Udnyttelse af vinkelrette ventrikulære hjælpeenheder i akut myokardieinfarkt kompliceret af kardiogen shock
Summary
Vinkelrette ventrikulære hjælpeanordninger bliver i stigende grad udnyttet hos patienter med akut myokardieinfarkt og kardiogent chok. Heri diskuterer vi virkningsmekanismen og de hæmodynamiske virkninger af sådanne anordninger. Vi gennemgår også algoritmer og bedste praksis for implantation, styring og fravænning af disse komplekse enheder.
Abstract
Kardiogent chok er defineret som vedvarende hypotension, ledsaget af tegn på end organ hypo-perfusion. Vinkelrette ventrikulære hjælpeanordninger (PVADs) bruges til behandling af kardiogen chok i et forsøg på at forbedre hæmodynamik. Impella er i øjeblikket den mest almindelige PVAD og aktivt pumper blod fra venstre ventrikel ind i aorta. PVADs losse venstre ventrikel, øge hjerte output og forbedre koronar perfusion. PVAD’er placeres typisk i laboratoriet for hjertekateterisation under fluoroskopisk vejledning via lårpulsåren, når det er muligt. I tilfælde af alvorlig perifer arteriel sygdom kan PVAD’er implanteres gennem en alternativ adgang. I denne artikel opsummerer vi PVAD’s virkningsmekanisme og de data, der understøtter deres anvendelse til behandling af kardiogenchok.
Introduction
Kardiogent chok (CS) defineres som vedvarende hypotension (systolisk blodtryk 30 minutter eller behovet for vasopressorer eller inotroper), hypomafusion i end organet (urinudgang 2 mmol/L), lungepropper (lungekapillær kiletryk (PCWP) ≥ 15 mmHg) og nedsat hjerteydelse (hjerteindeks <2.2 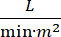 )1, 2 på grund af en primær hjertesygdom. Akut myokardieinfarkt (AMI) er den mest almindelige årsag til CS3. CS forekommer i 5-10 % af AMI og har historisk set været forbundet med betydeligdødelighed 3,4. Mekaniske kredsløbshjælpemidler (MCS) enheder såsom intra-aorta ballonpumpe (IABP), vinkelrette ventrikulære hjælpeanordninger (PVAD), ekstrakorporal membran iltning (ECMO) og perkutan venstre atrie til aorta enheder bruges ofte til patienter med CS5. Rutinemæssig brug af IABP har ikke vist nogen forbedring i kliniske resultater eller overlevelse i AMI-CS1. I betragtning af de dårlige resultater forbundet med AMI-CS, vanskelighederne med at gennemføre forsøg i AMI-CS, og de negative resultater af IABP brug i AMI-CS, klinikere er i stigende grad ser til andre former for MCS.
)1, 2 på grund af en primær hjertesygdom. Akut myokardieinfarkt (AMI) er den mest almindelige årsag til CS3. CS forekommer i 5-10 % af AMI og har historisk set været forbundet med betydeligdødelighed 3,4. Mekaniske kredsløbshjælpemidler (MCS) enheder såsom intra-aorta ballonpumpe (IABP), vinkelrette ventrikulære hjælpeanordninger (PVAD), ekstrakorporal membran iltning (ECMO) og perkutan venstre atrie til aorta enheder bruges ofte til patienter med CS5. Rutinemæssig brug af IABP har ikke vist nogen forbedring i kliniske resultater eller overlevelse i AMI-CS1. I betragtning af de dårlige resultater forbundet med AMI-CS, vanskelighederne med at gennemføre forsøg i AMI-CS, og de negative resultater af IABP brug i AMI-CS, klinikere er i stigende grad ser til andre former for MCS.
PVAD’er anvendes i stigende grad hos patienter med AMI-CS6. I denne artikel vil vi primært fokusere vores diskussion på Impella CP, som er den mest almindelige PVAD, der bruges i øjeblikket6. Denne enhed anvender en aksial flow Arkimedes-skrue pumpe, som aktivt og kontinuerligt driver blod fra venstre ventrikel (LV) ind i stigende aorta (Figur 1). Enheden er oftest placeret i hjertekateterisation laboratorium under fluoroskopisk vejledning via lårpulsåren. Alternativt kan det implanteres gennem en aksillær eller transkaval adgang, når det er nødvendigt7,8.
Protocol
Representative Results
Discussion
Minimering af risici og komplikationer ved PVAD (tabel 2)
De hæmodynamiske fordele ved PVAD kan neutraliseres betydeligt, hvis der opstår komplikationer fra storboring, såsom større blødning og akut lemmer iskæmi28,29. Det er derfor vigtigt at minimere enhedens risiko og komplikationer.
For at mindske komplikationer på adgangsstedet og reducere antallet af adgangsforsøg bør ultralyds- og fluoroskopisk vejle…
Disclosures
The authors have nothing to disclose.
Acknowledgements
Ingen
Materials
| 4 Fr-018-10 cm Silhouette Stiffened Micropuncture Set | Cook | G48002 | Microvascular access |
| 5 Fr Infiniti Pigtail Catheter | Cordis | 524-550S | pigtail catheter |
| Impella CP Intra-cardiac Assist Catheter | ABIOMED | 0048-0003 | Impella catheter kit |
References
- Holger, T., et al. Intraaortic Balloon Pump in Cardiogenic Shock Complicating Acute Myocardial Infarction. Circulation. 139 (3), 395-403 (2019).
- Hochman, J. S., et al. Early Revascularization in Acute Myocardial Infarction Complicated by Cardiogenic Shock. New England Journal of Medicine. 341 (9), 625-634 (1999).
- van Diepen, S., et al. Contemporary Management of Cardiogenic Shock: A Scientific Statement From the American Heart Association. Circulation. 136 (16), 232-268 (2017).
- Kolte, D. h. a. v. a. l., et al. Trends in Incidence, Management, and Outcomes of Cardiogenic Shock Complicating ST-Elevation Myocardial Infarction in the United States. Journal of the American Heart Association. 3 (1), 000590 (2014).
- Aditya, M., Sunil, R. V. Percutaneous Mechanical Circulatory Support Devices in Cardiogenic Shock. Circulation: Cardiovascular Interventions. 10 (5), 004337 (2017).
- Amit, A. P., et al. The Evolving Landscape of Impella Use in the United States Among Patients Undergoing Percutaneous Coronary Intervention With Mechanical Circulatory Support. Circulation. 141 (4), 273-284 (2020).
- Kajy, M., et al. Deploying Mechanical Circulatory Support Via the Axillary Artery in Cardiogenic Shock and High-Risk Percutaneous Coronary Intervention. The American Journal of Cardiology. 128, 127-133 (2020).
- Afana, M., et al. Transcaval access for the emergency delivery of 5.0 liters per minute mechanical circulatory support in cardiogenic shock. Catheterization and Cardiovascular Interventions. , 29235 (2020).
- Sandoval, Y., et al. Contemporary Arterial Access in the Cardiac Catheterization Laboratory. JACC: Cardiovascular Interventions. 10 (22), 2233-2241 (2017).
- Seto, A. H., et al. Real-Time Ultrasound Guidance Facilitates Femoral Arterial Access and Reduces Vascular Complications. JACC: Cardiovascular Interventions. 3 (7), 751-758 (2010).
- Mignatti, A., Friedmann, P., Slovut, D. P. Targeting the safe zone: A quality improvement project to reduce vascular access complications: Vascular Access Complications Postcardiac Catheterization. Catheterization and Cardiovascular Interventions. 91 (1), 27-32 (2018).
- Rihal, C. S., et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. Journal of the American College of Cardiology. 65 (19), 7-26 (2015).
- Burzotta, F., et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. International Journal of Cardiology. 201, 684-691 (2015).
- Basir, M. B., et al. Improved Outcomes Associated with the use of Shock Protocols: Updates from the National Cardiogenic Shock Initiative. Catheterization and Cardiovascular Interventions. 93 (7), 1173-1183 (2019).
- Kaki, A., et al. Access and closure management of large bore femoral arterial access. Journal of Interventional Cardiology. 31 (6), 969-977 (2018).
- Basir, M. B., et al. Effect of Early Initiation of Mechanical Circulatory Support on Survival in Cardiogenic Shock. The American Journal of Cardiology. 119 (6), 845-851 (2017).
- Tehrani, B. N., et al. Standardized Team-Based Care for Cardiogenic Shock. Journal of the American College of Cardiology. 73 (13), 1659-1669 (2019).
- Ouweneel, D. M., et al. Percutaneous Mechanical Circulatory Support Versus Intra-Aortic Balloon Pump in Cardiogenic Shock After Acute Myocardial Infarction. Journal of the American College of Cardiology. 69 (3), 278-287 (2017).
- Alushi, B., et al. Impella versus IABP in acute myocardial infarction complicated by cardiogenic shock. Open Heart. 6 (1), 000987 (2019).
- Ginwalla, M., Tofovic, D. S. Current Status of Inotropes in Heart Failure. Heart Failure Clinics. 14 (4), 601-616 (2018).
- O’Neill, W. W., et al. Analysis of outcomes for 15,259 US patients with acute myocardial infarction cardiogenic shock (AMICS) supported with the Impella device. American Heart Journal. 202, 33-38 (2018).
- O’neill, W. W., et al. The Current Use of Impella 2.5 in Acute Myocardial Infarction Complicated by Cardiogenic Shock: Results from the USpella Registry. Journal of Interventional Cardiology. 27 (1), 1-11 (2014).
- Hernandez, G. A., et al. Trends in Utilization and Outcomes of Pulmonary Artery Catheterization in Heart Failure With and Without Cardiogenic Shock. Journal of Cardiac Failure. 25 (5), 364-371 (2019).
- Thayer, K., et al. Pulmonary Artery Catheter Usage and Mortality in Cardiogenic Shock. The Journal of Heart and Lung Transplantation. 39 (4), 54-55 (2020).
- Fincke, R., et al. Cardiac power is the strongest hemodynamic correlate of mortality in cardiogenic shock: A report from the SHOCK trial registry. Journal of the American College of Cardiology. 44 (2), 340-348 (2004).
- Lim, H. S., Gustafsson, F. Pulmonary artery pulsatility index: physiological basis and clinical application. European Journal of Heart Failure. 22 (1), 32-38 (2020).
- Korabathina, R., et al. The pulmonary artery pulsatility index identifies severe right ventricular dysfunction in acute inferior myocardial infarction. Catheterization and Cardiovascular Interventions. 80 (4), 593-600 (2012).
- Lauten, A., et al. Percutaneous left-ventricular support with the Impella-2.5-assist device in acute cardiogenic shock: results of the Impella-EUROSHOCK-registry. Circulation. Heart Failure. 6 (1), 23-30 (2013).
- Dixon, S. R., et al. A Prospective Feasibility Trial Investigating the Use of the Impella 2.5 System in Patients Undergoing High-Risk Percutaneous Coronary Intervention (The PROTECT I Trial): Initial U.S. Experience. JACC: Cardiovascular Interventions. 2 (2), 91-96 (2009).
- Abu-Fadel, M. S., et al. Fluoroscopy vs. Traditional guided femoral arterial access and the use of closure devices: A randomized controlled trial. Catheterization and Cardiovascular Interventions. 74 (4), 533-539 (2009).
- Lata, K., Kaki, A., Grines, C., Blank, N., Elder, M., Schreiber, T. Pre-close technique of percutaneous closure for delayed hemostasis of large-bore femoral sheaths. Journal of Interventional Cardiology. 31 (4), 504-510 (2018).
- Basir, M. B., et al. Feasibility of early mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock: The Detroit cardiogenic shock initiative. Catheterization and Cardiovascular Interventions. 91 (3), 454-461 (2018).
- Udesen, N. J., et al. Rationale and design of DanGer shock: Danish-German cardiogenic shock trial. American Heart Journal. 214, 60-68 (2019).
- Clinical Research. Protected PCI Community Available from: https://www.protectedpci.com/clinical-research/ (2020)
- Seyfarth, M., et al. A Randomized Clinical Trial to Evaluate the Safety and Efficacy of a Percutaneous Left Ventricular Assist Device Versus Intra-Aortic Balloon Pumping for Treatment of Cardiogenic Shock Caused by Myocardial Infarction. Journal of the American College of Cardiology. 52 (19), 1584-1588 (2008).
- Schrage, B., et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation. 139 (10), 1249-1258 (2019).
- Casassus, F., et al. The use of Impella 2.5 in severe refractory cardiogenic shock complicating an acute myocardial infarction. Journal of Interventional Cardiology. 28 (1), 41-50 (2015).
- Joseph, S. M., Brisco, M. A., Colvin, M., Grady, K. L., Walsh, M. N., Cook, J. L. Women With Cardiogenic Shock Derive Greater Benefit From Early Mechanical Circulatory Support: An Update From the cVAD Registry. Journal of Interventional Cardiology. 29 (3), 248-256 (2016).
- Lauten, A., et al. Percutaneous Left-Ventricular Support With the Impella-2.5-Assist Device in Acute Cardiogenic Shock. Circulation: Heart Failure. 6 (1), 23-30 (2013).
- Ouweneel, D. M., et al. Impella CP Versus Intra-Aortic Balloon Pump in Acute Myocardial Infarction Complicated by Cardiogenic Shock: The IMPRESS trial. Journal of the American College of Cardiology. , 23127 (2016).
- Badiye, A. P., Hernandez, G. A., Novoa, I., Chaparro, S. V. Incidence of Hemolysis in Patients with Cardiogenic Shock Treated with Impella Percutaneous Left Ventricular Assist Device. ASAIO Journal. 62 (1), 11-14 (2016).

