Visualizing Synaptic Degeneration in Adult Drosophila in Association with Neurodegeneration
Summary
The goal of this procedure is to dissect the dorsal longitudinal muscle (DLM) tissue to assess the structural integrity of DLM neuromuscular junctions (NMJs) in neurodegenerative disease models using Drosophila melanogaster.
Abstract
Drosophila serves as a useful model for assessing synaptic structure and function associated with neurodegenerative diseases. While much work has focused on neuromuscular junctions (NMJs) in Drosophila larvae, assessing synaptic integrity in adult Drosophila has received much less attention. Here we provide a straightforward method for dissection of the dorsal longitudinal muscles (DLMs), which are required for flight ability. In addition to flight as a behavioral readout, this dissection allows for the both DLM synapses and muscle tissue to be amenable to structural analysis using fluorescently labeled antibodies for synaptic markers or proteins of interest. This protocol allows for the evaluation of the structural integrity of synapses in adult Drosophila during aging to model the progressive, age-dependent nature of most neurodegenerative diseases.
Introduction
Synaptic dysfunction is among the earliest known hallmarks of most major neurodegenerative diseases1,2,3,4,5,6. However, very little is known regarding how these structural and functional impairments relate to later stages of disease progression. Drosophila has proven to be a useful model system for understanding synapse growth and development using larval NMJs7,8,9. However, the third larval instar stage only lasts a few days, limiting their utility in studying progressive, age-dependent neurodegeneration. An alternative to assessing larval NMJs is to examine synaptic structures in adult Drosophila, such as the synapses formed on the Dorsal Longitudinal Muscles (DLMs) that are required for flight10,11,12,13,14,15,16. These tripartite synapses are structurally organized in a similar manner to mammalian synapses17, providing a unique advantage for assessing models of neurodegenerative diseases.
Here we describe a straightforward method for analyzing the structural integrity of adult NMJs in a Drosophila model of neurodegeneration. Previous DLM dissection methods and studies have emphasized the importance of preserving muscle tissue for a variety of applications18,19,20,21,22,23. Our protocol provides a comprehensive method to preserve both neuronal and muscle tissue to investigate neurodegenerative diseases. Another major component of studying these diseases is the ability to understand neuronal loss in an age dependent manner. Previous work provides a critical and in-depth understanding of how the DLM NMJs are formed during metamorphosis into early adulthood11,12,14,15,16,24. Our protocol establishes a method to build upon this work to investigate DLM NMJs in an age-dependent manner in aging and neurodegenerative diseases.
Protocol
1. Generation of transgenic flies
- To generate transgenic flies for this experiment, collect OK371-Gal425 virgin female flies and males of UAS-TDP-43M337V 26 (Figure 1A) by anesthetizing flies with CO2 on a pad to sort.
- Sort anesthetized flies into vials with standard Drosophila media for the cross. Place labeled vials at 25 °C for the next generation to emerge.
NOTE: Clear the adults from the vials before the progeny emerge to ensure the proper genotype. - Once progeny emerge, collect the transgenic flies into vials and sort by sex to begin aging for experimental conditions.
- Once flies are collected, transfer flies to fresh food every 2 days until the flies are 21 days old.
2. Dissection prep
- To prepare for dissections, obtain room temperature phosphate buffered saline (1x PBS), a 10 cm dissection dish coated with a silicone elastomer, straight edge dissecting scissors, one set of blunt dissection forceps, a P200 pipette and pipette tips, 2.0 mL microcentrifuge tubes, standard office scissors, 70% ethanol, a 6 cm Petri dish, and 32% formaldehyde diluted to 4% with 1x PBS.
- Label tubes for each genotype or condition and add 900 µL of 1x PBS (room temp) and 150 µL of 32% formaldehyde to each tube. Wear gloves and safety glasses when preparing the 4% formaldehyde fixative.
- Anesthetize 6‒10 flies per group directly from the vial with CO2 and submerge flies into a 6 cm Petri dish with 70% ethanol. Press flies down into the ethanol using a paint brush to ensure that specimens are fully submerged. This will remove the layer of oil on the outer cuticle.
3. Thorax isolation and fixation
- Before dissecting each specimen, add approximately 7‒10 mL of 1x PBS to the dissection dish coated with silicone elastomer. This volume should ensure that the tissue samples are completely submerged.
- Transfer one fly to the dissection dish from the 70% ethanol using blunt forceps and grasping either the wings or the legs.
- Focus the sample in the dissection dish under a dissecting microscope. Next submerge the sample in 1x PBS, and carefully remove the wings using blunt Dumont #5 fine forceps.
- Using Vannas straight edge spring dissection scissors, remove the legs by creating a small incision in the ventral side of the cuticle. In step 3.8, this incision will allow the formaldehyde to penetrate the tissue.
- Take the scissors in one hand and hold the forceps in the other to position the fly ventral side up. While holding the specimen in place with the blunt forceps, remove the head and abdomen with the dissection scissors.
- Transfer the isolated thorax using the modified pipette tip into the labeled tube, from step 3.2.
NOTE: Set the pipette to 40 µL to avoid adding extra 1x PBS to the fixative. - Repeat steps 3.2‒3.6 above for each specimen.
- Fix samples for 30 min at room temperature.
- Remove the fix using a Pasteur pipette and discard it in the proper waste container under the fume hood. Rinse samples three times with 1.5 mL of 1x PBS each using a Pasteur pipette. Complete a fourth rinse using only 750 µL and leave tissues in 1x PBS.
NOTE: At this point, tissue samples can remain at 4 °C for up to 3 days before proceeding to the next steps.
4. Flash freezing and thorax bisection
- Before beginning the bisections, fill a Dewar flask with liquid nitrogen wearing proper cryo-protective gloves and safety glasses. Obtain a blade breaker, feather blades, one pair of fine forceps, ice, ice-cold 1x PBS, and cryogenic tweezers.
- Prepare an ice bucket to keep 1x PBS ice-cold.
- Use the blade breaker to grab the feather blade at an angle, and bend the blade in order to break off a small piece. The blade breaker can then lock the blade in position for use as a small scalpel.
NOTE: One blade should last for all groups. Switch if blade breaks or becomes dull. - Add a clean pipette tip to the P200 and remove 1/5th of the tip to transport samples.
- Prepare a new microcentrifuge tube for each group and add 200 µL of 1x PBS to each tube. This second tube will be used to collect the final DLM preps.
- Remove all 1x PBS from tubes using a Pasteur pipette.
- Wearing proper protective equipment, submerge the tube into the liquid nitrogen flask for 10 s with the cryogenic tweezers.
NOTE: The tubes should be closed tightly to keep the tube from exploding. - Remove the tube from the liquid nitrogen and add approximately 300 µL of ice-cold 1x PBS to the samples with a Pasteur pipette. Keep the samples on ice.
- Add ice-cold 1x PBS to the 10 cm dissection dish coated with silicone elastomer and dispense the first thorax with the modified 200 µL pipette.
- Place the thorax ventral side up. In one hand use a dull pair of forceps to position the thorax and in the other use a fine pair of forceps to remove some of the thoracic ganglion to expose the midline of the thorax.
- Use the midline of the thorax as a guide to make a shallow cut through 1/3rd of the thorax with the blade.
- Remove the blade from the thorax and position the thorax at a 45° angle with the blunt forceps. Reinsert the blade and cut straight down the midline of the thorax. This will result in two hemithoraces.
- Take one hemithorax at a time and remove the excess tissue under DLM muscle fiber F (Figure 1B), the most ventral fiber. Use the blade to carefully make one or two cuts to remove the excess tissue without damaging the DLMs.
- Once isolated, transfer the hemithorax to the correct tube with 1x PBS.
- Repeat steps 4.6‒4.14 until 10 dissected hemithoraces per group are made.
5. Structural staining
- After bisecting the thorax samples, place the tissue in blocking buffer (1x PBS with 0.1% normal goat serum, and 0.2% Triton X-100 at pH 7.4) to permeabilize the tissue and prevent non-specific staining. Use a Pasteur pipette to remove excess 1x PBS and add 1.5 mL of blocking buffer to each tube. Block tissues for at least 1 h at 4 °C.
- Prepare the samples for structural staining using a fluorescently conjugated antibody, horseradish peroxidase 488 (anti-HRP-488) at a dilution of 1:200 and Phalloidin-647 at a dilution of 1:1000 in blocking buffer to stain motor neurons and muscle tissue, respectively. Make enough stain to have 150 µL per tube. Store the stain at 4 °C covered in foil or in a dark box until ready for staining.
- After blocking, remove the excess blocking buffer with a glass Pasteur pipette.
- Before dispensing the structural stain, vortex the stain. Add 150 µL of the stain to each tube. Place the samples in a dark box on the rotator at room temperature for 2 h.
- Remove the stain and wash the tissues four times in 1.5 mL of room temp 1x PBS with 0.3% Triton X-100 for 5 min on the rotator in a dark box. The samples are now ready to mount to a slide.
6. Mounting tissue
- After washing samples in PBST, prepare a microscope slide to mount tissue for staining. Prepare additional supplies including glass cover slips, a P200 pipette, 200 µL pipette tips, scissors, clear reinforcements, straight edge forceps, anti-fade fluorescent mounting media, nail polish, and a dark box to cover the slides.
- Label the slide to identify the samples and clean the slide with kimwipes to ensure there are no smudges.
- To ensure the hemithorax samples are not damaged by the cover slip, build a “bridge” using reinforcement labels. Take one reinforcement label, cut it in half, and place each half approximately 15 mm apart. This distance must be smaller than the width of the cover slip. Repeat this step four times to complete a “bridge” that is 5 labels high.
- Take the P200 pipette and modify a tip by cutting off 1/5th of the tip to transfer the samples to the slide. Samples should be transferred onto the slide in the center of the bridge.
- Take the edge of a lab wipe and remove any excess PBST. Using forceps, arrange the DLMs such that all samples are facing muscle side up and cuticle side down.
- Using a standard P200 pipette tip, apply 70 µL of mounting media to the slide, avoiding air bubbles. Dispense the media in a circular pattern inside the reinforcements starting from the outside into the center.
- Place a cover slip over the reinforcements.
- Use nail polish to coat the outside edges around the perimeter of the coverslip. Apply generously to form a complete seal of the tissue.
- Place the slide on a flat surface in the dark, allowing at least 10 min to dry and prevent photo-bleaching or loss of fluorescence. Slides may now be used for imaging immediately, or otherwise stored in a slide folder at -20 °C for later viewing.
7. Alternative: Staining with primary antibodies
NOTE: This section is optional and should be used directly between sections 4 and 5 if desired.
- To stain tissue with primary antibodies, submerge tissue in blocking buffer for at least 1 h.
- Prepare primary antibody with proper dilution in blocking buffer. At minimum, prepare enough antibody mixture to have 150 µL per group. Note that the samples are kept still. Store at 4 °C until ready for use.
- Remove excess blocking buffer with a Pasteur pipette. Briefly vortex the primary antibody and add 150 µL of antibody mixture to each group and place samples at 4 °C overnight.
- On the next day, remove primary antibody and wash tissue 4 times with PBST for 5 min each on a rotator.
- Prepare secondary stain in blocking buffer. Add the secondary stain to the sample and then keep it a room temperature for 2 h in a dark box on the rotator.
NOTE: The secondary staining can also include HRP and phalloidin. - After the 2 h incubation, to remove the secondary stain wash tissue 4 times for 5 min with PBST and proceed to mounting.
Representative Results
The generation of transgenic flies expressing human Tar-Binding Protein of 43 kDa mutant (TDP-43M337V) is represented by the schematic (Figure 1A). This demonstrates the application of the binary Gal4/UAS system in Drosophila27. The illustration depicts a hemithorax with six muscle fibers, A‒F going from the most dorsal fiber A to the most ventral F (Figure 1B)11,12. To assess synaptic integrity, NMJs were stained with HRP and Phalloidin (Figure 1C‒E). Motor neurons in TDP-43M337V mutants (Figure 1F) have little to no HRP staining by Day 21, while WT (Oregon-R) remains intact (Figure 1C). There are no visible differences in muscle staining (Figure 1D,G). The changes in gross morphology observed in TDP-43M337V mutants demonstrates how synaptic integrity can be implicated in a neurodegenerative disease model of amyotrophic lateral sclerosis (ALS) using the adult DLM model. In addition to structural staining, staining the DLM NMJs can also provide an assessment of synaptic integrity with presynaptic (Figure 2A‒R) and post synaptic (Figure 2S‒X) markers. Together, these results illustrate how this dissection protocol could be applied to studying DLM tissue in neurodegenerative diseases.
One key aspect of this dissection is the application of liquid nitrogen to flash freeze the tissue to make the bisection easier. The utility of the liquid nitrogen is demonstrated in WT flies with liquid nitrogen where muscle tissue has no damage or nicked fibers (Figure 3A‒C). Without liquid nitrogen, the tissue can be more difficult to dissect. For example, following this protocol and skipping the liquid nitrogen flash freezing step allows the tissue to be more susceptible to damage from the dissection tools such as damaged neurons (Figure 3D) or damaged muscle fibers (Figure 3E). The application of liquid nitrogen helps to prevent tissue damage that could occur when working with DLM tissue regardless of the genotype of the specimen (Figure 3C and 3F).

Figure 1: Progressive denervation of DLM synapses in a Drosophila model of ALS. (A) The generation of ALS transgenic flies expressing a human mutant form of Tar-Binding Protein of 43 kDa (TDP-43) are shown in the schematic. (B) The illustration depicts the shape and orientation of a hemithorax in an adult Drosophila. Using the protocol, we can observe the progressive loss of synaptic integrity of DLM NMJ synapses through structural staining of motor neurons with HRP (green) and muscle tissue with Phalloidin (magenta). Our model depicts the loss of synaptic integrity in an adult model of ALS through the generation of adult flies expressing a mutant from of human TDP-43M337V in motor neurons (Figure 1F‒H) in comparison to WT (Figure 1C‒E) flies in muscle fiber C. Arrows highlight examples of a WT synapse (Figure 1C) and an example of loss of synaptic integrity. Scale bar =20 µm at 63x magnification. Please click here to view a larger version of this figure.

Figure 2: Assessing synaptic integrity using presynaptic markers at adult NMJs. Synaptic integrity can also be assessed using presynaptic and postsynaptic markers in WT flies that are 14 days old in muscle fiber C. The presynaptic markers Synapsin (B), Syntaxin (H), and Bruchpilot (BRP) (N) are co-stained with HRP (A, G, M). The staining depicts the localization of these markers to the presynaptic terminals (C, I, O). At higher magnification, the images illustrate the localization of Synapsin (E), Syntaxin (K), and BRP (Q) with HRP (D, J, and P) in more detail (Figure F, L, and R). We also show a postsynaptic marker Glutamate Receptor III (GluRIII) (T) co-stained with HRP (S). The co-staining demonstrates the utility of these markers (U). At higher magnification the representative images exemplify the localization (X) of GluRIII (W) and HRP (V) to the postsynaptic muscle tissue and the presynaptic terminals, respectively. Scale bar for panels A‒C, G-I, M‒O, S‒U represent 20 µm at 63x magnification. Scale bar for panels D‒F, 2J-2L, 2P-2R, and 2V-2X represent 10 µm at 63x magnification. Please click here to view a larger version of this figure.
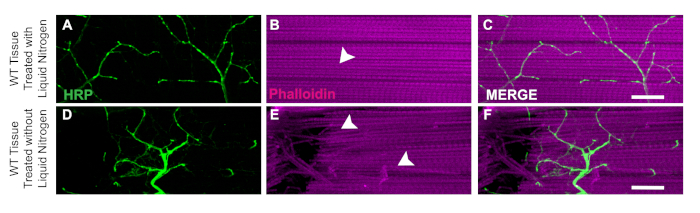
Figure 3: Utility of liquid nitrogen for DLM dissections. To demonstrate the utility of liquid nitrogen for the DLM dissections, we show a comparison of day 21 WT flies with and without liquid nitrogen from muscle fiber C. With liquid nitrogen, Phalloidin (B) remains intact and does not compromise the HRP staining (A, C). Without liquid nitrogen, muscle tissue becomes stringy and difficult to bisect (E) and HRP staining (D, F) becomes compromised due to technical error. White arrows show an area of no muscle damage in with liquid nitrogen (B) and damaged muscle tissue (E). Scale bar = 20 µm at 63x magnification. Please click here to view a larger version of this figure.
Discussion
Using the methods described in this protocol, we provide a straightforward approach for dissection of the DLM tissue and demonstrate how this can be applied to assess synaptic integrity through structural staining and synaptic markers in adult Drosophila. One critical step in the protocol that makes the DLM tissue easier to dissect is the flash freezing with liquid nitrogen. Without this step, the tissue is less firm and more difficult to cut precisely as observed in Figure 3. This protocol builds upon previous dissection methods to allow the preservation of both motor neurons and muscle tissue18,19,20,21,22,23. One limitation of this protocol is that when making the cut down the midline for the bisection, it can be difficult to get two clean preps per thorax. One way to ensure at least one hemithorax per fly, you can purposely cut off to one side of the thorax to get one clean prep. With this modification, one may also need to remove additional excess tissue from the cut to clean up the sample with the blade breaker. For those new to this technique, with continued practice, accuracy of the bisection will increase.
The method described here allows researchers to easily assess structural integrity of adult DLM NMJs at any time throughout their lifespan. A major advantage of this protocol is the ability to access synaptic integrity in neurodegenerative disease models by using synaptic markers. We demonstrate that this application can help visualize changes in gross morphology with structural staining (Figure 1C‒H). Additionally, synaptic integrity can be assessed with staining of presynaptic markers including but not limited to Synapsin28 (Figure 2A‒F), Syntaxin29 (Figure 2G‒L) and BRP30 (Figure 2M‒R). The postsynaptic muscle tissue can also be assessed using the Glutamate Receptor III subunit antibody31 (Figure 2S‒X), demonstrating the utility of this protocol.
Researchers can also utilize this dissection method to complement functional data to comprehensively examine the structural integrity of synapses associated with a wide variety of diseases. These synapses also allow for functional analysis through electrophysiological recordings32,33,34 and the flight assay10. This protocol can also provide ease of access to the tissue for many applications and assays. Future studies, for example, could use this protocol to quantify synaptic changes through quantification of the density and number of synapses15,16. While the protocol described here specifically examines synaptic integrity of motor neurons, complementary protocols for assessing muscle cell loss can also be performed with this dissection using TUNEL staining35. To examine neuronal loss, dissection of the thoracic ganglion36 could also be used with TUNEL staining. We expect that the dissection described here will have more applications to future studies assessing age-related pathologies as well as neurodegenerative diseases.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Institutes of Health (R01 NS110727) to D.T.B.
Materials
| 32% Formaldehyde | Electron Microscopy Sciences | 15714 | Tissue preservation |
| Alexa Fluor 568 goat anti mouse | Fisher Scientific | A11031 | Labels primary antibodies. Used at 1:200 concentration. |
| Alexa Fluor 568 goat anti rabbit | Fisher Scientific | A11036 | Labels primary antibodies. Used at 1:200 concentration. |
| anti- Bruchpilot (BRP) antibody | Developmental Studies Hybridoma Bank | NC82 | Stains the active zones in presynaptic neurons. Used at 1:25 concentration. |
| anti-GluRIII antibody | Gift from Aaron DiAntonio | N/A | Labels glutamate receptor subunits. Used at 1:1000 concentration. |
| anti-Synapsin antibody | Developmental Studies Hybridoma Bank | 3C11 | Labels the synaptic protein synapsin. Used at 1:50 concentration. |
| anti-Syntaxin antibody | Developmental Studies Hybridoma Bank | 8C3 | labels the synaptic protein syntaxin. Used at 1:10 concentration. |
| BenchRocker | Genesee Scientific | 31-302 | Rotating samples during staining |
| Blade Breaker | Fine Science Tools | 10053-09 | Used for holding feather blade |
| cover slips | Fisher Scientific | 12548A | For mounting tissue |
| cryogenic gloves | VWR | 97008-198 | protect hands from liquid nitrogen |
| cryogenic tweezers | VWR | 82027-432 | Hold 2.0 mL tube in liquid nitrogen |
| dewar flask-1900 mL | Thomas Scientific | 5028M54 | Hold liquid nitrogen |
| Feather Blades | Electron Microscopy Sciences | 72002-01 | Scalpel Blades |
| Fine Forecps x 2 | Fine Science Tools | 11252-20 | One fine pair for Clearing midline of thorax. The other pair can be dulled using a sharpening stone. |
| FITC-conjugated anti HRP | Jackson Laboratories | 123-545-021 | Stains Motor Neurons. Used at 1:100 concentration |
| freezer box (Black) | Fisher Scientific | 14100F | Protects samples from light |
| glass pasteur pipettes | VWR | 14637-010 | Used to transfer samples |
| glass slides | Fisher Scientific | 12550143 | For mounting tissue |
| mounting media (vectashield) anti-fade | VWR | 101098-042 | Mounting media retains fluorescent signaling |
| nail polish | Electron Microscopy Sciences | 72180 | Seals microscope slides |
| normal goat serum | Fisher Scientific | PCN5000 | Prevents non-specific binding of antibodies |
| paint brush | Genesee Scientific | 59-204 | Transferring flies |
| PBS | Fisher Scientific | 10-010-023 | Saline solution for dissecting and staining |
| Phalloidin 647 | Abcam | AB176759 | Stains F-Actin in muscle Tissue. Used at 1:1000 concentration |
| plastic petri dish (100 mm) | VWR | 25373-100 | Dissection dish |
| reinforcement labels | W.B. Mason | AVE05722 | Provides support for glass coverslip over the mounted tissue |
| sharpening block | Grainger | 1RDF5 | Keeping fine forceps sharp and also dulling separate pair |
| slide folder | VWR | 10126-326 | Sample storage |
| standard office scissors | W.B. Mason | ACM40618 | Cutting reinforcement labels |
| Sylgard 184 | Electron Microscopy Sciences | 24236-10 | Coating for dissection dish |
| Triton-X-100 | Electron Microscopy Sciences | 22140 | Helps to permeabilize tissue |
| Vannas Disssection Sissors | Fine Science Tools | 1500-00 | Ued for removing fly legs and making an incision on thorax |
Riferimenti
- Casas, C., Manzano, R., Vaz, R., Osta, R., Brites, D. Synaptic failure: focus in an integrative view of ALS. Brain Plasticity. 1, 159-175 (2016).
- Lodato, M. A., et al. Aging and neurodegeneration are associated with increased mutations in single human neurons. Science. 359, 555-559 (2018).
- López-Erauskin, J., et al. ALS/FTD-linked mutation in FUS suppresses intra-axonal protein synthesis and drives disease without nuclear loss-of-function of FUS. Neuron. 100, 816-830 (2018).
- Munsie, L., et al. Retromer-dependent neurotransmitter receptor trafficking to synapses is altered by the Parkinson’s disease VPS35 mutation p. D620N. Human Molecular Genetics. 24, 1691-1703 (2015).
- Oddo, S., et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Aβ and synaptic dysfunction. Neuron. 39, 409-421 (2003).
- Selkoe, D. J. Alzheimer’s disease is a synaptic failure. Science. 298, 789-791 (2002).
- Collins, C. A., DiAntonio, A. Synaptic development: insights from Drosophila. Current Opinion in Neurobiology. 17, 35-42 (2007).
- Jan, L., Jan, Y. Properties of the larval neuromuscular junction in Drosophila melanogaster. The Journal of Physiology. 262, 189-214 (1976).
- Zito, K., Parnas, D., Fetter, R. D., Isacoff, E. Y., Goodman, C. S. Watching a synapse grow: noninvasive confocal imaging of synaptic growth in Drosophila. Neuron. 22, 719-729 (1999).
- Babcock, D. T., Ganetzky, B. An improved method for accurate and rapid measurement of flight performance in Drosophila. Journal of Visualized Experiments. , e51223 (2014).
- Fernandes, J., Bate, M., Vijayraghavan, K. Development of the indirect flight muscles of Drosophila. Development. 113, 67-77 (1991).
- Fernandes, J., VijayRaghavan, K. The development of indirect flight muscle innervation in Drosophila melanogaster. Development. 118, 215-227 (1993).
- Fernandes, J. J., Keshishian, H. Patterning the dorsal longitudinal flight muscles (DLM) of Drosophila: insights from the ablation of larval scaffolds. Development. 122, 3755-3763 (1996).
- Fernandes, J. J., Keshishian, H. Nerve-muscle interactions during flight muscle development in Drosophila. Development. 125, 1769-1779 (1998).
- Hebbar, S., Fernandes, J. J. Pruning of motor neuron branches establishes the DLM innervation pattern in Drosophila. Journal of Neurobiology. 60, 499-516 (2004).
- Hebbar, S., Fernandes, J. J. A role for Fas II in the stabilization of motor neuron branches during pruning in Drosophila. Developmental Biolology. 285, 185-199 (2005).
- Danjo, R., Kawasaki, F., Ordway, R. W. A tripartite synapse model in Drosophila. PloS One. 6, (2011).
- Hunt, L. C., Demontis, F. Whole-mount immunostaining of Drosophila skeletal muscle. Nature Protocols. 8, 2496-2501 (2013).
- Kucherenko, M. M., et al. Paraffin-embedded and frozen sections of Drosophila adult muscles. Journal of Visualized Experiments. , e2438 (2010).
- Llamusi, B., et al. BSF and TBPH are mislocalized in the muscle sarcomere of a Drosophila myotonic dystrophy model. Disease Models & Mechanisms. 6, 184-196 (2013).
- Pantoja, M., Fischer, K. A., Ieronimakis, N., Reyes, M., Ruohola-Baker, H. Genetic elevation of sphingosine 1-phosphate suppresses dystrophic muscle phenotypes in Drosophila. Development. 140, 136-146 (2013).
- Schnorrer, F., et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature. 464, 287-291 (2010).
- Viswanathan, M. C., Blice-Baum, A. C., Schmidt, W., Foster, D. B., Cammarato, A. Pseudo-acetylation of K326 and K328 of actin disrupts Drosophila melanogaster indirect flight muscle structure and performance. Frontiers in Physiology. 6, 116 (2015).
- Hebbar, S., Fernandes, J. J. Glial remodeling during metamorphosis influences the stabilization of motor neuron branches in Drosophila. Biologia dello sviluppo. 340, 344-354 (2010).
- Mahr, A., Aberle, H. The expression pattern of the Drosophila vesicular glutamate transporter: a marker protein for motoneurons and glutamatergic centers in the brain. Gene Expression Patterns. 6, 299-309 (2006).
- Ritson, G. P., et al. TDP-43 mediates degeneration in a novel Drosophila model of disease caused by mutations in VCP/p97. Journal of Neuroscience. 30, 7729-7739 (2010).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118, 401-415 (1993).
- Klagges, B. R., et al. Invertebrate synapsins: a single gene codes for several isoforms in Drosophila. Journal of Neuroscience. 16, 3154-3165 (1996).
- Fujita, S. C., Zipursky, S. L., Benzer, S., Ferrus, A., Shotwell, S. L. Monoclonal antibodies against the Drosophila nervous system. Proceedings of the National Academy of Sciences of the United States of America. 79, 7929-7933 (1982).
- Wagh, D. A., et al. a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 49, 833-844 (2006).
- Marrus, S. B., DiAntonio, A. Preferential localization of glutamate receptors opposite sites of high presynaptic release. Current Biology. 14, 924-931 (2004).
- Augustin, H., Allen, M. J., Partridge, L. Electrophysiological recordings from the giant fiber pathway of D. melanogaster. Journal of Visualized Experiments. , e2412 (2011).
- Maccioni, R., et al. Standardized phytotherapic extracts rescue anomalous locomotion and electrophysiological responses of TDP-43 Drosophila melanogaster model of ALS. Scientific Reports. 8, 16002 (2018).
- Siddiqi, O., Benzer, S. Neurophysiological defects in temperature-sensitive paralytic mutants of Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 73, 3253-3257 (1976).
- Wang, Z. H., Clark, C., Geisbrecht, E. R. Drosophila clueless is involved in Parkin-dependent mitophagy by promoting VCP-mediated Marf degradation. Human Molecular Genetics. 25, 1946-1964 (2016).
- O’Sullivan, A., et al. Multifunctional Wing Motor Control of Song and Flight. Current Biology. 28, 2705-2717 (2018).

