Establishment and Maintenance of Gnotobiotic American Cockroaches (Periplaneta americana)
Summary
This protocol is used in establishing and maintaining gnotobiotic American cockroaches (Periplaneta americana) by surface sterilizing the egg cases (oothecae) prior to hatching. These gnotobiotic insects contain their vertically transmitted Blattabacterium endosymbionts but have axenic guts.
Abstract
Gnotobiotic animals are a powerful tool for the study of controls on microbiome structure and function. Presented here is a protocol for the establishment and maintenance of gnotobiotic American cockroaches (Periplaneta americana). This approach includes built-in sterility checks for ongoing quality control. Gnotobiotic insects are defined here as cockroaches that still contain their vertically transmitted endosymbiont (Blattabacterium) but lack other microbes that normally reside on their surface and in their digestive tract. For this protocol, egg cases (oothecae) are removed from a (nonsterile) stock colony and surface sterilized. Once collected and sterilized, the oothecae are incubated at 30 °C for approximately 4−6 weeks on brain-heart infusion (BHI) agar until they hatch or are removed due to contamination. Hatched nymphs are transferred to an Erlenmeyer flask containing a BHI floor, sterile water, and sterile rat food. To ensure that the nymphs are not housing microbes that are unable to grow on BHI in the given conditions, an additional quality control measure uses restriction fragment-length polymorphism (RFLP) to test for nonendosymbiotic microbes. Gnotobiotic nymphs generated using this approach can be inoculated with simple or complex microbial communities and used as a tool in gut microbiome studies.
Introduction
Gnotobiotic animals have proven to be invaluable tools for microbiome studies1,2,3. Germ-free and defined-flora animals have allowed elucidation of host-microbe interactions, including host immunological responses, gut epithelial maturation, and host metabolism1,4,5,6,7. Gnotobiotic animals inoculated with a simplified community have also assisted in a more complete understanding of microbe-microbe interactions in a gut community, specifically in unraveling cross-feeding and antagonistic relationships8,9,10,11. The current preferred model system for studies in the mammalian gut microbiome is the murine model. While this system has been vital in the discoveries outlined above, a key shortcoming is the cost involved. Specialized equipment and highly trained technicians are necessary to establish and maintain a gnotobiotic facility. This, in combination with extra care that must be given to every aspect of gnotobiotic animal maintenance, causes the a gnotobiotic animal to cost ten to twenty times more to breed than a standard animal model12. Due to high costs, many researchers may be unable to afford a gnotobiotic murine model. Additionally, while murine models may be the most widely accepted choice for studies looking to translate to human health, there are still many physiological and morphological differences between human and mouse guts13. Clearly no singular model is enough to answer the ever-increasing number of questions regarding the many aspects of the gut microbiome.
Insect models are a cheaper alternative due to their lower cost-of-maintenance in comparison to mammalian species. Extensive germ-free and gnotobiotic research in a variety of insect species has led to the development of multiple commonly used models. Mosquitos and Drosophila are common models for germ-free work due to their relevance to global diseases and genetic tractability14,15. Another emerging model system is that of the honey bee (Apis mellifera), given its importance in pollination and sociality research16. However, many of these commonly used insects lack the taxonomic complexity seen in mammalian gut communities17, limiting their ability to model higher order interactions. Not only is the total diversity of microbes found in the gut of American cockroaches more similar to mammals, but many of the microbes present in the cockroach gut belong to families and phyla that are commonly found in the gut microbiota of mammals and humans18. The hindgut of the cockroach is also functionally analogous to the large intestine of mammals, as it is a fermentation chamber densely packed with bacteria to assist in extraction of nutrients19,20. Finally, the omnivorous nature of cockroaches allows for a diversity of diet regimes that would not be possible with dietary specialists.
American cockroaches can be a useful model system for understanding gut microbial communities in higher organisms, but the cockroach’s status as a pest also makes this system relevant for pest control21. Leveraging fundamental knowledge of the gut community’s influence on cockroach health and physiology assists in developing new techniques for pest management.
The goal of this method is to outline a comprehensive description of the establishment and maintenance of gnotobiotic American cockroaches (Periplaneta americana), but this protocol could be used to generate nymphs of any oviparous cockroach. It includes a method for efficient, noninvasive collection of mature oothecae, and a nondestructive technique to monitor gnotobiotic status of the insects22,23,24. While previous methods of achieving and maintaining gnotobiotic cockroaches describe ootheca collection23,24,25,26,27, ootheca maturity is either interpreted in terms of species-specific cues (in Blattella germanica22,24,25), or not explicitly described27,28, making implementation difficult for those unfamiliar with the system. Since the method described here uses naturally dropped oothecae, the error of removing eggs prematurely is absent. This protocol contains both culture-dependent and culture-independent methods of quality control, and the culture-dependent method does not require sacrificing insects. Finally, this method brings together information from multiple gnotobiotic cockroach studies to create one, comprehensive protocol with all necessary information for achieving and maintaining gnotobiotic cockroaches.
Protocol
1. Preparation of materials
- Maintenance of stock cockroach cultures
NOTE: There are many ways to rear these robust insects. The specifics on providing shelter and water can be different depending on accessible materials (i.e., egg cartons instead of cardboard tubes). The following sterilization protocol will work for any stock tank setup.- Spread enough woodchip bedding in a 37.85 L (10 gallon) fish tank to cover the bottom of the tank with approximately 1 inch of bedding. Prepare housing by cutting (flat) cardboard to 2 in x 4 in pieces. Insert cardboard pieces into cardboard tubes (e.g., toilet paper tubes) and stack tubes at one end of the tank (Figure 1).
- Smear a thin layer of petroleum jelly on the top two inches of the inside of the tank to prevent insect escape.
NOTE: Be sure to properly coat the inside of the corners of the tank. - Add cockroaches by transferring (occupied) cardboard tubes from a previous stock tank, shaking them to release their inhabitants. For each transfer, move 100−200 mixed-age, mixed-sex cockroaches. Add dog food (20−30 pieces), monitor the amount of dog food in the tank and refill when low.
- Set up a water dish.
- Fill a small plastic reusable food container with double-distilled water (ddH2O). Cut cellulose sponges and holes in the lid of the food container to approximately the same size.
NOTE: The cellulose sponges prevent cockroaches from drowning in the water dish.
- Fill a small plastic reusable food container with double-distilled water (ddH2O). Cut cellulose sponges and holes in the lid of the food container to approximately the same size.
- Insert sponges into holes in the lid and place the lid on the filled container. Place the container in the tank and refill when low. Cover the tank with cotton cloth and secure it in place with an elastic band.
- As tanks begin to accumulate excessive quantities of frass and insect carcasses, set up new tanks and transfer cockroaches.
NOTE: Tanks are typically transferred every 6 months. Any remaining cockroaches/eggs in decommissioned stock tanks are euthanized by freezing at -20 °C for 1 h and the contents of the stock tank are then transferred to an autoclave bag and autoclaved (1 h, gravity cycle) prior to disposal. Stock tanks are sterilized with 2% bleach between uses.
- Disinfect a secondary container.
NOTE: This container does not include a filter, but instead allows free air exchange.- Spray the inside of both the lid and bottom with 2% bleach and allow them to soak for 10 min. Wipe out the bleach with a clean paper towel.
- Spray the inside of the lid and bottom with 70% ethanol and wipe dry with a clean paper towel. Replace the lid until use.
- Make BHI slants and flasks for incubating eggs and housing nymphs.
- Prepare BHI according to package instructions, adding 2% agar. Boil the BHI-agar solution until clarified.
- For slants, transfer 5 mL aliquots to 18 mm x 150 mm glass test tubes and cap. Sterilize via autoclave (sterilization time = 20 min, liquid cycle). Place autoclaved tubes at a 45° angle to cool into slants. Once solidified, refrigerate until use to prevent drying out.
- For flasks, transfer 10 mL aliquots of boiled BHI-agar solution into 250 mL Erlenmeyer flasks to completely cover the bottom of the flask. Cover the flask with foil and sterilize via autoclave (20 min, liquid cycle). Allow autoclaved flasks to cool and refrigerate until use to prevent drying out.
NOTE: No air filter is required for this setup. The foil cover is sufficient to allow gas exchange while preventing contamination from open air flow.
- Sterilize via autoclave: autoclavable rat chow broken into half-sizes (~1/2 inch pieces) in a foil-covered beaker (sterilization time = 1 h, gravity cycle), ddH2O in a capped bottle (sterilization time = 20 min, liquid cycle), and forceps in a foil-covered beaker (sterilization time = 20 min, gravity cycle).
NOTE: Do not overfill the rat chow beaker. Pellets will swell in the autoclave. - Set up a “maternity ward” tank.
NOTE: This tank contains the same materials as the stock tanks (cardboard tubes, water dishes with sponges, dog food, woodchip bedding; see Figure 1) and should be empty of cockroaches unless transferred for oothecae collection (see section 2). - Humidify an incubator by preparing a beaker full of supersaturated sodium chloride (NaCl) solution. Prepare this solution by adding 37 g of NaCl per 100 mL of ddH2O and stirring until dissolved.
NOTE: Typically, 500 mL of saturated salt solution typically humidifies an incubator with chamber dimensions 51 cm x 46 cm x 46 cm (H x W x D) for approximately a month before more water must be added.
2. Collection of oothecae
- Transfer females (in any number as appropriate for planned experiments) carrying oothecae (Figure 2) from the stock tank to the “maternity ward” by using forceps to move cardboard tubes that contain gravid females.
- If a carboard tube contains multiple insects in addition to the gravid female, first shake out the tube into an additional plastic container ringed with petroleum jelly, and then encourage the target insect to climb back into the cardboard tube alone.
- Transfer females back to the stock tank once they have dropped their oothecae. Retrieve the oothecae from the litter in the tank with forceps.
NOTE: Oothecae are often dropped within 24 h of the female being transferred.
3. Cleaning of oothecae
- Add oothecae to a 5 mL centrifuge tube containing 3 mL of sodium dodecyl sulfate (SDS). Vortex for 10 s. Repeat for a second wash step with a centrifuge tube containing fresh SDS.
NOTE: Up to five oothecae may be used per 3 mL of SDS. - Using a delicate task wipe, gently scrub the surface of each ootheca to remove any debris. More SDS may be added to assist in thorough cleaning. Place cleaned oothecae in a weighing boat until ready for sterilization.
NOTE: Protocol may be paused here, but leaving the oothecae in low humidity environments for extended periods of time (days to weeks) will cause them to dehydrate and lose viability.
4. Sterilization and incubation of oothecae
- Aliquot sterile water for post-sterilization rinse. For every ootheca to be sterilized, fill two 1.5 mL centrifuge tubes with 1 mL of sterile water.
- Add 10 µL of concentrated (32%) peracetic acid stock solution to 3.2 mL of ddH2O in a 5 mL centrifuge tube to create a 0.1% solution for sterilization. Cap and invert several times to mix.
CAUTION: Peracetic acid is harmful in contact with skin or lungs. Dilute in a fume hood.
NOTE: This must be done the same day as sterilization. If diluted in advance, the solution will quickly decompose and therefore not properly sterilize. As many as five cleaned oothecae may be sterilized in 3.2 mL of dilute acid. - Place (up to five) cleaned oothecae in the 0.1% peracetic acid solution for 5 min. Invert the tube several times every 60 s.
- In a laminar flow hood, use sterile forceps to transfer each ootheca to its own centrifuge tube with aliquoted sterile rinse water (step 4.1). Invert several times to mix. Repeat for a second rinse, then transfer each rinsed ootheca to its own BHI slant using sterile forceps.
- Place slants in the sterilized secondary container. Move the container into the humidified incubator at 30 °C for 4−5 weeks until hatched.
NOTE: Slants may be held upright by a small test tube rack or a medium/small beaker. - Check slants regularly (1−2x per week). If fungal or bacterial colony growth appears on the agar, remove the contaminated slant. When the four-week timepoint approaches, check slants every day.
NOTE: Once hatched, nymphs can survive for up to several weeks on BHI alone but will not grow optimally.
5. Maintenance of gnotobiotic nymphs
- In a laminar flow hood, aseptically transfer a pellet of sterilized rat chow to a prepared BHI flask (from step 1.3.3) with sterile forceps. As a sterility check, place the flask in the secondary container in a 30 °C incubator for 24 h, and do not use if growth appears.
- Add nymphs to the BHI flask with sterile food pellet. Shake them out of their BHI slant and let them fall into the flask in a laminar flow hood.
NOTE: The nymphs do not have traction on the glass walls of the test tube. Shaking the tube to knock them off of the slant and then tipping the tube to allow them to slide down the glass into the flask is effective. While nymphs can be transferred using forceps, the risk of fatal injury is high. - Water nymphs with 300 µL of sterile water once per week in a laminar flow hood by pipetting directly onto the BHI floor of the flask.
- As nymph feces begin to cover the BHI floor, transfer to a new BHI flask, adding the sterilized rat chow 24 h in advance (to verify sterility) as in step 5.1.
6. Quality control of sterility
- Remove one nymph from the BHI flask to sacrifice for a culture-independent quality control check of gnotobiotic status via restriction fragment-length polymorphism (RFLP). To do this, pour the nymph into a sterile centrifuge tube (similar to nymphal transfer from step 5.1) or place a sterile wooden applicator into the flask and wait for a nymph to begin climbing it, then transfer it to the centrifuge tube.
- Add 0.5 mL of 1x phosphate buffered saline (PBS) to the nymph in the centrifuge tube and homogenize with a sterile micropestle until all large pieces are broken up. Vortex well.
- Extract DNA from nymph homogenate using a bacterial DNA extraction kit (Table of Materials) as follows.
- Centrifuge the homogenized nymph for 10 min at 5,000 x g and remove the supernatant. Preheat a thermal shaker to 37 °C.
- Add 100 µL of 1x Tris-EDTA, and vortex to completely resuspend the pellet. Add 10 µL of lysozyme and mix, followed by a 30 min (no shaking) incubation in the preheated 37 °C thermal shaker.
- Add 25 mg of glass beads to samples, and vortex at maximum speed for 5 min. Preheat the thermal shaker to 55 °C.
- Allow the beads to settle before transferring the supernatant to a new 1.5 mL centrifuge tube with 100 µL of proteinase K buffer and 20 µL of proteinase K. Vortex to mix thoroughly.
- Incubate, with shaking at 600 rpm, in a 55 °C thermal shaker for 60 min. Centrifuge at 10,000 x g for 2 min, and transfer supernatant to a new 1.5 mL centrifuge tube. Preheat the thermal shaker to 65 °C. Begin preheating the elution buffer in a 65 °C hybridization oven.
- Add 220 µL of 100% ethanol. Vortex at maximum speed for 20 s. Break up any visible precipitate by pipetting up and down 10x.
- Insert a DNA column into a 2 mL collection tube, and transfer the sample into the column, including any precipitate that may have formed. Centrifuge at 10,000 x g for 1 min, discard filtrate from the collection tube, and replace the collection tube.
- Add 500 µL of binding buffer to the column, and centrifuge at 10,000 x g for 1 min. Discard filtrate from the collection tube and replace the collection tube.
- Add 700 µL of DNA wash buffer to the column, and centrifuge at 10,000 x g for 1 min. Discard filtrate from the collection tube and replace the collection tube. Repeat for a second wash step.
- Centrifuge empty column for 2 min to dry it, transferring the column to a new 1.5 mL centrifuge tube afterward. Add 50 µL of preheated elution buffer directly to the DNA column matrix, and incubate at 65 °C for 5 min.
- Centrifuge at 10,000 x g for 1 min to elute. Quantify the extracted DNA in the filtrate via spectrophotometry or fluorometry.
- Amplify and digest whole 16S gene. Visualize fragments using gel electrophoresis.
- Use 12.5 µL of 2x master mix, 0.5 µL of each primer 1492R (5′-GGTTACCTTGTTACGACTT) and 27F (5'-AGAGTTTGATCCTGGCTCAG), 5 ng of DNA, and molecular-grade water for a total reaction volume of 25 µL.
- Run the following thermocycler program: 94 °C for 60 s; followed by 35 cycles of 94 °C for 30 s, 50 °C for 45 s, 68 °C for 90 s; followed by 68 °C for 5 min.
- Purify the polymerase chain reaction (PCR) product using a DNA purification kit (Table of Materials).
- Add 120 µL of purifying buffer to the PCR product and vortex to mix. Briefly centrifuge to collect droplets inside the lid. Insert a DNA column into a 2 mL collection tube, transfer liquid to the prepared column and centrifuge at ≥13,000 x g for 1 min.
- Discard the filtrate and replace the collection tube. Add 700 µL of DNA wash buffer and centrifuge at ≥13,000 x g for 1 min. Discard the filtrate and replace the collection tube. Repeat for a second wash step.
- Centrifuge the empty column for 2 min to dry and transferring the column to a new 1.5 mL centrifuge tube. Add 50 µL of preheated elution buffer directly to the DNA column matrix, and incubate at room temperature for 2 min.
- Centrifuge at 10,000 x g for 1 min to elute. Quantify the extracted DNA in filtrate via spectrophotometry or fluorometry.
- Add 1 µg of purified PCR product to 5 µL of digestion buffer, 10 units RsaI, and molecular-grade water for a total reaction volume of 50 µL. Mix by pipetting up and down and incubate at 37 °C for 60 min.
- Separate the digested product via gel electrophoresis by running 20 µL of digested DNA on a 2% agarose gel. Visualize the gel to confirm gnotobiotic status.
NOTE: Gnotobiotic insects should only result in bands at 402 bp, 201 bp, and a smear from 163 to 148 bp, based on the 16S rDNA sequence of the endosymbiont, Blattabacterium. Any extra bands seen in the gel are indicative of contaminating microbial species.
7. Aseptic tracking of nymphal growth
- Record the body length to track nymphal growth by measuring the insects through the translucent BHI floor of the flask.
NOTE: Nymphs may be placed at 4 °C for 15 min to slow their movement, thereby making them easier to measure.
Representative Results
Stock tanks are set up as depicted in Figure 1. “Pregnant” females are identified by the ootheca attached to the posterior abdomen, as pictured in Figure 2. Incubation of oothecae on BHI agar allows for gnotobiotic quality control in a nondestructive fashion. In some cases, sterilization is unsuccessful, and growth appears around the oothecae as in Figure 3B. These oothecae should be removed and discarded. In our hands, a 10% average failure rate was observed for sterilization (n = 51). The oothecae hatch an average of 34 days after sterilization without growth on the medium, as seen in Figure 3A. We have observed typical hatch rates of 41% (n = 46) for sterilized, noncontaminated oothecae, with an average of 11 nymphs per ootheca. Larger nymphs are transferred to BHI flasks covered with foil, as in Figure 4. The foil prevents contamination, and the nymphs have room to grow. RFLP of the 16S rDNA from a homogenized nymph is used to confirm gnotobiotic status. Gnotobiotic nymphs have been observed to grow at a slower rate than their non-sterile counterparts, as represented Figure 5. Figure 6 displays results from successfully gnotobiotic insects as well as standard (nonsterile) nymphs.
While this test has not yet identified contamination in the absence of a positive culture result, this step has been carried out routinely during critical experiments to rule out the presence of contaminating oxygen-sensitive or fastidious microbes. Slower growth has been observed in the gnotobiotic cockroaches when compared to standard/nonsterile insects.

Figure 1: Cockroach stock culture setup.
Cardboard tubes can be seen stacked in the far end of the tank. Food and water are both near the front of the tank. Cotton cloth cover and elastic band have been removed for visibility. Please click here to view a larger version of this figure.

Figure 2: A “pregnant” American cockroach.
The arrow indicates the ootheca. Please click here to view a larger version of this figure.
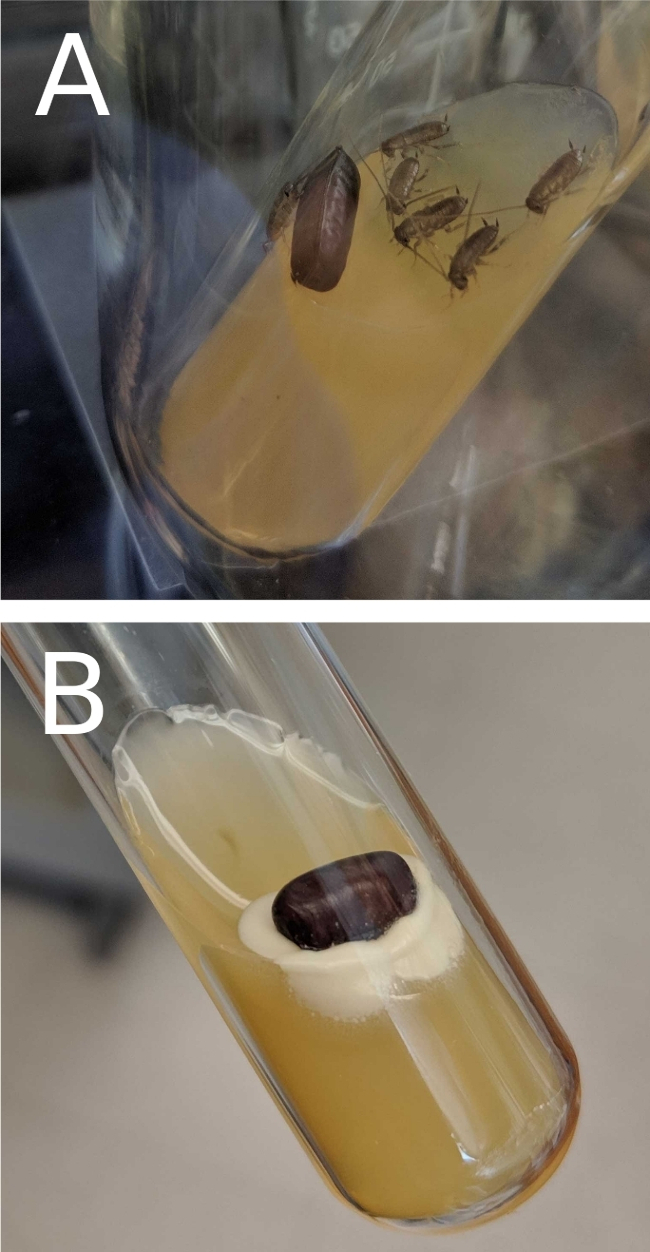
Figure 3: Images of successfully gnotobiotic nymphs hatched and unsuccessfully sterilized oothecae on BHI slants.
Oothecae were sterilized and incubated as described in this protocol. (A) The lack of microbial growth on the BHI slant indicates that the insects are free of culturable organisms. (B) Oothecae on slants that result in colony formation should be discarded as contaminated. Please click here to view a larger version of this figure.

Figure 4: Gnotobiotic rearing apparatus.
Insects are kept in sterile flasks covered with a foil lid to prevent contamination. The secondary container (green lid) is sterilized with 2% bleach followed by 70% ethanol. Air flow is not restricted in the secondary container. Please click here to view a larger version of this figure.
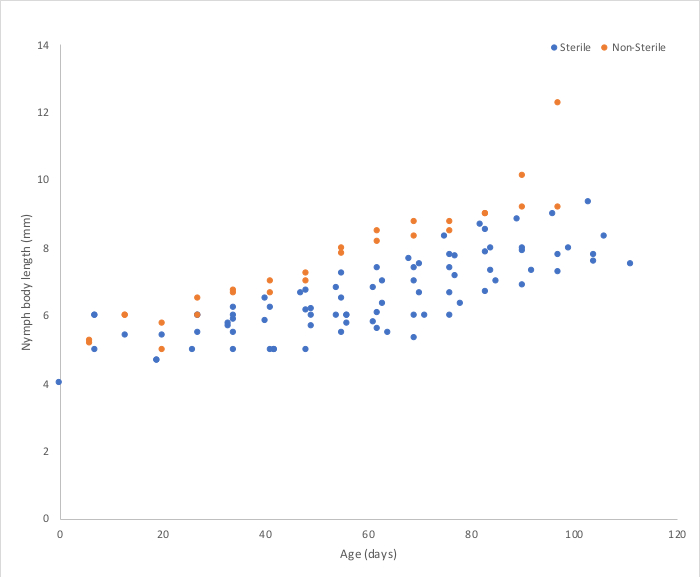
Figure 5: Representative growth rate data comparing body lengths of gnotobiotic and nonsterile nymphs. Both groups of insects were fed autoclaved rodent diet. Gnotobiotic insects (here: n = 105) are kept on BHI as described. Nonsterile insects (here: n = 50) live in flasks with autoclaved woodchip bedding with small dishes for water. Nonsterile nymphs grow an average rate of 0.059 mm/day, while gnotobiotic nymphs grow at 0.028 mm/day (p < 0.0001). Please click here to view a larger version of this figure.
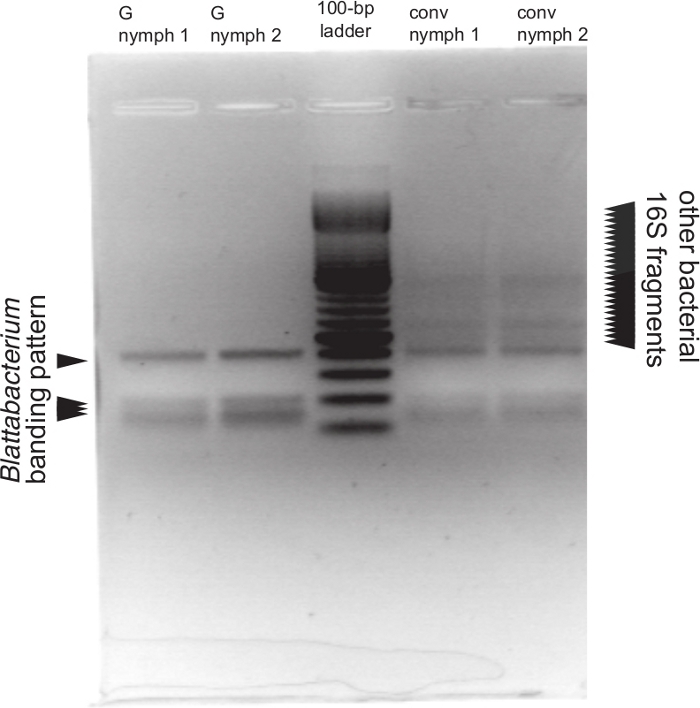
Figure 6: A representative gel image of RFLP results for quality control.
Whole-16S gene amplicons were digested with RsaI. DNA for PCR was extracted from nymphs homogenized in 1x PBS. “G nymph” lanes correspond to gnotobiotic nymphs, while “conv nymph” lanes correspond to conventional, nonsterile counterparts. Based on virtual restriction digest, the endosymbiont (Blattabacterium) is expected to have bands at the sizes 402 bp, 206 bp, and 163 bp, with a smear of bands between 163 bp and 148 bp. A gnotobiotic insect should show only the Blattabacterium banding pattern. A mixed bacterial community is expected to have a smear of bands with varying sizes, labeled here “other bacterial 16S fragments”. Please click here to view a larger version of this figure.
Discussion
Other methods describing generation of gnotobiotic cockroaches either did not describe oothecae collection or used benchmarks specific to other cockroach species to indicate when the oothecae could be removed from the mother23,25,26. Originally, oothecae were collected from the woodchip bedding in the stock tanks, resulting in very low hatch rates (~10%) compared to nonsterilized oothecae (47%)29. This is likely due to the fact that unhatched oothecae accumulate over time in the stock cage, and there is no way of verifying ootheca age or viability. Implementation of the “maternity ward” approach allows collection of freshly deposited oothecae of known age. This further facilitates experimental planning, as the researcher can anticipate likely hatch times for individual oothecae. Another modification from initial and published protocols includes the incubation of oothecae and nymphs in semi-sealed chambers also containing a supersaturated sodium chloride solution. The presence of the solution maintains a relative humidity of approximately 75%30. Oothecae are routinely incubated at 30 °C, which has been shown to minimize the number of days required for incubation while also maximizing the embryos’ viability and number of nymphs produced per ootheca31. After hatching, gnotobiotic nymphs are routinely cultured on the benchtop at laboratory room temperature and ambient conditions, although humidity-controlled chambers are again utilized for critical experiments. After establishment of these changes to ootheca collection and incubation, hatch rates increased to approximately 41% (n = 51), not including oothecae removed due to contamination. A potential route to further optimization of hatch rates may include extending the time between ootheca collection and sterilization. The cuticle of the egg case may not be fully tanned on initial release32, and therefore may be permeable to solutions used during sterilization within 24 h of being dropped.
The sterilization protocol using 0.1% peracetic acid was adapted from Doll et al.25. Other studies have documented alternative techniques for sterilizing oothecae23,26. Contamination rates are based on the nondestructive method of incubating the oothecae on a BHI slant. This approach is highly advantageous as it allows for quick identification and removal of contaminated oothecae. Most previous protocols test for culturable organisms by plating feces or nymph homogenate on bacteriological media and checking for growth22,23,25,27,28,33. In at least one case, the method for testing gnotobiotic status was not fully described26. Except Clayton who added a small slab of sterility testing medium to rearing bottles24, previous methods22,23 housed gnotobiotic insects on bacteriological media only for short periods of time to initially evaluate the sterilization protocol.
Continued housing of the resulting nymphs on BHI medium as a built-in quality-control measure allows their gnotobiotic status to be monitored in semi-real-time—a technique not seen in most previous methods22,23. This is especially useful for long-term experiments that require gnotobiotic nymphs to be accessed. If the BHI floor under nymphs appears contaminated with bacterial or fungal growth, the flask should be discarded. This type of contamination typically occurs when uncovering the flasks to water nymphs, but it may also arise from the feces in the case of insufficiently sterilized oothecae or food. The use of a laminar flow hood when watering improves the contamination rate caused by uncovering flasks.
As not all contaminating organisms may grow aerobically on BHI medium, an additional culture-independent method of sterility testing is required. One potential approach is microscopy27, but this approach can be labor-intensive. Other protocols use sequence-based techniques to detect organisms that may escape culture14,23,27,28. However, such approaches are often expensive and hard to interpret, as the results of high-throughput sequencing approaches can easily be impacted by low-level contamination of reagents34 and barcode hopping35. Instead, a new approach has been developed which uses PCR amplification of the 16S rRNA gene in combination with restriction fragment-length polymorphism to visualize both the endosymbiont and any contaminating gut symbionts. This technique includes an internal PCR control, since Blattabacterium’s 16S gene has been sequenced, and its banding pattern should be present in both gnotobiotic and nonsterile insects. As the endosymbiont’s restriction pattern can be predicted from its genome sequence36, there is no need to sequence the amplicons or the restriction fragments, unless identification of any contaminant is desired. The current version of this protocol calls for a nymph to be sacrificed for PCR/RFLP, but this technique could also be used on feces as a nondestructive measure. However, it will not include a built-in control, as the feces should not contain much Blattabacterium.
An additional easily modifiable yet critical component of rearing gnotobiotic animals is diet. While BHI agar can serve as a temporary food source for the insects, it has been found that it results in substantial growth deficits among nymphs when used as the sole food source for extended periods. While diverse diets have been tried, autoclavable rat chow is recommended as a routine diet for the maintenance of gnotobiotic insects. Diets not specifically formulated for sterilization were often difficult to render fully sterile, and many sterile or autoclavable laboratory animal diets were found to exhibit rapid fungal growth under nonsterile conditions. This tendency to degrade under nonsterile conditions rendered them unsuitable for use in experiments directly comparing gnotobiotic and nongnotobiotic insects. The recommended diet allows for the use of a consistent diet between gnotobiotic and standard nymphs, facilitating comparison of characteristics—such as growth rates—between the two groups.
As others have observed27, the gnotobiotic nymphs grow more slowly than their nonsterile counterparts. A comparison between body lengths of gnotobiotic (n = 105) and nonsterile nymphs (n = 50) fed the same, autoclaved rodent diet and kept at room temperature reveals that nonsterile nymphs grow an average of 0.059 mm/day, while gnotobiotic nymphs grow 0.028 mm/day (p < 0.0001) (Figure 5). The presence of gut microbiota in P. americana has been shown to alter the insects’ metabolic rate37, and gut communities in general are thought to affect nutrient absorption38,39. These reasons support the observed differences in growth rate of gnotobiotic and nonsterile nymphs.
A possible limitation to this technique is that gnotobiotic nymphs may not reach sexual maturity, as the oldest sterile cohorts are more than 10 months old and have reached only the seventh instar (out of 10; 11 being adulthood) as approximated by body length40. These oldest cohorts are not on the autoclaved rat diet but instead eat irradiated rat chow, a diet that contains too much moisture to feed to nonsterile cohorts without excessive mold growth. Nonsterile nymphs on a nonsterilized dog food diet were found to reach adulthood after 9−10 months under laboratory conditions (room temperature and humidity). Cohorts of gnotobiotic and nonsterile nymphs on the shared, autoclaved rat chow are currently less than 7 months old, nonsterile insects are estimated to be seventh instar (average: 16.7 mm) while sterile insects are estimated to be fifth instar (average: 11.2 mm). As a result, we cannot, as of yet, verify whether our gnotobiotic cockroaches can successfully reproduce. However, given the ease with which new gnotobiotic cohorts can be established using this approach, this method shows great promise even in the absence of proven reproduction of gnotobiotic insects.
In conclusion, this protocol provides a versatile tool that allows microbiome researchers to operate their own, low-cost gnotobiotic “facility” using common laboratory materials. This approach may be used to generate gnotobiotic cockroaches for experiments examining the role of the microbiota in shaping host behavior, immunity, development, and stress responses21,26,27. These gnotobiotic insects may also be inoculated with either synthetic or xenobiotic communities and subsequently used as subjects for gut microbiome studies23,28. Further, elements of this approach, including the use of bacteriological media-lined incubation chambers as a built-in sterility check, are generalizable to other model systems and can facilitate routine maintenance of gnotobiotic animals in smaller-scale facilities.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM133789. The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health. The authors would like to acknowledge Josey Dyer for tracking sterilization rates, hatch rates, and growth rates of the gnotobiotic cockroaches.
Materials
| 2X master mix | New England BioLabs | M0482 | OneTaq MasterMix |
| Autoclavable rat chow | Zeigler | NIH-31 Modified Auto | |
| Bacterial DNA extraction kit | Omega Bio-Tek | D-3350 | E.Z.N.A. Bacterial DNA kit; includes lysozyme, glass beads, proteinase K, buffers (proteinase K, binding, wash, elution), DNA columns, 2-mL collection tube |
| binding buffer | Omega Bio-Tek | PD099 | included in Omega Biotek's bacterial DNA extraction kit ("HBC" buffer) |
| brain-heart infusion (BHI) broth | Research Products International | B11000 | |
| Delicate task wipes | KimWipe | JS-KCC-34155-PK | KimWipes |
| DNA purification kit | Omega Bio-Tek | D6492 | E.Z.N.A. Cycle Pure kit; D6493 may also be used; includes buffers (purifying ,wash, elution) |
| elution buffer | Omega Bio-Tek | PDR048 | included in Omega Biotek's bacterial DNA extraction kit |
| glass beads | Omega Bio-Tek | n/a | included in Omega Biotek's bacterial DNA extraction kit |
| Hybridization oven | UVP | 95-0330-01 | we use a hybridization oven for preheating elution buffer, but a water bath could probably also be used |
| Laminar flow biological safety cabinet | NuAire, Inc. | NU-425-400 | Protocol refers to this as "laminar flow hood" for brevity |
| lysozyme | Omega Bio-Tek | n/a | included in Omega Biotek's bacterial DNA extraction kit |
| peracetic acid stock solution (32%) | Sigma-Aldrich | 269336 | |
| Petroleum jelly | Vaseline | n/a | |
| proteinase K buffer | Omega Bio-Tek | PD061 | included in Omega Biotek's bacterial DNA extraction kit ("TL buffer") |
| purifying buffer | Omega Bio-Tek | PDR042 | included in Omega Biotek's CyclePure kit ("CP" buffer) |
| RsaI | New England BioLabs | R0167 | Includes CutSmart (digestion) buffer |
| Secondary container | n/a | n/a | a plastic container with a lid (such as a Kritter Keeper) works well for this (25cm long x 15cm wide x 22cm high); it should be large enough to fit BHI slants and test tubes |
| spectrophotometer | ThermoFisher | ND-2000 | Catalog info is for NanoDrop2000 |
| thermal shaker | Eppendorf | EP5386000028 | Thermomixer R |
| Tris-EDTA | Fisher | BP1338-1 | 10 nm Tris, 1 mM EDTA, pH 8 |
| wash buffer | Omega Bio-Tek | PDR044 | included in Omega Biotek's bacterial DNA extraction kit ("DNA wash" buffer) |
| Woodchip bedding | P.J. Murphy Forest Products | Sani-Chips |
Riferimenti
- Yi, P., Li, L. The germfree murine animal: An important animal model for research on the relationship between gut microbiota and the host. Veterinary Microbiology. 157 (1-2), 1-7 (2012).
- Nature Biotechnology. Laying better plans for mice. Nature Biotechnology. 31 (4), 263-263 (2013).
- Nicklas, W., Keubler, L., Bleich, A. Maintaining and Monitoring the Defined Microbiota Status of Gnotobiotic Rodents. ILAR Journal. 56 (2), 241-249 (2015).
- Faith, J. J., Ahern, P. P., Ridaura, V. K., Cheng, J., Gordon, J. I. Identifying Gut Microbe-Host Phenotype Relationships Using Combinatorial Communities in Gnotobiotic Mice. Science Translational Medicine. 6 (220), 11 (2014).
- Moon, C., et al. Vertically transmitted faecal IgA levels determine extra-chromosomal phenotypic variation. Nature. 521 (7550), 90-93 (2015).
- Eun, C. S., et al. Induction of Bacterial Antigen-Specific Colitis by a Simplified Human Microbiota Consortium in Gnotobiotic Interleukin-10-/- Mice. Infection and Immunity. 82 (6), 2239-2246 (2014).
- Cherbuy, C., et al. Microbiota matures colonic epithelium through a coordinated induction of cell cycle-related proteins in gnotobiotic rat. American Journal of Physiology: Gastrointestinal and Liver Physiology. 299 (2), 348-357 (2010).
- Van Den Abbeele, P., et al. Arabinoxylans and inulin differentially modulate the mucosal and luminal gut microbiota and mucin-degradation in humanized rats. Environmental Microbiology. 13 (10), 2667-2680 (2011).
- Stecher, B., Berry, D., Loy, A. Colonization resistance and microbial ecophysiology: using gnotobiotic mouse models and single-cell technology to explore the intestinal jungle. FEMS Microbiology Reviews. 37 (5), 793-829 (2013).
- Sugahara, H., et al. Probiotic Bifidobacterium longum alters gut luminal metabolism through modification of the gut microbial community. Scientific Reports. 5 (1), 13548 (2015).
- Martín, R., Bermúdez-Humarán, L. G., Langella, P. Gnotobiotic Rodents: An In Vivo Model for the Study of Microbe-Microbe Interactions. Frontiers in Microbiology. 7, 409 (2016).
- Mallapaty, S. Gnotobiotics: getting a grip on the microbiome boom. Lab Animal. 46 (10), 373-377 (2017).
- Nguyen, T. L. A., Vieira-Silva, S., Liston, A., Raes, J. How informative is the mouse for human gut microbiota research. Disease Models & Mechanisms. 8 (1), 1-16 (2015).
- Correa, M. A., Matusovsky, B., Brackney, D. E., Steven, B. Generation of axenic Aedes aegypti demonstrate live bacteria are not required for mosquito development. Nature Communications. 9 (1), 4464 (2018).
- Trinder, M., Daisley, B. A., Dube, J. S., Reid, G. Drosophila melanogaster as a High-Throughput Model for Host–Microbiota Interactions. Frontiers in Microbiology. 8, 751 (2017).
- Zheng, H., Steele, M. I., Leonard, S. P., Motta, E. V. S., Moran, N. A. Honey bees as models for gut microbiota research. Lab animal. 47 (11), 317-325 (2018).
- Engel, P., Moran, N. A. The gut microbiota of insects – diversity in structure and function. FeMS Microbiology Reviews. 37 (5), 699-735 (2013).
- Tinker, K. A., Ottesen, E. A. The Core Gut Microbiome of the American Cockroach, Periplaneta americana, Is Stable and Resilient to Dietary Shifts. Applied and Environmental Microbiology. 82 (22), 6603-6610 (2016).
- Zurek, L., Keddie, B. A. Contribution of the colon and colonic bacterial flora to metabolism and development of the american cockroach Periplaneta americana L. Journal of Insect Physiology. 42 (8), 743-748 (1996).
- Cruden, D. L., Markovetz, A. J. Microbial aspects of the cockroach hindgut. Archives of Microbiology. 138, 131-139 (1984).
- Pietri, J. E., Tiffany, C., Liang, D. Disruption of the microbiota affects physiological and evolutionary aspects of insecticide resistance in the German cockroach, an important urban pest. PLoS ONE. 13 (12), 0207985 (2018).
- Benschoter, C., Wrenn, R. Germfree techniques for establishment and maintenance of a colony of aseptic German cockroaches. Annals of the Entomological Society of America. 65 (3), 641-644 (1972).
- Tegtmeier, D., Thompson, C. L., Schauer, C., Brune, A. Oxygen Affects Gut Bacterial Colonization and Metabolic Activities in a Gnotobiotic Cockroach Model. Applied and Environmental Microbiology. 82 (4), 1080-1089 (2016).
- Clayton, R. A simplified method for the culture of Blattella germanica under aseptic conditions. Nature. 184 (4693), 1166-1167 (1959).
- Doll, J. P., Trexler, P. C., Reynolds, L. I., Bernard, G. R. The Use of Peracetic Acid to Obtain Germfree Invertebrate Eggs for Gnotobiotic Studies. American Midland Naturalist. 69 (1), 231 (1963).
- Wada-Katsumata, A., et al. Gut bacteria mediate aggregation in the German cockroach. Proceedings of the National Academy of Science. 112, 15678-15683 (2015).
- Jahnes, B. C., Herrmann, M., Sabree, Z. L. Conspecific coprophagy stimulates normal development in a germ-free model invertebrate. PeerJ. 7, 6914 (2019).
- Mikaelyan, A., Thompson, C. L., Hofer, M. J., Brune, A. Deterministic Assembly of Complex Bacterial Communities in Guts of Germ-Free Cockroaches. Applied Environmental Microbiology. 82 (4), 1256-1263 (2016).
- Katoh, K., et al. Group-housed females promote production of asexual ootheca in American cockroaches. Zoological Letters. 3, 3 (2017).
- Greenspan, L. Humidity fixed points of binary saturated aqueous solutions. Journal of Research of the National Bureau of Standards. 81 (1), 89-96 (1976).
- Bressan-Nascimento, S., Oliveira, D. M. P., Fox, E. G. P. Thermal requirements for the embryonic development of Periplaneta americana (L.) (Dictyoptera: Blattidae) with potential application in mass-rearing of egg parasitoids. Biological Control. 47 (3), 268-272 (2008).
- Nation, J. L. . Insect Physiology and Biochemistry, Second Edition. , (2008).
- House, H. L. Nutritional studies with Blattella germanica (L.) reared under aseptic conditions I. Equipment and technique. The Canadian Entomologist. 81 (5), 94-100 (1949).
- Stinson, L. F., Keelan, J. A., Payne, M. S. Identification and removal of contaminating microbial DNA from PCR reagents: impact on low-biomass microbiome analyses. Letters in Applied Microbiology. 68 (1), 2-8 (2018).
- Kircher, M., Sawyer, S., Meyer, M. Double indexing overcomes inaccuracies in multiplex sequencing on the Illumina platform. Nucleic Acids Research. 40, 3 (2011).
- Sabree, Z. L., Kambhampati, S., Moran, N. A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proceedings of the National Academy of Sciences of the United States of the America. 106 (46), 19521-19526 (2009).
- Ayayee, P. A., Ondrejech, A., Keeney, G., Muñoz-Garcia, A. The role of gut microbiota in the regulation of standard metabolic rate in female Periplaneta americana. PeerJ. 6, 4717 (2018).
- Krajmalnik-Brown, R., Ilhan, Z. E., Kang, D. W., DiBaise, J. K. Effects of gut microbes on nutrient absorption and energy regulation. Nutrition in clinical practice : official publication of the American Society for Parenteral and Enteral Nutrition. 27 (2), 201-214 (2012).
- Rowland, I., et al. Gut microbiota functions: metabolism of nutrients and other food components. European Journal of Nutrition. 57 (1), 1-24 (2018).
- Gier, H. T. Growth rate in the cockroach Periplaneta americana (Linn). Annals of the Entomological Society of America. 40, 303-317 (1947).

