Stereotaxic Intracranial Delivery of Chemicals, Proteins or Viral Vectors to Study Parkinson’s Disease
Summary
We describe how to successfully inject solutions into specific brain areas of rodents using a stereotaxic frame. This survival surgery is a well-established method used to mimic various aspects of Parkinson’s disease.
Abstract
Parkinson’s disease (PD) is a progressive disorder traditionally defined by resting tremor and akinesia, primarily due to loss of dopaminergic neurons in the substantia nigra. Affected brain areas display intraneuronal fibrillar inclusions consisting mainly of alpha-synuclein (asyn) proteins. No animal model thus far has recapitulated all characteristics of this disease. Here, we describe the use of stereotaxic injection to deliver chemicals, proteins, or viral vectors intracranially in order to mimic various aspects of PD. These methods are well-established and widely used throughout the PD field. Stereotaxic injections are incredibly flexible; they can be adjusted in concentration, age of animal used for injection, brain area targeted and in animal species used. Combinations of substances allow for rapid variations to assess treatments or alter severity of the pathology or behavioral deficits. By injecting toxins into the brain, we can mimic inflammation and/or a severe loss of dopaminergic neurons resulting in substantial motor phenotypes. Viral vectors can be used to transduce cells to mimic genetic or mechanistic aspects. Preformed fibrillar asyn injections best recapitulate the progressive phenotype over an extended period of time. Once these methods are established, it can be economical to generate a new model compared to creating a new transgenic line. However, this method is labor intensive as it requires 30 minutes to four hours per animal depending on the model used. Each animal will have a slightly different targeting and therefore create a diverse cohort which on one hand can be challenging to interpret results from; on the other hand, help mimic a more realistic diversity found in patients. Mistargeted animals can be identified using behavioral or imaging readouts, or only after being sacrificed leading to smallercohort size after the study has already been concluded. Overall, this method is a rudimentary but effective way to assess a diverse set of PD aspects.
Introduction
Parkinson's disease (PD) is a relatively common progressive neurodegenerative disease affecting up to 1 % of people over the age of 601. PD is heterogenousbut clinically characterized mainly by motor symptoms including resting tremor, bradykinesia, akinesia, rigidity, gait disturbance and postural instability. The majority of motor symptoms typically appear when 60-70% of striatal dopamine (DA) is lost as a result of progressive and distinct neurodegeneration in the substantia nigra (SN) pars compacta2,3. Surviving dopaminergic neurons contain intracellular inclusions known as Lewy bodies4. These aggregates primarily consist of alpha-synuclein (asyn), a small but highly expressed protein in neurons in the brain5.
The underlying mechanism of neurodegeneration in PD is still unknown. Aging is still the biggest risk factor for this disorder6. Furthermore, humans are the only species that develops PD naturally. Therefore, in order to investigate PD pathology and test new drugs to prevent disease progression, a wide array of animal models have been developed7. Ideally, animal models of PD should display an age dependent, progressive loss of DA neurons in the SN, accompanied by intracellular inclusions followed by motor dysfunction and be responsive to DA replacement therapies. None of the currently available animal models fully recapitulate all clinical symptoms and pathology of PD. As each model presents with different aspects of the disease, it is important to carefully consider the appropriate model to use in an experiment based on the questions asked.
Historically, animal models were based on toxicants, including 6-hydroxydopamine (6-OHDA) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), and pesticides, such as rotenone and paraquat8. Each toxicant has a different mechanism of action and ranges from DA neuron specific to generally harmful to brain cells. Toxins can either be given orally, injected intraperitoneally or directly into the brain using stereotaxic injections depending on blood brain barrier permeability. Unlike other models, toxin models guarantee a high degree of nigrostriatal dopaminergic cell loss and behavioral phenotypes. Some models may even present with subtle pathology. These features make toxin PD models a great tool for studying replacement therapies and the effects of environmental toxins on the onset of PD9,10.
Additionally, numerous transgenic mouse models have been generated using a variety of promoters and PD related genes11. Most mice present with nigrostriatal pathology but without clear evidence of neurodegeneration. Transgenic models have the advantage of being consistent between animals and cohorts and once generated are easy to maintain and distribute. While they do not result in neurodegeneration, they are nevertheless useful models to investigate cellular changes caused by genetic variants and possible drug candidates in a complex in vivo system12.
In contrast to transgenic models, viral vector mediated expression of PD related genes offers a more flexible approach13. Stereotaxic injections allow for various brain areas, cell types, and expression levels to be chosen for a broad range of animal species such as mice, rats, pigs and non-human primates. Initially, recombinant viral vectors encoding for asyn were used to transduce neurons located in the rat SN. Protein accumulation and cellular dysfunction precede progressive dopaminergic cell loss resulting in behavioral deficit. Differences in targeting can lead to a large variation of cell loss between animals (30-80%), which is responsible for variable behavioral deficits seen in only approximately 25% of injected rats14.
A recently established model is the intracranial injection of preformed asyn fibrils (PFFs) or aggregate extracts from mouse or patient brain tissue15,16. Multiple studies indicate that the injection of PFFs or extracts result in a wide-spread asyn pathology in the animal brain as well as a loss of dopaminergic neurons in the SN. Accumulation of asyn appears within neurons innervating the injected area. Unlike viral vector-based models, the PFF model develops slowly over several months followed by motor deficits at 6 months. This model has great potentialfor studying the mechanism or prevention of asyn pathology17,18.
All models mentioned above have been well-established and used numerous times to study various aspects of the human disorder. Stereotaxic injections of substances directly into the brain have played a large part in the development of these animal models not only in the field of PD but also other neurological disorders. While it is labor-intensive, stereotaxic surgery has the advantages of being highly flexible in age of animals used, brain region targeted and substance injected, and can be adjusted depending on the research question asked. For example, substances can be injected singly or in combination (vector + fibrils or toxicant + vector) to recapitulate more aspects of the disease or assess treatments19,20. Additionally, substances can be injected unilaterally leaving the uninjected side as an internal control for evaluating behavior as well as neurodegeneration. Therefore, this manuscript will outline detailed steps to generate PD models using stereotaxic injections.
Protocol
All experiments in this study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health and approved by the Animal Care and Use Committees of the US National Institute on Aging.
Before starting, please make sure to have acquired the appropriate training and ethical approval from your institute necessary to perform this procedure. Additionally, anesthetics (e.g., ketamine and buprenorphine, or fentanyl and medetomidine) used should be acquired and handled according to relevant rules of your institution.
1. Preparation (duration 1 hour)
- Bring animals to the surgery room and let them acclimatize while setting up surgical area. Put on appropriate personal protection equipment (PPE).
NOTE: This can be dependent on local safety regulations and substance injected. Viral vectors mostly need biosafety level 2 PPE. Generally, at least wear a mask, gloves and disposable lab coat. In specific circumstances additional gloves and goggles should be used. - Sterilize the surgical tools using either an autoclave or a glass bead sterilizer. For rodents you need scalpel handle, hemostatic forceps, Dumont tweezers, curved forceps, wound closing kit (clips) or scissors and forceps (for sutures) and a drill head.
- Disinfect the surgical area and stereotaxic instrument with 70% ethanol, and cover the area next to stereotaxic instrument with either clean paper towels or a sterile absorbent sheet.
- Place the following on towels: eye drops (e.g., Liquigel), hair trimmer, cotton swabs, diluted iodixanol, disposable surgical blade, surgical drill with disinfected drill bit, marker or ear clipper, sterile H2O in a 1.5 mL tube, 1.5 mL tube with 3 % H2O2 in sterile H2O (1.35 mL of H2O + 0.15 mL of 30 % H2O2, freshly prepared), a 1 mL syringe with 33G needle and sterile PBS or saline, syringe with analgesic and antidote if injectable anesthesia is used (e.g., atipamezole + buprenorphine/ketoprofen; freshly prepared and approved in the ethical permit) and autoclaved surgical tools.
- Fill up isoflurane and check O2 and N2 pressures, if using gas anesthesia. Add a paper towel to the bottom of the inhalation chamber to keep it dry and clean.
Mix and freshly prepare the anesthetic (e.g., fentanyl + medetomidine (for rats) or ketamine + xylazine solution (for mice)) if using injectable anesthesia and if approved in the ethical permit. - Assemble the Hamilton glass syringe and glass capillary (Figure 1A 1.).
- Place the pulled glass capillary on the table. Place your index finger where capillary gets thinner with nail ending where capillary should be cut.
NOTE: Depending on the depth of your injection anywhere between 0.5 – 2 cm. - Use the edge of a ceramic tile to carefully scratch the needle part a few times. Take a glass capillary in one hand and use your thumb and index finger to grab the thin needle part. Pull from middle till end until the needle breaks off bluntly.
NOTE: If it does not work or the needle breaks off edged, repeat a few times. If it still does not break off, repeat tile scratching. Make sure the needle part is not too thin (>50 µm in diameter) and does not bend easily.
- Place the pulled glass capillary on the table. Place your index finger where capillary gets thinner with nail ending where capillary should be cut.
- Seal the glass capillary to the Hamilton syringe.
- Attach 1 cm shrink tubing over the blunt metal needle attached to the Hamilton glass syringe. Then put the thick end of glass capillary over the Hamilton metal needle.
- Place shrink tubing over half the glass capillary, ensuring at least 1 cm space between the end of the Hamilton metal needle and the conical transition of the capillary. This will be the space holding the desired injectable solution later.
- Use a lighter or match to heat up the shrink tubing and seal the injection needle.
NOTE: There are several different holder options that allow you to fix the Hamilton syringe to the stereotaxic arm on the left. Be sure you can still read the numbers on the Hamilton syringe. If you use a pump to inject the solution, attach the pump to the stereotaxic arm and fix the Hamilton syringe onto the pump.
- Remove the metal plunger. Insert a 27G needle attached to a 1 mL syringe filled with saline or PBS on the top part of the Hamilton syringe. Flush the Hamilton syringe with PBS to check if it is sealed and to flush out all air bubbles.
NOTE: Air bubbles will disturb your take-up and injection of your solution as air compresses more than liquid. - Once everything is checked and ready, open the little screw on the z-axis arm (Figure 1A 2.) of the stereotaxic instrument and rotate the arm in 90˚ increments clockwise to move glass syringe/capillary out of the way (to avoid the capillary to break while fixing the animal to the stereotaxic instrument).
NOTE: Check that all the stereotax angles and settings are set to desired parameters (normally 0˚), toothbar and earbars are set to desired coordinates and the Hamilton syringe/capillary are all straight. - Turn on the heat pad on the stereotax platform, using additional padding on top if it is too warm. Place an empty, clean cage with an additional heat pad next to surgical area.
2. Surgery (duration average 1 hour per animal)
- For gas anesthesia
- Turn on isoflurane to 5% with a N2 + O2 air flow (about 1 L/min) and open the valveto the inhalation chamber. Place the animal into the chamber and make sure it is sealed well. Observe the breathing until it has slowed down considerably (deep breathing).
- Once animal is unconscious, open the valve to stereotaxic instrument and close chamber valve. Set isoflurane to 2%.
- Open the chamber and take out the animal holding it at the neck, open its mouth with the forceps and place the animal onto the tooth bar (Figure 1A 6.) with the front upper teeth fitting into the hole.
NOTE: This step is time sensitive as the animal can wake up again by not breathing in isoflurane. - Place the nose cone over the snout and observe breathing to ensure a steady rhythm without forced breathing. Make sure the animal is deeply anesthetized by checking the absence of the pedal withdrawal reflexes of the hindlimbs before proceeding.
NOTE: Keep checking throughout the procedure so the animal doesn't stop breathing or wake up. If necessary, adjust % of isoflurane in small increments. To test pedal withdrawal reflex of the hindlimbs use a pair of tweezers and pinch the hindpaw. If the animal retracts the limb (reflex to remove from pain stimuli) the anesthesia is too low. - Fix the head of the animal in place using the two earbars (Figure 1A 7.). When done correctly, the ears should be pointing sideways over the ear bars. If the head is still moving adjust the ear bars or nose cone to fix the head tighter in place.
NOTE: Be gentleto avoid breaking the animals' eardrums by inserting the earbars too deep. Rats tend to blink when the earbar is inserted correctly.
- For injectable anesthesia:
- Inject the animal with an appropriate / recommended dose of anesthesia i.p. (300 µg/kg of fentanyl and 300 µg/kg medetomidine for rats; 100 mg/kg ketamine and 10 mg/kg xylazine for mice) and place it back into the home cage.
- Check animals' pedal withdrawal reflexes 5 min after injection. If absent fix the head of the animal in place using the two earbars (Figure 1A 7.). When done correctly, the ears should be pointing sideways over the ear bars. Afterwards, place the animalonto the toothbar (Figure 1A 6.) and screw down the lever. If the head is still moving adjust the ear bars or lever cone to fix the head tighter in place.
NOTE: It is possible to break an animal'seardrum when earbars are inserted too deep. Do not screw on lever too tight. It can break the animals' nose. - If the pedal withdrawal reflexes are observed, wait another 5-10 min. If still observed, inject another 10% of the previous anesthetic.
NOTE: Increase the anesthetic dose slowly with a waiting period in between to avoid the death of the animal due to asphyxiation. - Make sure that the skull is flat by adjusting tooth bar and/or ear bar heights.
NOTE: Be careful while adjusting the bars as the head will change angle and the nose cone/lever can break the animals' nose.
- Put eye drops on each eye (e.g., Liquigel) to avoid their drying out during the surgery. Shave the head of the animal from eyes to ears.
- Disinfect the surgical area using 10% iodopovidone. Additionally, inject a local anesthetic (e.g., 0.5% lidocaine) intradermally at the site of incision. It is recommended to wait for 2-3 min for it to act before continuing.
- Using the scalpel, make one continuous incision from between the eyes to between the ears.
NOTE: Be careful not to cut too far back as cutting the neck muscles will complicate recovery. - Use the cotton tips to open the wound and clean the area from blood. For rats, use the hemostatic forceps to keep the wound open, by clamping them into the subcutaneous tissue and letting them hang down each side. For mice, if necessary, use micro clips.
NOTE: This is the most painful part of the procedure. Clamping the subcutaneous tissue instead of the skin will increase healing. - Move the syringe/capillary (Figure 1A 1.) 180° counter-clockwise on the z-axis (Figure 1A 2.) and bring it over the animal's head. Make sure to close the knob completely so the z-axis arm does not wobble during surgery.
- Use the microscope and the knobs on each stereotaxic arm (Figure 1A 3.-5.) to move the blunt end of the glass capillary (attached to the Hamilton glass syringe; Figure 1A 1.) right on top of bregma (touching but not pressing on the bone). Set all values on the digital display to zero (Figure 1A 8.).
NOTE: Bregma (Figure 1B) is the point where the sagittal and coronal sutures of the parietal and frontal bones meet. This will be the point of origin. - Looking through the microscope, move the blunt end of the capillary right on top of lambda. If the z-axis (dorsal/ventral = DV) value is within +/-0.1 mm the head of the animal is leveled. If it is higher or lower use the tooth bar to adjust the head accordingly until within +/-0.1 mm.
NOTE: Lambda (Figure 1B) is defined as the point where the sagittal and coronal sutures of the parietal and occipital bones meet. This method could also be used to level the left and right side of the skull if ear bars can be adjusted individually. Be careful while adjusting bars as the head will change angle and nose cone / lever can break the animals' nose. - Use the Allen brain atlas (Figure 1C, D) to identify the coordinates for your desired target region. Use the y-axis (anterior/posterior = AP) and x-axis (medial/lateral = ML) arms to move the syringe/capillary to the correct position.
NOTE: Dye injections (Figure 2A-E) should be performed before surgical procedures on separate animals to ensure that the coordinates are correct for the strain/ age of the animals used. - Look through the microscope and prepare to carefully drill a hole where the blunt end of the capillary meets the bone. Before starting, move the capillary up enough on the y-axis so it will not get damaged while drilling. Start drilling in a bigger circle and get smaller as you drill deeper. While drilling, rest the hand on an ear bar to keep it steady.
NOTE: Do not drill all the way to the brain tissue. Leave a thin layer of bone to avoid damage to the dura. Instead use a tweezer to carefully remove the remaining thin layer of bone. - Using the microscope, check that the blunt end of the needle can touch the dura / brain tissue without being diverted by bone pieces.
- Clean the syringe / capillary to avoid contamination of the injection solution.
- Move syringe/capillary all the way up, and dip it into the 1.5 mL tube containing 3% H2O2. Make sure to remove all debris and blood off the glass. Then, dip the capillary into the 1.5 mL tube containing H2O to wash off the H2O2.
- Remove the metal plunger from the Hamilton syringe and place a gauze under the syringe/capillary, on top of the animal's head. Use the 1 mL syringe filled with PBS to wash the Hamilton syringe by inserting the needle in the top and squirting out PBS. After this, insert the metal plunger into the Hamilton syringe.
- Draw up 1 µL of air. This will keep the PBS in the Hamilton syringe and the injection solution from mixing in the capillary. Then draw up the desired amount of injection solution. A maximum of 1 µL and 2 µL per deposit is recommended for mice and rats respectively.
NOTE: A total of 3 µL /6 µL per hemisphere should not be exceeded as the pressure in the brain will rise too much. Using a pen, a little mark can be made very carefully where the meniscus of the solution is. This mark can then be used as a reference point to gauge if the solution enters the brain. - For rats, use a 25G needle to puncture the dura. Insert the capillary into the brain to the desired DV coordinate. Move the syringe/capillary 0.1 mm deeper than intended and draw back up to make space for the solution.
- Inject solution either by hand (0.1 µL per 10-15 sec) or with a pump (0.4-0.6 µL/min). Check the meniscus to ensure that the solution is fully injected.
NOTE: If the solution does not move into the brain, move the syringe/capillary up and down 0.2 mm. If it still does not inject repeat steps 2.13-2.14. Most likely the needle is clogged with brain tissue debris. - Hold the syringe/capillary in place for another 5 min to let the injected solution distribute and the pressure go down. During this waiting period, inject the analgesic and mark the animal if necessary.
- Move the syringe/capillary up 0.2 mm and hold in place for another 2 min. Draw the syringe/needle out very slowly to avoid pulling up any injected solution due to the negative pressure.
- Once the syringe/capillary is out of the brain, clean it as described in step 2.13 to prevent tissue from drying and clogging the capillary.
- Continue injecting more brain areas or finish the surgery.
- Move the syringe needle aside by turning it 180° on the z-axis to avoid breakage while closing the wound. Then, close the wound either with clips, tissue glue or sutures. If injectable anesthesia was used, inject the animal with the antidote.
NOTE: Animals not housed singly tend to clean each other's head and possibly remove clips, glue or stitches. - Remove the animalfrom the stereotaxic frame and place it into the clean cage on top of the heat pad. Monitor the animal to ensure it wakes up without complications. Provide easy access to food and water for the first 24 h.
3. Post-OP care (duration 3-7 days)
- Check the weight (10% weight loss is acceptable), fur, feces and food/water intake of the animal over the next 2-3 days (up to 10 days for toxicant injection) to ensure proper recovery. If necessary, isolate the animal and provide wet food, saline injections and a heating pad, essential for some toxicant injections as animals might have additional systemic issues.
- Inject animals with analgesic for another 1-2 days after surgery to ensure pain relief according to local regulations. If sutures, clips or glue does not come off by itself, use isoflurane to anesthetize the animal and remove sutures, clips or glue 1-week post-op.
Representative Results
To avoid mistargeting, before every experiment, verify the coordinates using dye injections. Animals were injected with 0.2-0.5 µL tryptophan blue using the same protocol, capillary was rapidly withdrawn after injection and the brain was quickly frozen to avoid diffusion. After sectioning on the microtome, the injection site can be seen in blue (Figure 2 C,E). To ensure effective targeting, dye injections should be carried out successfully on 2-3 animals prior to actual experiment.
Stereotaxic injections can be used to create three main Parkinson's disease models with varying degrees of pathology and neurodegeneration. Typically, in the PD field, neurodegeneration is evaluated by quantifying tyrosine hydroxylase (TH), a marker for dopaminergic neurons, as well as a general neuronal marker (e.g., Neuronal Nuclei [NeuN] or HuC) as downregulation of TH due to a toxic environment can give the illusion of nigral cell death.
When injecting toxicants such as 6-OHDA the goal is to eradicate as many nigral dopaminergic cells as possible. 6-OHDA is taken up by monoamine transporters and blocks mitochondrial respiration21. Desipramine is given before each surgery to ensure the toxicant is only taken up by dopamine neurons and not by noradrenergic or serotonergic cells. To evaluate the success of each injection, fixed midbrain sections should be Immunolabeled for TH and NeuN. As toxins act quickly, this model only takes 2-3 weeks after injection to be fully developed. If 6-OHDA injections into the medial forebrain bundle (MFB) were successful, dopaminergic cell loss should be 80% and greater compared to PBS injected controls (Figure 2 F-G). The MFB is a region where nigrostriatal projections bundle together and therefore allow for targeting many dopaminergic neurons with a single injection. Toxicants can also be injected directly into the nigra or striatum for less severe cell death.
Viral vector-based models highly depend on the (sero-)type of the vector used. AAV2 was the most commonly injected as it was easy to produce and has a high affinity towards dopaminergic neurons. Engineering of expression promoters, viral capsids and envelope proteins has opened doors to more specific targeting of brain regions and cell populations. Most viral vectors in PD models are directly injected into the SN to transduce dopaminergic neurons in the pars compacta. Expression of most vectors stabilizes 3 weeks after injection and can be visualized by immunolabeling for the exogenous protein. Usually, GFP is expressed as a control at titers not causing neurodegeneration (Figure 2H). PD-related genes are used to mimic the genetic aspect of the disease, for example duplications or triplications of asyn. Overexpression of asyn in nigral neurons causes dopaminergic cell death (Figure 2I).
The PFF model is the newest addition to the PD tool kit. Aggregated asyn can be acquired either in vitro by weeklong shaking of recombinant asyn protein to generate PFFs or by isolating aggregates from animal models or patient brains. Injected PFFs or brain extracts are then causing accumulation in neurons projecting to the injection site. This model is unique in that it relies on triggering normal endogenous asyn to phosphorylate and accumulate rather than driving aggregation via elevating endogenous asyn. Asyn PFFs are commonly injected into the striatum. Several weeks following injections, asyn pathology can be visualized in prefrontal cortex, striatum, cortex and SN by immunolabeling for phosphorylated asyn (Figure 2K). Control injections of PBS or serum albumin do not result in those PD typical intraneuronal accumulation (Figure 2J).
The stereotaxic injection method can be challenging and expensive, and common errors can usually only be detected after the animals have been sacrificed. The capillary thickness of the blunt end can vary from 10-100 µm depending on the pulling method. If the capillary is too thin, it can clog while entering the brain and lead to a failure in injection (Figure 2L). In this particular case, the needle tract can be identified by scar tissue and uncleared blood cells while no effect of the injection is visible. Another common error is mistargeting (Figure 2M). With age, bregma can be difficult to determine, incorrect coordinates or reading errors can all lead to incorrectly placed injections. While targeting is less complicated in bigger animals (e.g., rats) and larger brain areas (e.g., striatum), they become increasingly more challenging in mice SN injections for example. Another common mistake is dosage. Control substances should not cause more than 10-15% cell death on their own. If expression of GFP (Figure 2N) or PBS injection is toxic, parameters need to be changed to ensure that pathology and cell death are specific to the substance injected.
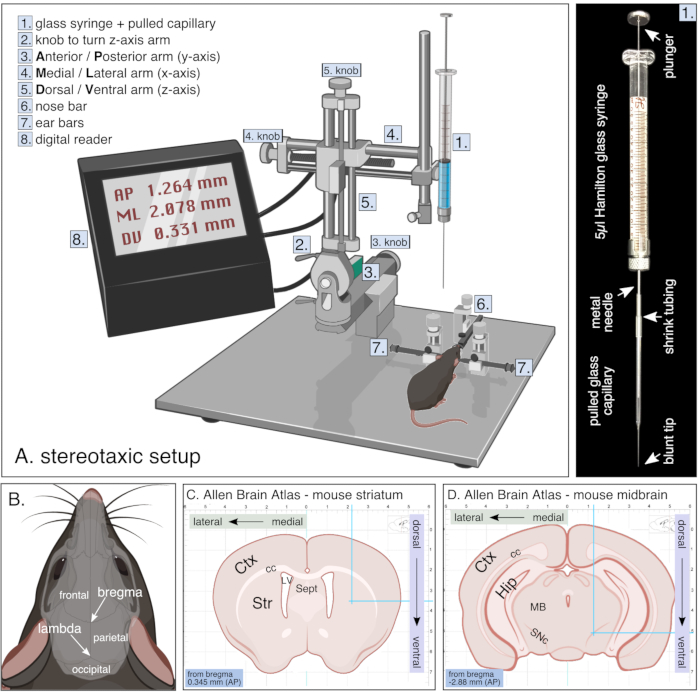
Figure 1. Setup for stereotaxic surgery. (A) Schematic of a stereotaxic setup during survival surgery including digital reader and glass syringe which is further detailed on the right. (B) Skull anatomy of a rodent showing bone plates (frontal, parietal and occipital bones) with corresponding sutures resulting in bregma and lambda. (C,D) Adapted images from the Allen brain atlas webpage showing coronal sections of mouse striatum (C) and midbrain (D) with corresponding coordinate grid in mm. Images adapted from biorender. Please click here to view a larger version of this figure.
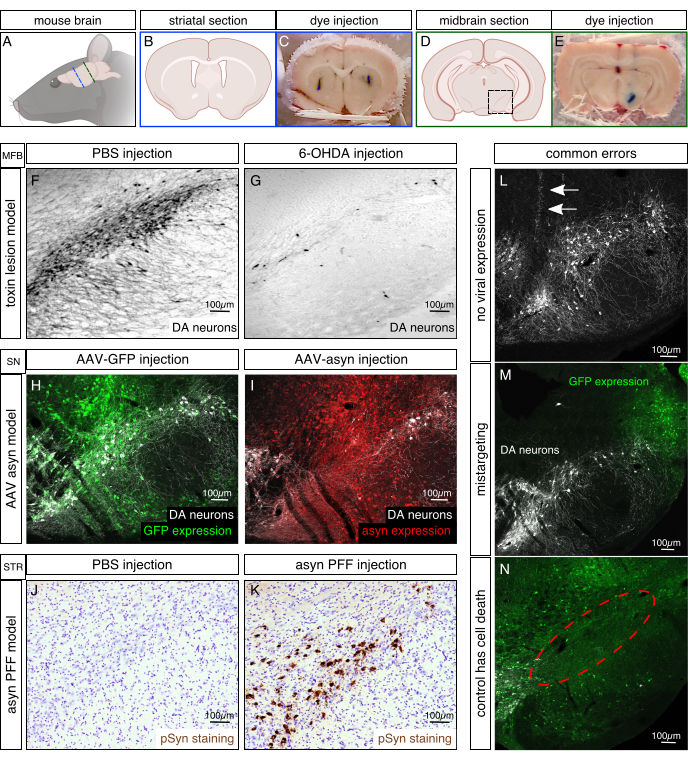
Figure 2. Histological examples of Parkinson's disease animal models. (A) Schematic of the orientation of a mouse brain. Blue line indicates location of coronal striatal section for B and C. Green line indicates location of coronal midbrain section in D and E. (B,D) Illustrations of coronal mouse brain section showing the striatal area in B and the midbrain area in D. Black box represents magnified ventral midbrain (substantia nigra) region in F-N. (C,E) Coordinates were tested for their accuracy prior to experiment by injecting 0.2 µL tryptophan blue. Brain was immediately taken out after surgery, frozen and cut on a microtome to analyze targeting. Examples for correct targeting of striatum and substantia nigra are shown in C and E, respectively. (F,G) Representative images of the 6-hydroxydopamine (6-OHDA) model are shown by immunolabeling for the dopaminergic marker tyrosine hydroxylase (TH) on nigral sections. 6-OHDA (4 µL of 3 µg/µL; G) or PBS as control (F) was injected into the medial forebrain bundle (MFB) and rats were sacrificed 6 weeks after surgery. Half an hour prior to injection, 25 mg/kg desipramine was given i.p. to prevent noradrenergic cell death. (H,I) Example of viral vector based animal model shows transduced cells in green (Immunolabeled against GFP transgene [control vector], H) or red (Immunolabeled for asyn, I), and DA neurons in white. AAVs with a serotype 6 (1 µL of 7 x 1013 viral genomes /mL each) were injected directly into the substantia nigra pars compacta (SN) and mice were sacrificed 4 weeks after surgery. (J,K) Representative images of rats injected with 8 µg of mouse asyn PFF (K) or with PBS as a control (J) were Immunolabeled for phosphorylated asyn (brown) and cell nuclei with cresyl violet (purple). Rats were injected into the striatum (2 x 2 µL deposits) and sacrificed 8 weeks after injection. Images adapted from Duffy et al.21. (L,M,N) Common errors of stereotaxic experiments are no viral solution injected (L, needle tract indicated by arrows), mistargeting (M, in this case too lateral) and control injection causes cell death (N, missing cells indicated by red circle). Transduced neurons are Immunolabeled in green for GFP and white for TH. Please click here to view a larger version of this figure.
Discussion
Stereotaxic injection, as any surgical procedure, has the main difficulty to guarantee the wellbeing and survival of the animal. Therefore, it is essential to monitor the animal closely throughout the procedure. Looking out for breathing irregularities, loss of breathing, or reoccurrence of reflexes and movements should be the main focus, especially for inexperienced surgeons. Additionally, the application of analgesics is crucial to help with the recovery process. Surgeries involving toxicants can be especially difficult to recover from and additional wet food should be supplied.
Besides animal welfare, the main technical complication of stereotaxic injection is mistargeting. Thus, before starting each experiment, pilot animals should be used to verify the coordinates, even when they have been correct in previous experiments. Breeding, diet and age can influence head and brain shape and size and can lead to gross over/underestimation of coordinates. Since the targeting in smaller animals is more challenging, it is crucial to level the head properly to guarantee reproducible injection results. New technology where the stereotaxic instrument is connected to a computer program can help adjust coordinates for each animal by measuring specific points on top of the skull and reduce error. Errors in reading coordinates, especially after long hours of surgery, can also be reduced by digital tools and are worth the financial investment. In larger animals such as pigs and primates, MRI or CT scans are used to calculate more precise coordinates and reduce the chance of mistargeting.
Another challenging obstacle can be clogged capillaries during injection. Shape, size and thickness of each capillary can vary, especially if hand-pulled. If the glass is too thin, the capillary could break or bend during surgery, or clog by taking up tissue in the way of the injection path. If the capillary is too thick, it can disrupt and destroy many structures while going into the brain and leave scar tissue behind. Sutter Inc. therefore provides a cookbook (https://www.sutter.com/PDFs/pipette_cookbook.pdf) for making various shaped and sized capillaries although mostly optimized for electrophysiology. Newer pulling machines need to be operated manually for longer capillaries as used in surgeries. Another great alternative is to order pre-pulled capillaries with the precise specifications needed for your injections. Ideally the diameter of a blunt glass capillary can go up to 80-100 µm but shouldn’t be smaller than 20 µm while the length depends on your injection depth (DV coordinates).It is important to use glass as a material for your injection capillaries. Glass can be used with a finer gauge than metal needles and injectable substances are less prone to interact with or bind to glass.
As each animal is slightly different from one another, targeting will also vary from animal to animal creating a diverse cohort with a range of cell death and pathology. This diverse cohort can make it more difficult to interpret results or achieve a good N number. Consequently, each study has to be powered appropriately in order to be successful. More consistent strategies to develop in vivo models include genetic modifications to create transgenic animals. This method is more expensive and difficult in its beginning stages, even with CRISPR technology, but can pay off later on as these models have very little intra-animal variation and need minimal extra work. Furthermore, it is fairly uncomplicated to transfer transgenic animals from laboratory to laboratory making it a tool that can be easily available to many scientists. Weaknesses of the transgenic approach, at least in the field of Parkinson's disease, are the lack of dopaminergic cell death11, appropriate control lines in general and flexibility to change and adjust the model based on your hypothesis or novel evidence. Additionally, cross breedings will produce many more animals than needed for a set experiment. Once set up, stereotaxic animal models can be more cost efficient and flexible as toxicants, viral vectors and fibril strains can be altered more readily. Both methods have a valid place in the field of neurodegeneration and should be chosen by their appropriateness to address the scientific question asked.
The use of toxicants in PD animal models can result in consistent, severe and reproducible dopaminergic degeneration. Errors while using toxicants occur mainly while handling these substances. Most toxicants, such as 6-OHDA, are light and temperature sensitive and are prone to oxidation. This will render the substance ineffective even before the injection and can result in low to no toxicity. Therefore, solutions have to be freshly prepared adding ascorbic acid to prevent oxidation and have to be shielded from light exposure before and during the injection22. MPTP can be given systemically to animals causing substantial DA cell loss in the SN when given in acute doses over several days23. This model will also result in behavioral deficits but mostly lacks pathological aspects. Disadvantage of using MPTP are its lack of toxicity in rats and some mouse lines, and its neurotoxic risk among researchers and animal caretakers. Because of this risk, it is absolutely necessary to establish a proper working procedure before using MPTP in laboratories. While toxicants are a more straightforward tool, viral vectors need to be cloned and produced correctly in order to function efficiently. Cloning can be complicated as the plasmids contain two repetitive sequences necessary for packaging of the virus that could be lost recombinantly during replication. Additionally, packaging limitations only allow for an expression cassette to be 4.5 or 11 kbp for AAV or Lentivirus, respectively. This limitation can sometimes be bypassed by using multiple vectors or creative editing. Future vector engineering will undoubtedly be focused on extending the size of vector genomes. The production of viral particles can be difficult as the end product needs to be pure but also have a high titer24. Quality control is necessary before each batch of viral vector can be used to avoid increased inflammation or low expression. Furthermore, a dose-response curve should be generated as the GFP expressing vectors can also result in cell function abnormalities and cell death25. Similar to the vector models, quality control of PFFs is of utmost importance. The production of preformed fibrils has long been of great debate as different protocols result in different strains of fibrillar aggregates and may cause diverse pathological patterns26,27. Injections of poorly assembled fibrils will not result in the desired pathological phenotype. It is also worth mentioning that most laboratories use PBS as a control as injection of monomeric asyn can also cause several intracellular aggregates at longer time points28. To prevent initial pitfalls using these tools, viral vectors or PFF should be acquired from experienced researchers or companies. This can drastically reduce the initial failure rate and provide time to gain experience using stereotaxic injections.
Since stereotaxic injections is such a versatile method, new technology is constantly being developed to make this technique easier and more streamlined; from motorized robotic injection arms, over automated drilling to 3D reconstruction of the animal's head. This will allow for faster and more accurate injections in the future. Furthermore, new viral vectors are constantly being developed to improve spread and specificity while increasing their genome capacity. Additionally, new insights into PD but also neurodegeneration in general will help us improve our injectable tools to create these models. Recent scientific findings have let us to develop the PFF model and future discoveries, genetic or mechanistic, will help us advance PD models and guide us on our quest on finding a cure.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was supported in part by the Intramural Research Program of the National Institute of Health, National Institute on Aging. CES is supported by NS099416. The authors wish to acknowledge support by the NIMH IRP Rodent Behavioral Core (ZIC MH002952 and MH002952 to Yogita Chudasama) and by the NICHD IRP Microscopy and Imaging Core.
Materials
| Allen brain atlas | Allen Institute | mouse brain – reference atlas | |
| analgesic: ketoprofin OR buprenorphine | |||
| anesthetic: Isoflurane OR ketamine / xylazine OR fentanyl / medetomidine | |||
| blades – surgical sterile | Oasis Medical | No 10 | |
| capillaries – glass | Stoelting | 50811 | |
| capillary puller | Sutter Instruments | P-97 | |
| cotton-tipped applicators | Stoelting | 50975 | |
| drill – dental | Foredom | MH-170 | |
| Ethanol 70% | |||
| eye drops (Liquigel) | CVS | NDC 0023-9205-02 | Carboxymethylcellulose Sodium (1%), Boric acid; calcium chloride; magnesium chloride; potassium chloride; purified water; PURITE® (stabilized oxychloro complex); sodium borate; and sodium chloride |
| forceps – full curved | Stoelting | 52102-38P | |
| forceps – hemostatic delicate | Stoelting | 52110-13 | |
| gauze – cotton absorbent | |||
| H2O – sterile | |||
| H2O2 30% | Sigma Aldrich | 216763 | |
| Hamilton 5ul syringe | Hamilton Company | 7634-01 | |
| Hamilton blunt metal needle | Hamilton Company | 7770-01 | |
| heat pad – far infrared | Kent Scientific | 2665967 | |
| Iodine solution (Dynarex) 10% | Indemedical | 102538 | |
| isoflurane | Baxter | 1001936040 | |
| lidocaine 0.5% | |||
| lighter / matches | |||
| microscope (Stemi 508 Boom stand) | Zeiss | 435064-9000-000 | |
| PBS sterile | Gibco – Thermo Fischer | 10010-023 | |
| pump (injector) | Stoelting | 53311 | |
| scalpel handle | Stoelting | 52171P | |
| shaver – electrical | andis | 64800 | |
| solution to inject / material to implant | |||
| stereotax – small animal digital | Kopf | Model 940 | |
| sterilizer – glass bead | BT Lab Systems | BT1703 | |
| tubing – heat-shrink | Nelco | NP221-3/64 | |
| tweezers – dumont fine curved | Roboz | RS-5045A | |
| underpad – absorbent | |||
| vaporizer for isoflurane (package) | Scivena Scientific | M3000 | |
| wound clips and applier / remover | Stoelting | 59040 | |
| wound glue (Vetbond) | 3M corporation | 1469SB |
Riferimenti
- Tanner, C. M., Goldman, S. M. Epidemiology of Parkinson’s disease. Neurologic Clinics. 14 (2), 317-335 (1996).
- Fearnley, J. M., Revesz, T., Brooks, D. J., Frackowiak, R. S., Lees, A. J. Diffuse Lewy body disease presenting with a supranuclear gaze palsy. Journal of Neurology, Neurosurgery & Psychiatry. 54 (2), 159 (1991).
- Kordower, J. H., et al. Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain. 136 (8), 2419-2431 (2013).
- Braak, H., Sandmann-Keil, D., Gai, W., Braak, E. Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by α-synuclein immunocytochemistry. Neuroscience Letters. 265 (1), 67-69 (1999).
- Spillantini, M. G., Schmidt, M. L., Lee, V. M. Y., Trojanowski, J. Q., Jakes, R., Goedert, M. α-Synuclein in Lewy bodies. Nature. 388 (6645), 839-840 (1997).
- Thomas, B., Beal, M. F. Parkinson’s disease. Human Molecular Genetics. 16 (2), 183-194 (2007).
- Cenci, M. A., Björklund, A. Animal models for preclinical Parkinson’s research: An update and critical appraisal. Progress in Brain Research. 252, 27-59 (2020).
- Bové, J., Prou, D., Perier, C., Przedborski, S. Toxin-induced models of Parkinson’s disease. NeuroRX. 2 (3), 484-494 (2005).
- Kikuchi, T., et al. Human iPS cell-derived dopaminergic neurons function in a primate Parkinson’s disease model. Nature. 548 (7669), 592-596 (2017).
- Baltazar, M. T., Dinis-Oliveira, R. J., Bastos, M. d. e. L., Tsatsakis, A. M., Duarte, J. A., Carvalho, F. Pesticides exposure as etiological factors of Parkinson’s disease and other neurodegenerative diseases-A mechanistic approach. Toxicology Letters. 230 (2), 85-103 (2014).
- Breger, L. S., Armentero, M. T. F. Genetically engineered animal models of Parkinson’s disease: From worm to rodent. European Journal of Neuroscience. 49 (4), 533-560 (2019).
- Mandler, M., et al. Next-generation active immunization approach for synucleinopathies: implications for Parkinson’s disease clinical trials. Acta Neuropathologica. 127 (6), 861-879 (2014).
- Löw, K., Aebischer, P. Use of viral vectors to create animal models for Parkinson’s disease. Neurobiology of Disease. 48 (2), 189-201 (2012).
- Kirik, D., et al. Parkinson-Like Neurodegeneration Induced by Targeted Overexpression of α-Synuclein in the Nigrostriatal System. Journal of Neuroscience. 22 (7), 2780-2791 (2002).
- Luk, K. C., Kehm, V. M., Zhang, B., O’Brien, P., Trojanowski, J. Q., Lee, V. M. Y. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in miceSpread of pathological α-synuclein in vivo. The Journal of Experimental Medicine. 209 (5), 975-986 (2012).
- Luk, K. C., et al. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. 338 (6109), 949-953 (2012).
- Henderson, M. X., et al. Characterization of novel conformation-selective α-synuclein antibodies as potential immunotherapeutic agents for Parkinson’s disease. Neurobiology of Disease. 136, 104712 (2019).
- Hoban, D. B., et al. Impact of α-synuclein pathology on transplanted hESC-derived dopaminergic neurons in a humanized α-synuclein rat model of PD. Proceedings of the National Academy of Sciences. 117 (26), 15209-15220 (2020).
- Thakur, P., et al. Modeling Parkinson’s disease pathology by combination of fibril seeds and α-synuclein overexpression in the rat brain. Proceedings of the National Academy of Sciences. 114 (39), 8284-8293 (2017).
- Björklund, T., Carlsson, T., Cederfjäll, E. A., Carta, M., Kirik, D. Optimized adeno-associated viral vector-mediated striatal DOPA delivery restores sensorimotor function and prevents dyskinesias in a model of advanced Parkinson’s disease. Brain. 133 (2), 496-511 (2010).
- Duffy, M. F., et al. Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. Journal of Neuroinflammation. 15 (1), 129 (2018).
- Glinka, Y., Gassen, M., Youdim, M. B. H. Mechanism of 6-hydroxydopamine neurotoxicity. Journal of neural transmission. Supplementum. 50, 55-66 (1997).
- Meredith, G. E., Rademacher, D. J. MPTP mouse models of Parkinson’s disease: an update. Journal of Parkinson’s disease. 1 (1), 19-33 (2011).
- Fripont, S., Marneffe, C., Marino, M., Rincon, M. Y., Holt, M. G. Production, Purification, and Quality Control for Adeno-associated Virus-based Vectors. Journal of Visualized Experiments. 143, (2019).
- Landeck, N., Buck, K., Kirik, D. Toxic effects of human and rodent variants of alpha-synuclein in vivo. European Journal of Neuroscience. 45 (4), 536-547 (2017).
- Polinski, N. K., et al. Best Practices for Generating and Using Alpha-Synuclein Pre-Formed Fibrils to Model Parkinson’s Disease in Rodents. Journal of Parkinson’s Disease. 8 (2), 303-322 (2018).
- Patterson, J. R., et al. Generation of Alpha-Synuclein Preformed Fibrils from Monomers and Use In Vivo. Journal of Visualized Experiments. 148, (2019).
- Paumier, K. L., et al. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiology of Disease. 82, 185-199 (2015).

.
