Delivery of Modified mRNA in a Myocardial Infarction Mouse Model
Summary
This protocol presents a simple and coherent way to transiently upregulate a gene of interest using modRNA after myocardial infarction in mice.
Abstract
Myocardial infarction (MI) is a leading cause of morbidity and mortality in the Western world. In the past decade, gene therapy has become a promising treatment option for heart disease, owing to its efficiency and exceptional therapeutic effects. In an effort to repair the damaged tissue post-MI, various studies have employed DNA-based or viral gene therapy but have faced considerable hurdles due to the poor and uncontrolled expression of the delivered genes, edema, arrhythmia, and cardiac hypertrophy. Synthetic modified mRNA (modRNA) presents a novel gene therapy approach that offers high, transient, safe, nonimmunogenic, and controlled mRNA delivery to the heart tissue without any risk of genomic integration. Due to these remarkable characteristics combined with its bell-shaped pharmacokinetics in the heart, modRNA has become an attractive approach for the treatment of heart disease. However, to increase its effectiveness in vivo, a consistent and reliable delivery method needs to be followed. Hence, to maximize modRNA delivery efficiency and yield consistency in modRNA use for in vivo applications, an optimized method of preparation and delivery of modRNA intracardiac injection in a mouse MI model is presented. This protocol will make modRNA delivery more accessible for basic and translational research.
Introduction
Gene therapy is a powerful tool involving delivery of nucleic acids for the treatment, cure, or prevention of human diseases. Despite the progress in the diagnostic and therapeutic approaches for heart disease, there has been limited success in the delivery of genes in myocardial infarction (MI) and heart failure (HF). As straightforward as the process of gene therapy seems, it is a markedly complex approach considering the many factors that need to be optimized before employing a particular delivery vehicle. The correct delivery vector should be non-immunogenic, efficient, and stable inside the human body. Efforts in this field have generated two types of delivery systems: viral or non-viral. The widely used viral systems, including gene transfer by adenovirus, retrovirus, lentivirus, or adeno-associated virus, have shown exceptional transduction capacity. However, their use in clinics is limited due to the strong immune response induced1, risk of tumorigenesis2, or the presence of neutralizing antibodies3, all of which remain a major obstacle to broad and effective application of viral vectors in human gene therapy. On the other hand, despite their impressive expression pattern, the delivery of naked plasmid DNA displays a low transfection efficiency, while mRNA transfer presents high immunogenicity and susceptibility to degradation by RNase4.
With the extensive research in the field of mRNA, modRNA has become an attractive tool for delivery of genes to the heart and various other organs due to its numerous advantages over traditional vectors5. Complete replacement of uridine with naturally occurring pseudouridine results in more robust and transient protein expression, with minimal induction of innate immune response and risk of genomic integration6. Recently established protocols use an optimized amount of anti-reverse cap analog (ARCA) that further enhances the protein translation by increasing the stability and transability of the synthetic mRNA7.
Previous reports have shown the expression of various reporter or functional genes delivered by modRNA in the rodent myocardium after MI. With modRNA applications, significant areas of the myocardium, including both cardiomyocytes and noncardiomyocytes, have been successfully transfected post-cardiac injury8 to induce angiogenesis9,10, cardiac cell survival11, and cardiomyocyte proliferation12. A single administration of modRNA encoded for mutated human follistatin-like 1 induces the proliferation of mouse adult CMs and significantly increases cardiac function, decreases scar size, and increases capillary density 4 weeks post-MI12. A more recent study reported improved cardiac function after MI with application of VEGFA modRNA in a swine model10.
Thus, with the increased popularity of modRNA in the cardiac field, it is essential to develop and optimize a protocol for the delivery of modRNA to the heart post-MI. Herein is a protocol describing the preparation and delivery of purified and optimized modRNA in a biocompatible citrate-saline formulation that provides robust, stable protein expression without stimulating any immune response. The method shown in this protocol and video demonstrates the standard surgical procedure of a mouse MI by permanent ligation of the left anterior descending artery (LAD), followed by three site intracardiac injections of modRNA. The aim for this paper is to clearly define a highly accurate and reproducible method of modRNA delivery to the murine myocardium to make modRNA application widely accessible for cardiac gene therapy.
Protocol
All animal procedures outlined here have been approved by Icahn School of Medicine at Mount Sinai Institutional Care and Use Committee.
1. Synthesis of modRNA
NOTE: The details of modRNA synthesis can be found in Kondrat et al.13.
- Order the plasmid templates (Table of Materials) and generate a clean PCR product to use as the mRNA template.
- Prepare the modRNAs by transcription in vitro with a customized ribonucleoside blend of the following (Table of Materials): T7 transcription kit, 6 mM anti-reverse cap analog (ARCA) 30-O-Me-m7G(50) ppp(50)G, 75 mM guanosine triphosphate, 75 mM adenosine triphosphate, 75 mM cytidine triphosphate, 100 mM pseudouridine-5-triphosphate, and T7 DNase enzyme.
- Purify the modRNA made in step 1.2 with a transcription cleanup kit (Table of Materials) and treat with antarctic phosphatase. Next, repurify the modRNA with the transcription cleanup kit and quantify it using a spectrophotometer.
- Precipitate the modRNA with ethanol and ammonium acetate and resuspend in 10 mM Tris-HCl and 1 mM EDTA.
- Concentrate the modRNA for in vivo delivery by centrifugal filters and measure the quality using an automated electrophoresis platform (Table of Materials).
2. Preparation of modRNA injection for in vivo delivery
- Calculate the volume needed for 100 µg of luciferase (Luc)/Cre modRNA injection based on the modRNA concentration.
- Mix the calculated volume of modRNA with equal parts of sucrose solution prepared in nuclease free water (0.3 g/mL) and 0.1 M citrate solution (pH = 7) (20 µL each recommended).
- Bring the final volume of the injection to 60 µL by adding saline solution.
3. Myocardial infarction surgery
- Anesthesia and preparation of the animal
NOTE: This study uses male and female CFW mice 8−12 weeks old.- Anesthetize the mouse with 2% isoflurane using an induction chamber and place the animal on its back in a dorsal position with a facemask over its nose and mouth to keep up the anesthesia. Maintain the anesthesia by mechanical ventilation using a respirator equipped with a 7−8 mm airway tube and a filter.
- To immobilize the mouse on the surgery platform, secure its limbs with surgical tape. shave the neck area and the left side of the ribcage and disinfect using 70% ethanol and povidone iodine. Repeat the disinfecting step twice. Check its reflexes, pinching the tail and hind feet to assess the depth of anesthesia.
- Continuously monitor and maintain the core body temperature at normothermia (37.5−38 °C).
- Maintain an open airway by performing intratracheal intubation. To do this, place the mouse in a ventral position with its mouth facing towards the experimenter and gently pull out the tongue. Adjust the angle of the neck and straighten the intubation path and push in the intubation cannula.
- In case the animal is unable to breathe through it, tracheostomy can be performed. Perform a cervical incision along the midline isolating the skin, muscle, and tissue outlining the trachea using a microscope. When the trachea is clearly visible, insert the endotracheal tube into the tissue between two cartridge rings below the glottis by making a small hole and holding the cranial part of the trachea using microsurgical forceps.
- Observe the thoracic movement of the mouse to verify that both lungs are receiving oxygen. The respiratory rate (RR) should be approximately 120 breaths per minute, with an inspiratory pressure of 17−18 cm H2O.
- Left anterior descending (LAD) artery ligation
- To perform the LAD ligation, turn the mouse carefully so it is lying on its right side. Lift the skin and perform a left sided thoracotomy between the 3rd and the 4th rib and dissect the tissue and muscle carefully. Use a cautery to prevent bleeding.
- Open the thorax carefully and once it is open, find the heart, without touching the lung with any sharp object. Place the retractors into the incision to keep the thoracic cavity open and have a clear view of the heart. Now move the lungs to the edge of the incision and remove the part of the pericardial sac that is covering the heart to access the anterior surface of the heart.
- Carefully identify the LAD, which can be seen as a deep light red vessel located between the pulmonary artery and the left auricle. Ligate the LAD proximal with one single suture of a 7-0 silk suture. Place a chest tube (28 G, venal catheter), between the 4th and the 5th rib.
- Close the thoracic incision in layers. Use the 6-0 silk running sutures to adapt the ribs and use 5-0 silk running sutures to close the skin. Make sure to leave a proper window for future echocardiographic measurement.
- Carefully drain the thorax with warm isotonic solution (9 g of sodium chloride in 1 L of water) using a 2 mL syringe. Place the mouse on its back. If performing tracheostomy, take the endotracheal tube out and using 7-0 silk sutures adapt the tracheal cartridge rings with one single stitch. Place the face mask on the mouse and close the skin using 5-0 silk running sutures.
- For the recovery phase, disconnect the intubation cannula from the ventilator and allow spontaneous breathing. Place the animal under a heat lamp until it attains consciousness. Do not leave the animal unattended after surgery until it is fully awake.
- Manage pain therapy with buprenophine at 0.1 mg/kg body weight, injected subcutaneously for the next 3 days at 12 h intervals.
4. Cardiac delivery of modRNA
- Deliver a total of 60 µL of the modRNA prepared in step 2.3 intramuscularly (IM) using an insulin syringe (31 G) following an MI.
- Inject 20 µL of the modRNA at three different sites of the heart muscle surrounding the infarct area (two on either side of ligation and one in the apex). Perform the injections immediately after the LAD, before closing the chest.
NOTE: Representative images of modRNA injection sites can be seen in the Figure 1.
- Inject 20 µL of the modRNA at three different sites of the heart muscle surrounding the infarct area (two on either side of ligation and one in the apex). Perform the injections immediately after the LAD, before closing the chest.
5. Protein expression validation in heart post MI
- Luciferase modRNA expression using a bioluminescence imaging system
NOTE: A total of 100 µg of Luc mRNA prepared in 60 µL of the sucrose citrate buffer is directly injected into the heart of CFW mice after the MI.- At 24 h post MI and modRNA injection, anesthetize the mice with 2% isoflurane using an induction chamber and intraperitoneally inject luciferin (150 mg/g body weight) to validate the Luc signal in vivo.
- Image the mice using a bioluminescence imaging system every 2 min until the Luc signal reaches saturation.
- Quantify the collected imaging data with imaging analysis software. Use the mice injected with saline only as a baseline reading for Luc expression and subtract the background signal collected from the saline-injected mice.
NOTE: Luc signal is expressed in p/s/cm2/sr x 106.
- Immunostaining for Cre transfection validation
- To validate the transfection with Cre, sacrifice the animals 24 h postinjection using an intraperitoneal injection of 100 mg/kg ketamine and 10 mg/kg xylazine followed by cervical dislocation. Before making an incision, disinfect the chest and abdomen using a 70% alcohol swab.
- Open the thoracic cavity by making a transverse incision ~1 cm lower than the sternum and move the scissors towards the head, cutting through the rib cage.
- Open the chest and inject 1 mL of sterile PBS in the right ventricular chamber to remove excess blood. Excise the heart immediately and place it in the sterile PBS to wash away the remaining blood.
- Fix the heart in 4% paraformaldehyde (PFA) for 24 h, followed by overnight incubation in 30% sucrose solution at 4 °C. The following day embed the hearts in optimal cutting temperature medium and section them at a thickness of 10 µm using a cryostat.
- Stain the sections with primary antibody against cardiac troponin I (cTNI) and label them with a fluorescent secondary antibody. To identify the nucleus, stain with 4',6-diamidino-2-phenylindole (DAPI) for 5 min. Image the slides using a fluorescent microscope.
6. Statistical analysis
- Plot a bar graph based on the Luc expression in saline and Luc-injected mice and report the values as mean ± SEM. Compare the two groups using an unpaired t-test (****p < 0.0001).
Representative Results
Eight to ten-week-old mice were anesthetized with isoflurane and intubated. After the animal was under anesthesia, the left thoracic region was shaved and sterilized with ethanol, and the heart was exposed for LAD ligation. The left coronary artery was occluded by firmly knotting the suture under the artery (diagram representation Figure 1A). After a successful infarction (indicated by the paling of the left ventricular free wall), a direct injection of 100 µg of Luc or Cre modRNA dissolved in sucrose citrate buffer was delivered directly into the myocardium at three different sites (Figure 1B) surrounding the injury area using an insulin syringe. The MI procedure with modRNA injections lasted for 30−45 min per animal. The animals showed approximately 90% survival rate postprocedure. After the procedure, the chest and the skin were firmly sutured in layers and the animal was removed from ventilation as soon as it started breathing normally.
After conducting the LAD ligation and subsequent delivery of Luc modRNA injection, we validated the Luc modRNA transfection by checking for Luc protein expression 24 h postinjection using a bioluminescence imaging system (Figure 2A). We established in previous publications that although the protein expression can be seen until day 6 posttransfection, the highest transfection efficiency of modRNA is observed at 24 h8. Similarly, we successfully detected the Luc signal in the heart treated with Luc modRNA injection (1.76 x 108) compared to the mice injected with sucrose citrate buffer after MI (3.0 x 105) (Figure 2B).
Further, we sought to validate the modRNA expression by checking its translation and biodistribution in a transgenic Rosa26mTmG mouse. This mouse model system expresses the cell membrane-localized tdTomato (mT) fluorescence expression in all body cells/tissues and changes to cell membrane-localized EGFP (mG) fluorescence expression upon Cre recombination. Thus, to observe the expression of the Cre modRNA, 100 μg Cre modRNA was injected directly into the myocardium post-MI in Rosa26mTmG male and female mice, and the animals were sacrificed 24 h postsurgery. Hearts were fixed and processed for immunostaining with cardiomyocyte marker cTNI and nuclear marker DAPI (Figure 3A). Successful Cre expression was evident due to the appearance of green colored cells (Figure 3B), which were a result of recombination with the Cre modRNA delivered to the mouse, represented by the change of the tdTomato color to EGFP around the Cre injection site (Figure 3C).
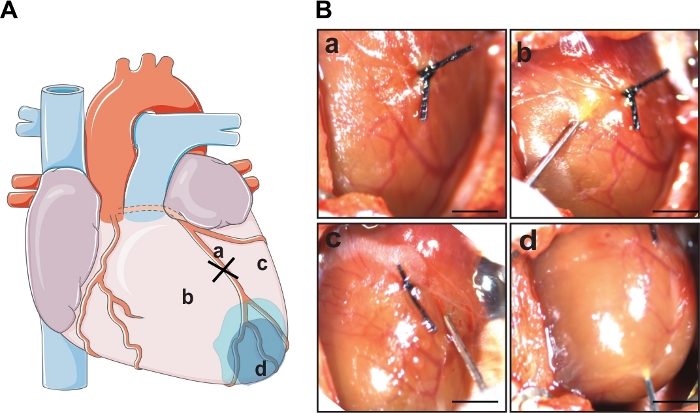
Figure 1: LAD ligation and cardiac delivery of modRNA. (A) Schematic diagram showing the area of LAD ligation and three sites of modRNA injection. (B) Representative images of the whole mouse heart post a successful MI induced by permanent ligation (a) and sites of modRNA injection following the MI. The 100 µg of modRNA dissolved in 60 µL of sucrose citrate buffer was delivered in the border zone area surrounding the infarction, two on either side of the ligation (b,c) and one in the apex (d). Scale bar = 1 cm. Please click here to view a larger version of this figure.
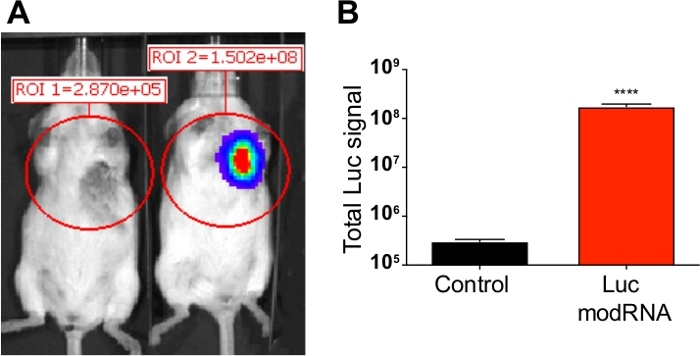
Figure 2: Luc expression analysis post modRNA injection. Sucrose citrate buffer containing 100 µg of Luc modRNA was injected directly into myocardium of CFW mice in an open-chest surgery. The bioluminescence imaging system was used to calculate Luc protein expression at 24 hours after injection. (A) Comparative bioluminescent images of control mice (transfected with buffer only) vs. mice injected with Luc modRNA. (B) Quantification of Luc signal compared with the control mice measured after 24 hours using bioluminescence imager. Error bar represents SEM with p < 0.0001. Please click here to view a larger version of this figure.
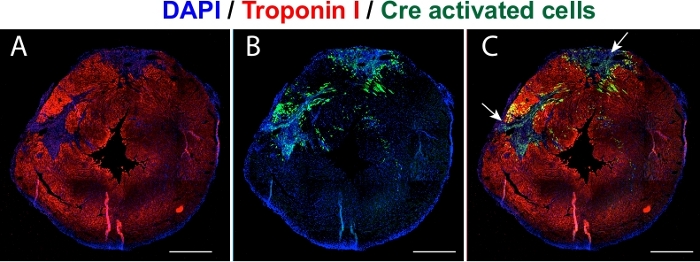
Figure 3: Validation of Cre expression in vivo. Representative images of transfected heart cross sections (short-axis view) validating the expression of Cre modRNA in Rosa26mTmG mouse 24 hours postinjection. (A) The cardiomyocytes immunostained with cTNI (red). (B) Green colored cells represent the Cre transfected cells. (C) Merged image showing Cre activated cells around the two injections. Blue is the nuclear stain DAPI. Scale bar = 1 cm. Please click here to view a larger version of this figure.
Discussion
Gene therapy has shown tremendous potential to significantly advance the treatment of cardiac disease. However, traditional tools employed in the initial clinical trials for treatment of HF have shown limited success and are associated with severe side effects. Modified RNA presents a nonviral gene delivery that is continuously gaining popularity as a gene transfer tool in the heart. ModRNA requires no nuclear localization of genes for translation, and thus offers an efficient and fast expression of the protein. Further, as the mRNA does not integrate into the genome host, gene delivery by modRNA skips the risk of mutagenesis. Consequently, owing to its advantages over conventional gene delivery vehicles, modRNA has become one of the most attractive platforms for delivery of single gene or gene combinations to the heart. However, to date there is no standardized protocol for the preparation and transfer of the modRNA into the heart postinjury. Thus, the aim here was to establish a standard and optimized protocol for intracardiac delivery of modRNA in rodent heart to improve the use of modRNA as a gene therapy tool and to make it suitable for therapeutic purposes.
In this protocol, modRNA is prepared by replacing uridine with pseudouridine, followed by capping with ARCA at the 5’ end. These changes in the secondary structure of mRNA have been shown to render higher protein translation compared to various other nucleotide modifications8. Moreover, this altered mRNA structure escapes the immunogenicity after IM injections in mice by limiting its recognition by toll-like receptors and nucleases6. Here, we showed that naked mRNA delivery with sucrose citrate buffer produces strong protein translation in the heart using a mouse MI model. In our previous studies, we established the superiority of modRNA delivered with sucrose citrate buffer in the heart in comparison to encapsulation of modRNA in nanoparticles, such as in vivo fectamine or in vivo jetPEI, which may hinder modRNA translation into protein8. The high preservation of the RNA by citrate and extra energy provided by the sucrose for endocytosis in addition of rescuing the single-stranded modRNA clumping could be the reason for the marked increase in translation of modRNA delivered in sucrose citrate buffer.
Although the use of modRNA has escalated in preclinical and clinical studies10,14 in the past decade, certain areas need to be investigated for improved success of modRNA in the clinic. First, synthesizing modRNA in the high quantities required for therapeutic investigations can be cost-prohibitive. Thus, it is essential to develop clinical-grade and cost-effective modRNA to move the field toward a clinical RNA therapy phase in humans. Second, a clinically applicable delivery method for modRNA needs to be identified. Considering the damage caused by the cardiac IM injections, less invasive catheter-based delivery methods might be more plausible in transferring the large amount of modRNA necessary to transfect large hearts.
In conclusion, this work demonstrated the successful delivery of RNA containing pseudouridine modifications in the mouse heart post MI. The delivery of modRNA in sucrose citrate buffer yielded a strong protein expression 24 hours after the injection. This protocol enables the researchers to follow a standardized delivery and protein evaluation in the heart post injury and thus provide more accessibility in the preparation and delivery of modRNA for their research.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Ann Anu Kurian for her help with this manuscript. This work was funded by a cardiology start-up grant awarded to the Zangi laboratory and also by NIH grant R01 HL142768-01
Materials
| Adenosine triphosphate | Invitrogen | AMB13345 | Included in Megascript kit |
| Antarctic Phosphatase | New England Biolabs | M0289L | |
| Anti-reverse cap analog, 30-O-Mem7G(50) ppp(50)G | TriLink Biotechnologies | N-7003 | |
| Bioluminescense imaging system | Perkin Elmer | 124262 | IVIS100 charge-coupled device imaging system |
| Blunt retractors | FST | 18200-09 | |
| Cardiac tropnin I | Abcam | 47003 | |
| Cytidine triphosphate | Invitrogen | AMB13345 | Included in Megascript kit |
| Dual Anesthesia System | Harvard Apparatus | 75-2001 | |
| Forceps- Adson | FST | 91106-12 | |
| Forceps- Dumont #7 | FST | 91197-00 | |
| Guanosine triphosphate | Invitrogen | AMB13345 | Included in Megascript kit |
| In vitro transcription kit | Invitrogen | AMB13345 | 5X MEGAscript T7 Kit |
| Intubation cannula | Harvard Apparatus | ||
| Megaclear kit | Life Technologies | ||
| Mouse ventilator | Harvard Apparatus | 73-4279 | |
| N1-methylpseudouridine-5-triphosphate | TriLink Biotechnologies | N-1081 | |
| NanoDrop Spectrometer | Thermo Scientific | ||
| Olsen hegar needle holder with suture scissors | FST | 12002-12 | |
| Plasmid templates | GeneArt, Thermo Fisher Scientific | ||
| Sharp-Pointed Dissecting Scissors | FST | 14200-12 | |
| Stereomicroscope | Zeiss | ||
| Sutures | Ethicon | Y433H | 5.00 |
| Sutures | Ethicon | Y432H | 6.00 |
| Sutures | Ethicon | 7733G | 7.00 |
| T7 DNase enzyme | Invitrogen | AMB13345 | Included in Megascript kit |
| Tape station | Aligent | 4200 | |
| Transcription clean up kit | Invitrogen | AM1908 | Megaclear |
| Ultra-4 centrifugal filters 10k | Amicon | UFC801096 |
Riferimenti
- Muruve, D. A. The innate immune response to adenovirus vectors. Human Gene Therapy. 15 (12), 1157-1166 (2004).
- Donsante, A., et al. Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Therapy. 8 (17), 1343-1346 (2001).
- Calcedo, R., Wilson, J. M. Humoral Immune Response to AAV. Frontiers in Immunology. 4, 341 (2013).
- Diebold, S. S., et al. Nucleic acid agonists for Toll-like receptor 7 are defined by the presence of uridine ribonucleotides. European Journal of Immunology. 36 (12), 3256-3267 (2006).
- Magadum, A., Kaur, K., Zangi, L. mRNA-Based Protein Replacement Therapy for the Heart. Molecular Therapy. 27 (4), 785-793 (2019).
- Kariko, K., et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Molecular Therapy. 16 (11), 1833-1840 (2008).
- Hadas, Y., et al. Optimizing Modified mRNA In Vitro Synthesis Protocol for Heart Gene Therapy. Molecular Therapy- Methods and Clinical Development. 14, 300-305 (2019).
- Sultana, N., et al. Optimizing Cardiac Delivery of Modified mRNA. Molecular Therapy. 25 (6), 1306-1315 (2017).
- Zangi, L., et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nature Biotechnology. 31 (10), 898-907 (2013).
- Carlsson, L., et al. Purified VEGF-A mRNA Improves Cardiac Function after Intracardiac Injection 1 Week Post-myocardial Infarction in Swine. Molecular Therapy Methods Clinical Development. 9, 330-346 (2018).
- Huang, C. L., et al. Synthetic chemically modified mRNA-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Molecular Pharmaceutics. 12 (3), 991-996 (2015).
- Magadum, A., et al. Ablation of a Single N-Glycosylation Site in Human FSTL 1 Induces Cardiomyocyte Proliferation and Cardiac Regeneration. Molecular Therapy – Nucleic Acids. 13, 133-143 (2018).
- Kondrat, J., Sultana, N., Zangi, L. Synthesis of Modified mRNA for Myocardial Delivery. Methods in Molecular Biology. 1521, 127-138 (2017).
- Gan, L. M., et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nature Communication. 10 (1), 871 (2019).

