heat = 400; pull = 125; velocity = 75; time = 150); and microgavage needles (air pressure = 500; heat = 400; pull = 100; velocity = 75; time = 150). Use a microloader tip to load 3 µL of nuclease-free H2O into the pulled needle.
- Introduce the needle into the injector and fasten it properly. Adjust the needle to a suitable angle for injection. Set the injection pressure between 600–900 hPa for microinjection needle and 200–300 hPa for gavage needle.
- Place a drop of mineral oil onto the black circle of the calibration slide. Use fine forceps to clip the tip of the needle. Inject one drop into the mineral oil to measure the size of the droplet.
- For microinjection, adjust the injection time to obtain a droplet with a diameter of 0.10–0.12 mm, which equals a volume of 0.5–1.0 nL. For microgavage, obtain a droplet with a diameter of 0.18–0.20 mm, which equals a volume of 3–5 nL.
- Prepare a 1.5 % agarose plate with agarose (Table of Materials, 8050) in a 10 cm Petri dish in 30% Danieau's medium using a plastic mold as the microgavage mold. Store at 4 °C to prevent desiccation until needed. Warm to RT or 28 °C prior to the experiment.
2. Preparation and Labeling of C. difficile and Spores with Fluorescent Dye
- Prepare a 1 mM stock solution of a fluorescent dye (Table of Materials). Because the dye is sold in 50 µg aliquots of powder, add 69 µL of DMSO to the vial to obtain a 1 mM stock concentration.
- Prepare a 100 µM working solution of the fluorescent dye by adding 2 µL of 1 mM stock solution to 18 µL of DMSO in a centrifuge tube, and mix well.
- Culture C. difficile (R20291, a ribotype 027 strain) by inoculating 10 mL of BHIS liquid medium with two to three colonies from a plate in an anaerobic hood without shaking overnight. BHIS is BHI supplemented with 0.5% (w/v) yeast extract and 0.1% (w/v) L-cysteine. Dissolve 1 g of L-cysteine in 10 mL of ddH2O and sterilize by filtration, then add to the autoclaved medium to obtain a final concentration of 1 g/L. Plates are prepared by adding 15 g/L agar-agar to the medium before autoclaving. Selective culturing of C. difficile is done by using chromID C. difficile plates (Table of Materials).
- Staining C. difficile with the fluorescent dye
- Harvest C. difficile at an OD600 of 1.0–1.2 and wash 1x with 1 mL of 1x PBS (5,000 x g for 3 min at RT). Resuspend in 1 mL of 1 x PBS.
- Add 3 µL of working solution of the fluorescent dye into 1 mL of bacteria suspension. Incubate the sample for 15 min at RT in the dark. Wash the stained C. difficile once with 1 mL PBS to remove residual dye and resuspend in 1x PBS to an OD600 of 1.0 (1.0 OD600 is approximately equivalent to 108 cfu/mL).
3. Injection of Stained C. difficile into Zebrafish Larvae
- Anesthetize 20–30 zebrafish larvae at 5 days post-fertilization (referred to here as 5 dpf) with 0.02%–0.04% tricaine (tricaine powder is dissolved in double-distilled water and adjusted to pH = 7 with 1 M Tris-HCL solution) in 30% Danieau's's medium ~10 min before injection. Transfer the anesthetized larvae to a fresh 10 cm Petri dish and remove any excess 30% Danieau's's medium.
- Place a drop of 0.8% low melting agarose onto the zebrafish larvae to cover. Gently adjust the larvae to a lateral position. Place the Petri dish on ice for 30–60 s to allow the low melting agarose to solidify. Add 30% Danieau's medium containing 0.02%–0.04% tricaine to cover the agarose.
- Prepare the injection solution. Add 1 µL of 0.5% phenol red in PBS solution into 9 µL of the dye-stained C. difficile inoculum to visualize the injection process.
- Load a calibrated microinjection needle with the injection solution using a microloader. Mount the loaded needle onto a micromanipulator and position it under a stereomicroscope.
- Adjust the injection pressure between 600–900 hPa. Set the injection time to 0.1–0.3 s to obtain 0.5–1.0 nL. Set the needle in the micromanipulator at a ~45° angle pointing toward the embedded larvae.
- Place the needle tip above the gastrointestinal tract close to the urogenital pore. Pierce through agarose then the muscle with the needle tip, then insert it into the intestinal lumen and inject 0.5–1.0 nL of C. difficile. Use a fluorescence microscope to monitor the injected larvae and pick up the properly injected larvae for confocal imaging.
4. Generation of Gnotobiotic Zebrafish Larvae
- Use the well-established natural breeding method to generate gnotobiotic zebrafish embryos, including: in vitro fertilization, washing with antibiotic-containing medium (1 µg/mL amphotericin B, 10 µg/mL kanamycin, and 20 µg/mL ampicillin), washing with 0.1% wt/vol polyvinyl pyrrolidone-iodine (PVP-I) solution, and incubation of the embryos in a cell culture hood12.
- Maintain all gnotobiotic zebrafish larvae under gnotobiotic conditions until 5 dpf or just before the gavage. After the gavage, zebrafish larvae will be transferred into a standard incubator but with sterile 30% Danieau's medium.
5. Gavage of Zebrafish Larvae
- Calibrate the microgavage needle as described in step 1.2.
- Measure the diameter of the tip of the needle by placing the needle on a calibration slide with one drop of mineral oil. Ensure that the tip is 30–40 µm in diameter, blunt, and smooth. Discard the sharp or rough needles.
NOTE: Sharp edges of needles can be blunted by quick flaming. - Prepare the gavage solution as described in step 3.3.
- Load and mount the needle onto a micromanipulator as described in step 3.4. Adjust the micromanipulator to position the needle at a 45° angle.
- Anesthetize the zebrafish larvae referred to in step 3.1. When the larvae stop moving, transfer them to the groove of a microgavage mold using a Pasteur pipette.
- Place a drop of 0.8% low melting agarose onto the zebrafish larvae to cover. Gently adjust the larvae with heads facing upright at 45° angles in the groove and tails against the wall of the groove. Ensure that the angles of the heads are approximately the same so that they are aligned with the angle of the gavage needles. Place the microgavage mold on ice for 30–60 s, allowing the low melting agarose to solidify in order to stabilize the positions of the larvae.
- Adjust the injection pressure between 200–300 hPa. Set the injection time to 0.1–0.3 s to obtain an injection volume of 3–5 nL of C. difficile.
- Gently operate the needle through the agarose then into the mouth of zebrafish larvae, through the esophagus. Once the tip of the needle is inside the anterior intestinal bulb, press the injection pedal to release the bacteria. Fill the lumen of the intestine with the delivered volume. Do not let it overflow from the esophagus or cloaca. Gently withdraw the needle from the mouth of the zebrafish.
- Following gavage, rescue the infected zebrafish larvae from the agarose with a flexible microloader tip by first cutting the agarose away then by lifting the larvae. Transfer these larvae into sterile 30% Danieau's medium. Rinse the larvae in sterile medium twice. Transfer the larvae to a fresh 10 cm Petri dish. The larvae will be maintained for up to 11 dpf.
6. Confocal Microscopy Analysis of Injected Zebrafish Larvae
- Anesthetize zebrafish larvae referred to step 3.1. Make a hole in the bottom of a 35 mm Petri dish with a glass slide attached to the hole, referred as the imaging chamber. Transfer embryos to the bottom of the imaging chamber with an adequate amount of 30% Danieau's medium.
- Add 200–300 μL of 1% low melting agarose to cover the anesthetized larvae. Since an inverted confocal microscope is used, place the infected region of the larvae against the glass slide as closely as possible.
- Let the agarose solidify on ice for 30–60 s. Submerge the agarose with 30% Danieau's containing 0.02%–0.04% tricaine.
- Image the larvae with a confocal laser scanning microscope (Table of Materials).
7. Dissection of Larval Zebrafish Intestine to Recover Viable C. difficile
- Isolate gastrointestinal tracts from larvae to analyze bacterial load. Start by euthanizing zebrafish larvae with 0.4 % tricaine.
- Rinse the zebrafish briefly with sterile 1x PBS and transfer them to a fresh agarose plate.
- Dissection of zebrafish
- Insert a needle into the dorsal trunk of zebrafish larvae close to the head to immobilize the zebrafish. Remove the head behind the gills with a lancet.
- Insert the second needle into the middle of the dorsal trunk. Insert the third needle into the abdomen of the zebrafish and pull the intestine out of the body cavity.
NOTE: Extreme care is needed to isolate the intact intestine. If it is difficult to do so, perform additional pulls to separate the rest of the intestine from the remaining internal organs. - Use a microinjection needle to transfer 10–15 intestines into a 1.5 mL tube containing 200 µL sterile of 1x PBS.
- Homogenize the intestines with a pestle to disrupt the tissue and prepare homogenates. Ensure the pestle reaches the bottom of the tube to disrupt all intestines completely.
- Incubate the homogenates in C. difficile rearing medium containing D-cycloserine and cefoxitin, with or without taurocholate (TCA, a germinant of C. difficile spores) in an anaerobic chamber.
- Incubate the plate anaerobically for 48 h at 37 °C.
- Use bacterial culture for 16S rRNA-PCR.
- Resuspend a colony in 50 µL of H2O and boil it at 95 °C for 15 min. Pellet the lysed debris by centrifugation (14,000 rpm for 2 min at RT) and use 2 µL of the supernatant as a template in 25 µL of PCR-reaction using C. difficile-specific primers (Cdiff16Sfw: 5' GTG AGC CAG TAC AGG 3'; Cdiff16Srev: 5' TTA AGG AGA TGT CAT TGG 3').
- When bacteria from liquid culture are used, harvest 1 mL of culture and wash once with 1 mL of PBS (14,000 rpm for 2 min at RT). Resuspend the pellet in 100 µL of H2Odd and treat as done above. To further characterize bacterial colonies, streak on a BHIS- or chromID-plate (Table of Materials).
Representative Results
C. difficile is strictly anaerobic, but the chromophore of fluorescent proteins usually requires oxygen to mature. To overcome this problem, a fluorescent dye was used to stain live C. difficile cells that were actively growing (R20291, a ribotype 027 strain; Figure 1A). Using the Gal4/UAS system, stable transgenic zebrafish lines were generated for live imaging, in which the mpeg1.1 or lyZ promoters drove the expression of EGFP fluorescent protein in macrophages and neutrophils (respectively) in a Gal4-dependent manner.
The stained C. difficile was injected into the zebrafish intestinal tract at 5 dpf, and the infected sites were imaged after 1 h of incubation. Time-lapse imaging showed that both neutrophils and macrophages reached the infection sites (Figure 1B), and the number of these two innate immune cells increased until the C. difficile was cleared. Clearing occurred by phagocytosis and digestion of the labeled C. difficile. Figure 1C shows that an activated macrophage phagocytized two C. difficile bacteria (see Supplementary Movie 1).
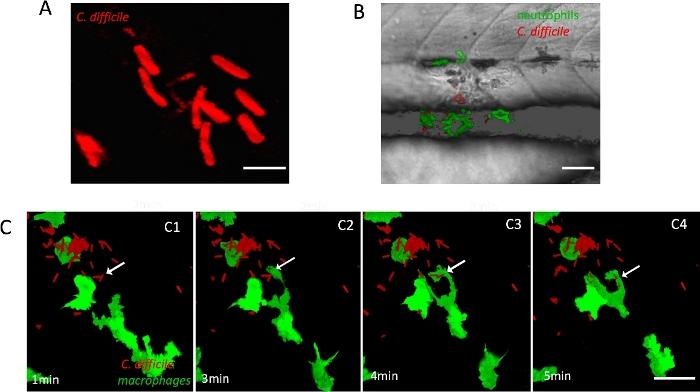
Figure 1: Staining and infection of C. difficile in zebrafish. (A) Fluorescently labeled C. difficile bacteria within infected zebrafish after microinjection. Scale bar = 5 µm. (B) Confocal Z-stack projection showing green fluorescent neutrophils in double transgenic zebrafish Tg(lyZ: KalTA4)bz17/Tg(4xUAS-E1b:EGFP)hzm3 accumulated at the C. difficile infection site at 2 h post-infection (hpi). Neutrophils are shown in green and C. difficile in red. Scale bar = 50 µm. (C) Confocal time-lapse imaging showing GFP-labelled macrophages phagocytosing red fluorescent stained C. difficile. Scale bar = 20 µm. Please click here to view a larger version of this figure.
Although microinjection is the most common method to infect zebrafish larvae with pathogens, this method invariably causes tissue damage, which can influence experimental results. To avoid this issue, microgavage was used to deliver fluorescence-labeled C. difficile into the intestinal lumen of macrophage and neutrophil reporter lines at 5 dpf, which mimics the natural path of CDI (Figure 2A). However, neutrophils and macrophages did not show obvious migration to the gastrointestinal tract for up to 12 h after microgavage (Figure 2B). In the meantime, fluorescence of the labeled C. difficile disappeared after around 5 h post-microgavage, likely due to either 1) enzymatic destruction, 2) inappropriate pH levels in the intestine of zebrafish, or 3) onset of spore formation and accompanying membraneous changes in the bacteria (Figure 2B). Therefore, an intestinal dissection method was established to detect C. difficile.
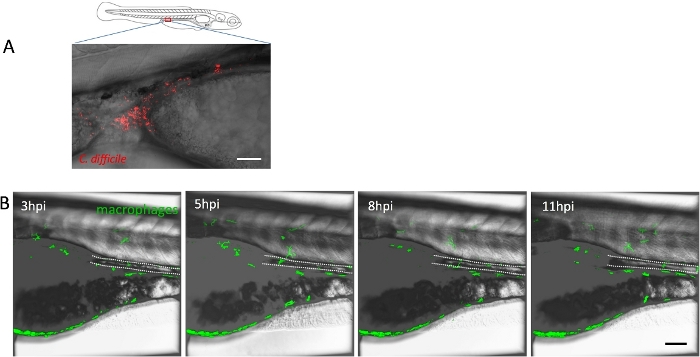
Figure 2: Microgavage of zebrafish larvae with C. difficile. (A) Representative image of zebrafish larvae at 5 dpf gavaged with fluorescence-stained C. difficile. The image was recorded around 3 h post-gavage, showing that C. difficile were present in the posterior intestine of zebrafish. Scale bar = 100 µm. (B) Confocal time-lapse imaging demonstrating macrophage motility. Double transgenic zebrafish larvae Tg(mpeg1.1: KalTA4)bz16/Tg(4xUAS-E1b:EGFP)hzm3 at 5 dpf were gavaged with fluorescence-labeled C. difficile. Confocal time-lapse imaging was performed for up to 12 h. Scale bar = 200 µm. Please click here to view a larger version of this figure.
To confirm whether C. difficile was able to inhabit zebrafish, zebrafish intestines were separated at various timepoints after the gavage. Then, the isolated intestines were homogenized with plastic pestles, and homogenates were incubated in C. difficile medium containing D-cycloserine and cefoxitin, with or without TCA. However, actively growing C. difficile cells were only detected up to 24 h post-infection both with and without TCA (data not shown). It is speculated that the indigenous microbial communities in conventional larvae prevented C. difficile invasion because of their colonization resistance.
Gnotobiotic zebrafish larvae were also used. Intestinal samples 24 hpi were dissected and showed bacterial growth in medium with TCA or without TCA, while no bacteria grew in the control group (Figure 3A). However, at later timepoints (i.e., 48 hpi, 72 hpi, and 120 hpi) incubated samples only grew in the medium containing TCA, which suggested that total C. difficile in the gut had formed spores (Figure 3B). This provides a possible explanation for the loss of fluorescence labeling.
16S rDNA PCR was then used to identify the grown bacteria as C. difficile, which produced specific PCR amplicons of predictable size (~800 bp; Figure 3C). This result was further verified by sequencing of these PCR products. Afterwards, bacterial cultures were spread on a BHIS plate. Several single colonies were then transferred onto a chromID-plate, which supported only C. difficile growth. The bacteria appeared as typical black colonies, which further indicated that bacteria from the zebrafish intestines were C. difficile (Figure 3D).
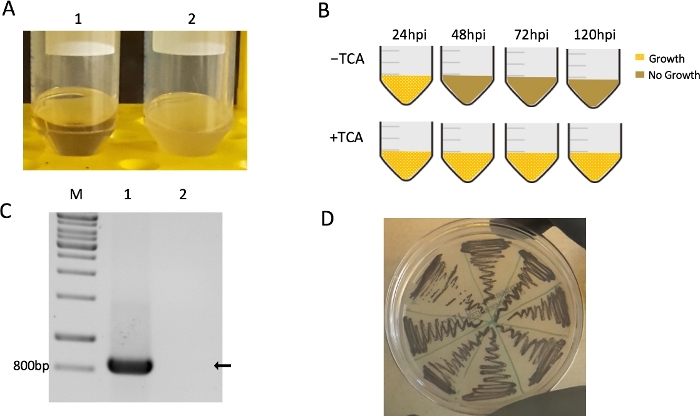
Figure 3: Detection of C. difficile in zebrafish intestine after infection. For each of the independent experiments, 15–20 gnotobiotic zebrafish larvae at 5 dpf were infected with 3–5 nL of 108 CFUs/mL C. difficile or PBS as a negative control by microgavage. (A) The homogenates of dissected intestines were cultured. Bacteria grew in the medium containing TCA 72 h after infection of gnotobiotic zebrafish larvae (1, non-infected control group; 2, R20291-infected zebrafish; n = 3). (B) Schematic illustration of overall experiment results after 24 h, 48 h, 72 h, and 120 h post-infection with C. difficile (n = 3) (C) Bacterial samples were tested by 16S rDNA PCR at 72 h post-microgavage (1, non-infected control group; 2, R20291-infected zebrafish; n = 3). (D) The growth of C. difficile on chromID-plate; note the black color of C. difficile, which is a feature of these plates (n = 3). Please click here to view a larger version of this figure.
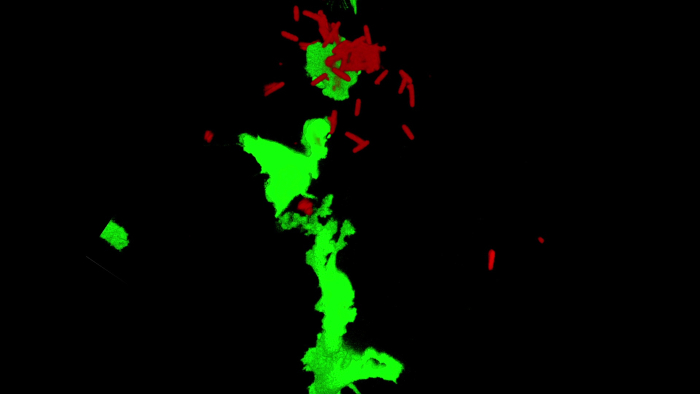
Supplementary Movie 1. Please click here to view this file (Right click to download).
Discussion
The presented methods modify and extend an existing approach to infect zebrafish larvae by performing both injection and microgavage10,14. It also demonstrates an approach to study anaerobic pathogens with zebrafish larvae22. In addition, the protocol facilitates the analysis of innate immune cell responses in vivo upon CDI and upon colonization of C. difficile in zebrafish. The method is reproducible and easy to conduct in a routine laboratory or clinical environment.
To monitor the phagocytosis of C. difficile by leukocytes, two stable transgenic zebrafish lines were used (e.g., Tg[mpeg1.1: KalTA4]bz16) to visualize macrophages (KalTA4: a zebrafish-optimized Gal4-variant) and Tg(lyz: KalTA4)bz17 and visualize neutrophils when crossed with the transgenic background of Tg(4xUAS: EGFP)hzm3 carriers. Due to the anaerobic environment that is required for the growth of C. difficile, genetic tools to trace C. difficile are limited. Although a codon-optimized mCherryOpt has been reported to label them, C. difficile bacteria must be fixed before exhibiting fluorescence prior to its use in live imaging settings13. Therefore, a fluorescent dye was used here to stain live C. difficile with red fluorescence, which can be combined with the numerous available green fluorescent transgenic zebrafish strains. This method can easily be applied to other intestinal anaerobic bacteria, such as Bacteroides fragilis and Helicobacter hepaticus. The representative results demonstrate that both neutrophils and macrophages can recognize and phagocytose C. difficile in infected zebrafish.
Both immersion and microinjection methods have been regularly used to infect zebrafish20,21,22. Using immersion methods is straightforward, yet this approach makes it difficult to accurately control the invasion time of bacteria into the intestine. Microinjection is the most common method to infect zebrafish embryos with pathogens, but it invariably causes tissue damage. Hence, microgavage was used to mimic the natural infection route.
However, it was found that dye-stained C. difficile became undetectable around 5 h post-microgavage. The reasons for this are currently unclear but may be related to either the 1) intolerance of the fluorophore under intestinal conditions or 2) initiation of spore formation of C. difficile cells. It was further found that C. difficile were not detectable in the culture of whole-larvae homogenates. Therefore, the gut of each infected zebrafish was dissected then cultured to determine whether C. difficile still inhabited the intestinal tissue of zebrafish.
Because of the indigenous microbiota of conventional mice, only mice pretreated with an antibiotic cocktail or gnotobiotic mice are susceptible to C. difficile16. Likewise, it was found that C. difficile was only detected in the intestines of gnotobiotic zebrafish but not in conventional wild-type zebrafish 24 h post-infection. Interestingly, intestinal samples of 48 h, 72 h, and 120 h post-infection zebrafish only grew in media containing TCA. As described above, TCA stimulates C. difficile spore germination in vitro. This result suggests that active C. difficile cells already formed vegetative spores in the zebrafish intestine, and spore-derived colonies were detected using the microgavage approach.
Intriguingly, germ-free zebrafish still did not present any symptoms of CDI, such as intestinal neutrophil influx or even death of zebrafish. This shows that infection by injection may activate innate immune cells by wounding and that only activated macrophages and neutrophils are able to quickly detect C. difficile. In addition, a likely explanation for the lack of prominent CDI in zebrafish is based on the structural differences between zebrafish and mammalian intestines17. The zebrafish gut lacks intestinal crypts, where C. difficile are frequently located18. Along with the lower maintenance temperature of zebrafish compared to that in mammals, it was inferred that the onset of CDI in zebrafish does not occur as fast as in mammalian models. However, two anaerobic bacteria of the human microbiota, Lactobacillus paracasei and Eubacterium limosum, have been proven to grow inside the zebrafish gut19. The technical advances presented here will encourage applications of this method to studying C. difficile and other bacteria or pathogens derived from the mammalian gut in molecularly tractable zebrafish larvae in vivo.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We are grateful to Timo Fritsch for excellent animal care. We thank the members of the Köster and Steinert labs for support and helpful discussions. We thank Dr. Dandan Han for critical reading the manuscript. We gratefully acknowledge funding by the Federal State of Lower Saxony, Niedersächsisches Vorab (VWZN2889).
Materials
| Agarose | Sigma-Aldrich | A2576 | Ultra-low gelling agarose |
| Agarose low-melting (LM) | Pronadisa | 8050 | It is used in agarose plates |
| BacLight Red Bacterial Stain | Thermo Fisher Scientific | B35001 | Fluorescent dye |
| Brain-Heart-Infusion Broth | Carl Roth GmbH | X916.1 | |
| Brass (wild-type) | deficient in melanin synthesis, used to generate stable transgenic lines | ||
| Calcium nitrate (Ca(NO3)2) | Sigma-Aldrich | C1396 | |
| Capillary Glass | Harvard Apparatus | 30-0019 | Injection needles |
| Clostridioides difficile | R20291,, a ribotype 027 strain, TcdA+/TcdB+/CDT+ production | ||
| DMSO | Carl Roth GmbH | A994 | |
| FIJI | open-source platform | Image processing | |
| HEPES | Carl Roth GmbH | 6763 | |
| Horizontal needle puller | Sutter instrument Inc | P-87 | |
| L-cysteine | Sigma-Aldrich | 168149 | |
| Leica Application Suite X (LAS X) | Leica | Image processing | |
| Magnesium sulfate (MgSO4) | Carl Roth GmbH | P026 | |
| Micro injector | eppendorf | 5253000017 | |
| Microinjection molds | Adaptive Science Tools | TU1 | |
| Leica SP8 confocal microscope | Leica | ||
| Phenol Red | Sigma-Aldrich | P0290 | |
| Potassium chloride (KCl) | Carl Roth GmbH | 5346 | |
| Sodium chloride (NaCl) | Carl Roth GmbH | 9265 | |
| Taurocholate | Carl Roth GmbH | 8149 | |
| Tg(lyZ: KalTA4)bz17/Tg(4xUAS-E1b:EGFP)hzm3 | stable transgenic line in which in which the lyZ promoters drive the expression of EGFP fluorescent protein in neutrophils | ||
| Tg(mpeg1.1: KalTA4)bz16/Tg(4xUAS-E1b:EGFP)hzm3 | stable transgenic line in which in which the mpeg1.1 drive the expression of EGFP fluorescent protein in macrophages | ||
| Tricaine | Sigma-Aldrich | E10521 | |
| Yeast extract | BD Bacto | 212750 |
Riferimenti
- Rupnik, M., Wilcox, M. H., Gerding, D. N. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nature Reviews Microbiology. 7, 526-536 (2009).
- Yang, Z., et al. Mechanisms of protection against Clostridium difficile infection by the monoclonal antitoxin antibodies actoxumab and bezlotoxumab. Infection and Immunity. 83, 822-831 (2015).
- Kelly, C. P., Kyne, L. The host immune response to Clostridium difficile. Journal of Medical Microbiology. 60, 1070-1079 (2011).
- Britton, R. A., Young, V. B. Interaction between the intestinal microbiota and host in Clostridium difficile colonization resistance. Trends in Microbiology. 20, 313-319 (2012).
- Goorhuis, A., et al. Emergence of Clostridium difficile Infection Due to a New Hypervirulent Strain, Polymerase Chain Reaction Ribotype 078. Clinical Infectious Diseases. 47, 1162-1170 (2008).
- Pépin, J., et al. Emergence of fluoroquinolones as the predominant risk factor for Clostridium difficile-associated diarrhea: a cohort study during an epidemic in Quebec. Clinical Infectious Diseases. 41, 1254-1260 (2005).
- Merrigan, M. M., Sambol, S. P., Johnson, S., Gerding, D. N. Prevention of Fatal Clostridium difficile -Associated Disease during Continuous Administration of Clindamycin in Hamsters. The Journal of Infectious Diseases. 188, 1922-1927 (2003).
- Chen, X., et al. A Mouse Model of Clostridium difficile-Associated Disease. Gastroenterology. 135, 1984-1992 (2008).
- Page, D. M., et al. An evolutionarily conserved program of B-cell development and activation in zebrafish. Blood. 122, 1-12 (2014).
- Benard, E. L., et al. Infection of Zebrafish Embryos with Intracellular Bacterial Pathogens. Journal of Visualized Experiments. (61), e3781 (2012).
- Theriot, C. M., Young, V. B. Interactions Between the Gastrointestinal Microbiome and Clostridium difficile. Annual Review of Microbiology. 69, 445-461 (2015).
- Pham, L. N., Kanther, M., Semova, I., Rawls, J. F. Methods for generating and colonizing gnotobiotic zebrafish. Nature Protocols. 3, 1862-1875 (2008).
- Ransom, E. M., Ellermeier, C. D., Weiss, D. S. Use of mCherry red fluorescent protein for studies of protein localization and gene expression in Clostridium difficile. Applied and Environmental Microbiology. 81 (5), 1652-1660 (2015).
- Cocchiaro, J. L., Rawls, J. F. Microgavage of Zebrafish Larvae. Journal of Visualized Experiments. (72), e4434 (2013).
- Chen, X., et al. A Mouse Model of Clostridium difficile-Associated Disease. Gastroenterology. 135 (6), 1984-1992 (2008).
- Hutton, M. L., Mackin, K. E., Chakravorty, A., Lyras, D. Small animal models for the study of Clostridium difficile disease pathogenesis. FEMS Microbiology Letters. 352, 140-149 (2014).
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Developmental & Comparative Immunology. 64, 82-92 (2016).
- Goulding, D., et al. Distinctive profiles of infection and pathology in hamsters infected with Clostridium difficile strains 630 and B1. Infection and Immunity. 77, 5478-5485 (2009).
- Toh, M. C., et al. Colonizing the Embryonic Zebrafish Gut with Anaerobic Bacteria Derived from the Human Gastrointestinal Tract. Zebrafish. 10, 194-198 (2013).
- Bloemberg, G. V., et al. Comparison of static immersion and intravenous injection systems for exposure of zebrafish embryos to the natural pathogen Edwardsiella tarda. BMC Immunology. 12, 58 (2011).
- Díaz-Pascual, F., Ortíz-Severín, J., Varas, M. A., Allende, M. L., Chávez, F. P. In vivo host-pathogen interaction as revealed by global proteomic profiling of zebrafish larvae. Frontiers in Cellular and Infection Microbiology. 7, 1-11 (2017).
- Valenzuela, M. J., et al. Evaluating the capacity of human gut microorganisms to colonize the zebrafish larvae (Danio rerio). Frontiers in Microbiology. 9, (2018).

