Simultaneous Calcium Imaging and Glucose Stimulation in Living Zebrafish to Investigate In Vivo β-Cell Function
Summary
Here, the study presents a protocol for calcium imaging and glucose stimulation of the pancreatic β-cells of the zebrafish in vivo.
Abstract
The pancreatic β-cells sustain systemic glucose homeostasis by producing and secreting insulin according to the blood glucose levels. Defects in β-cell function are associated with hyperglycemia that can lead to diabetes. During the process of insulin secretion, β-cells experience an influx of Ca2+. Thus, imaging the glucose-stimulated Ca2+ influx using genetically encoded calcium indicators (GECIs) provides an avenue to studying β-cell function. Previously, studies showed that isolated zebrafish islets expressing GCaMP6s exhibit significant Ca2+ activity upon stimulation with defined glucose concentrations. However, it is paramount to study how β-cells respond to glucose not in isolation, but in their native environment, where they are systemically connected, vascularized, and densely innervated. To this end, the study leveraged the optical transparency of the zebrafish larvae at early stages of development to illuminate β-cell activity in vivo. Here, a detailed protocol for Ca2+ imaging and glucose stimulation to investigate β-cell function in vivo is presented. This technique allows to monitor the coordinated Ca2+ dynamics in β-cells with single-cell resolution. Additionally, this method can be applied to work with any injectable solution such as small molecules or peptides. Altogether, the protocol illustrates the potential of the zebrafish model to investigate islet coordination in vivo and to characterize how environmental and genetic components might affect β-cell function.
Introduction
The pancreatic β-cells exhibit the unique capability for insulin secretion in response to glucose. After a carbohydrate-rich meal, the blood sugar increases and enters the β-cells, where it is quickly metabolized to produce ATP. The increase in the intracellular ratio of ATP/ADP leads to the closure of the ATP-dependent K+ channels, depolarizing the cell membrane and activating the voltage-dependent Ca2+ channels. The rapid increase in intracellular Ca2+ stimulates insulin-granule secretion by the β-cells.
Imaging of the islet cells within the intact pancreas in mice is demanding and requires exteriorization of the whole organ. A powerful alternative for non-invasive in vivo imaging is to transplant islets into the anterior chamber (AC) of the eye of mice1. Transplanted islets into the AC of mice mimics an in vivo environment, allowing longitudinal studies and Ca2+ imaging in vivo2,3. Nevertheless, the process of islet revascularization can take several weeks after islet transplantation4. Thus, preserving the original native environment, where the β-cells are vascularized and connected to the metabolic status of the organism, and achieving in vivo single-cell imaging resolution remain very challenging and time consuming.
To overcome these limitations, researchers have developed zebrafish transgenic lines expressing GECIs in β-cells, which allow for real-time visualization of Ca2+ influx in β-cells5,6,7,8,9. Using these tools, it was found that in cell culture, zebrafish β-cells show a conserved response to elevated glucose, similar to mouse and human islets. Moreover, the optical clarity of the zebrafish larvae has allowed to examine the function of β-cells in their native environment. Importantly, as early as 4 days post fertilization (dpf), the β-cells of the zebrafish primary islet express markers of maturity, such as the zebrafish orthologue of urocortin3 (ucn3l) and show in vitro responses to glucose in the physiological range6. In addition, the zebrafish islet is densely vascularized and innervated10. The genetic ablation of β-cells at this stage leads to glucose intolerance. Furthermore, the zebrafish shows conserved responses to glucose-lowering agents such as insulin and sulfonylureas, showing that the primary islet is a glucose responsive and systemically connected tissue controlling glucose levels. These special characteristics make the zebrafish a unique model to study islet β-cell activity in vivo11,12.
Theprotocol for Ca2+ imaging and simultaneous glucose injection directly in the circulation of zebrafish allows investigating the β-cell's glucose responsiveness in vivo. This protocol enables the injection of defined glucose concentrations in combination with high temporal and spatial resolution, which altogether reveal the coordinated response of β-cells to glucose and the presence of first-responder β-cells in vivo13. Moreover, the protocol can be adapted to any injectable solution such as chemical compounds or small peptides. Overall, this methodology illustrates the strength of the zebrafish model to investigate β-cell coordination in vivo and to characterize how environmental and genetic components might affect β-cells' function.
Protocol
The previously established transgenic lines used in this study were Tg(ins:GCaMP6s;cryaa:mCherry)6, Tg(ins:cdt1-mCherry;cryaa:CFP)14. All experiments were carried out in compliance with European Union and German laws (Tierschutzgesetz) and with the approval of the TU Dresden and the Landesdirektion Sachsen Ethics Committees (approval numbers: AZ 24D-9168,11-1/2013-14, TV38/2015, T12/2016, and T13/2016, TVV50/2017, TVV 45/2018, and TVV33-2019). In this study, all live imaging in vivo and glucose injections, as well as experimental procedures were performed with zebrafish larvae that did not exceed the 5 days post fertilization (dpf) stage, as stated in the animal protection law (TierSchVersV §14). According to the EU directive 2010/63/EU, the use of these earlier zebrafish stages reduces the number of experimental animals, according to the principles of the 3Rs.
1. Preparation
NOTE: This protocol pertains to the in vivo Ca2+ imaging of the zebrafish primary islet from Tg(ins:cdt1-mCherry;cryaa:CFP); Tg(ins:GCaMP6s;cryaa:mCherry) double transgenic larvae. In these animals, the insulin promoter drives the expression of cdt1-mCherry, which labels the nuclei of individual β-cell in red fluorescence. GCaMP6s shows changes in green fluorescence in response to the intracellular Ca2+ levels. The specific expression of the GCaMP6s in β-cells allows characterization of the glucose-responsiveness of individual cells without the interference from other cell types or tissues.
- Prepare E2 embryonic media15.
- Prepare 0.003% (200 µm) 1-pheniyl-2-thiourea (PTU) in 10% Hanks's saline15.
- Prepare 1% low-melting agarose (LMA) in E2 embryonic medium by heating in a microwave and filtering it with a <25 µm pore-size filter. Keep the LMA at >37 °C for up to 1 week.
NOTE: All the solutions should be filtered to prevent clogging of the glass capillary used for injection. - Prepare a 0.4% solution of tricaine methane sulfonate (MS222) with 979 mL of sterile H2O and 21 mL of 1 M Tris (pH 9). Adjust pH to 7.
- Dissolve 1.8 g of D-glucose in 50 mL of PBS to prepare 200 mM D-glucose solution and filter it with <25 µm pore-size filters. Prepare a working solution of D-Glucose (25 mM) by a 1:8 dilution of the 200 mM D-Glucose in 1x PBS (filtered). Keep at 4 °C for long-term storage. Add 5 µL of phenol-red to 1 mL of 25 mM D-Glucose to be able to visualize the solution upon injection.
NOTE: This solution can be kept at 4 °C for 1 week. However, the 25 mM working solution should be prepared fresh. Discard the stock if there is any sign of contamination. - Prepare pulled microinjection glass capillaries using a capillary puller or procure commercially available micro-glass capillaries. The settings for needle pulling, is described in the Table of Materials.
NOTE: Microinjection capillaries made of borosilicate glass without internal filament were found to offer better results in comparison to similar capillaries with internal filament. - Procure 35 mm diameter glass-bottom dishes for mounting the zebrafish larvae. Glass-bottom dishes with a glass cover of 0.17 mm were used in this protocol, as they allow imaging with glass-corrected objectives in confocal systems.
- Procure micro-loading pipet tips. 20 µL micro-loaders with 100 mm length were used in this protocol.
- Procure a set of micro-tweezers.
- Procure 90 mm Petri dishes for sorting and growing zebrafish.
- Procure mineral oil.
- Procure a pneumatic micro-pump or a system to deliver 1-5 nL of liquid volumes through the glass-capillaries.
- For zebrafish sorting and mounting, use a stereo microscope equipped with a light source and with blue and red filter cubes for fluorescence (CFP: excitation 420-450 nm, emission 460-490 nm, TRITC: excitation: 532-554 nm, emission: 570-613 nm; or Texas-Red: excitation: 540-580 nm, emission: 592-667 nm). Sort the double transgenic Tg(ins:cdt1-mCherry;cryaa:CFP); Tg(ins:GCaMP6s;cryaa:mCherry) larvae using the stereoscope. Select the embryos with blue and red fluorescence in the retina due to the expression of CFP and mCherry under the crystallin promoter.
- For imaging the Ca2+ influx in β-cells, use an inverted confocal microscope equipped with a 10x (0.8 NA) air and a 40x (1.0 NA) water objectives. Use a stage containing the plate holder for the 35 mm diameter glass-bottom dishes.
- Procure a 3D manual or motorized manipulator with a capillary holder. Insert the glass capillary into the capillary holder and mount the capillary holder into the 3D manipulator.
NOTE: The 3D manipulator allows precise movement of the glass capillary and insertion into the zebrafish larvae.
2. Zebrafish larvae mounting
NOTE: The double transgenic Tg(ins:cdt1-mCherry;cryaa:CFP); Tg(ins:GCaMP6s;cryaa:mCherry) embryos are treated with 0.03% (20 µM) 1-phenyl 2-thiourea (PTU) to inhibit pigmentation from 24 h post fertilization onward. At 4.5 dpf, anesthetize the larvae using 0.04% of the final concentration of Tricaine just before mounting.
- To maintain anesthesia during the imaging session, add to the 1% low-melting agarose a final concentration of 0.04% Tricaine and 20 µM PTU. Keep the LMA agarose at >37 °C until usage. Use this solution on the same day of preparation.
- Sort the anesthetized double transgenic Tg(ins:cdt1-mCherry;cryaa:CFP); Tg(ins:GCaMP6s; cryaa:mCherry) larvae. Place 3-5 fish on a glass-bottom Petri dish and remove most of the liquid.
NOTE: Do not let the larvae dry at this step and always keep them immersed in a minimum amount of E2. - Add 500 µL of LMA on the glass bottom Petri dish containing the fish, and with the help of the micro-tweezers gently move the fish so that the right side of the fish is directly in contact with the glass-bottom. Remove the excess agarose and let it solidify for 1-3 min. Observe that the agarose becomes slightly opaque at this point (Figure 1B).
NOTE: If the agarose is too hot, it could damage the larvae. To avoid this, let the agarose to cool down at room temperature for 30-45 s before adding it to the larvae. - Once the agarose becomes solid, with the help of the micro-tweezers, carefully remove the agarose around the area of the heart to prevent the breakage of the needle used for subsequent injection upon contact with the agarose (Figure 1C).
- Add 500 µL of E2 containing a final concentration of 0.04% Tricaine and 20 µM PTU.
NOTE: Make sure to avoid drying of the larvae during the live imaging.
3. Simultaneous injection and Ca2+ imaging of β-cells
NOTE: Those larvae that have excessive yolk on top of the pancreas due to a developmental delay should not be used for imaging as the lipids of the yolk interfere with the image quality. In addition, low laser powers are used (0.5%-5%) to avoid photobleaching and phototoxicity.
- Using a stereo microscope and micro-tweezers, cut off the tip of the glass capillary.
NOTE: Do not cut more than just the tip of the capillary, as the diameter of the capillary should be smaller than the inflow tract of the zebrafish heart. - Using the loading tips, fill the needle with the 25 mM D-Glucose solution.
NOTE: The capillary must be uniformly filled, taking special care to avoid the formation of any air bubbles as they interfere with the delivery of appropriate injection volumes. - Mount the glass capillary into the capillary holder and connect it to the micro-pump. Maintain the micropump at an injection pressure between 500-1,000 hPa, compensation pressure = 0 hPa, and delivery time = 1 s.
- To calibrate the injection volume, use a stereo microscope equipped with a graded eye-piece. Place a drop of mineral oil into a glass slide under the stereoscope. Adjust the zoom of the stereoscope to a power of 4 with a 1.5x objective. Insert to inject the glass capillary into the mineral oil.
NOTE: 5 nL is equivalent to 5 units of the graduation showed in the eye-piece of the stereoscope. Increase or decrease the injection pressure of the micro-pump to adjust the drop volume. - Mount the capillary holder into the 3D manipulator.
NOTE: The 3D manipulator should allow fine movement control in x, y, and z directions of the capillary holder. - Place the dish containing the mounted larvae carefully on the plate holder of the confocal microscope. Use a 10x, 0.8 NA air objective for locating the larvae using the bright field option.
- Orient the zebrafish with the heart side toward the direction where the capillary and the 3D manipulator are located. By observing the red fluorescence, make sure that the islet is at the center of the field of view.
NOTE: From this point onwards, the stage should stay fixed (Figure 2). - Using the 3D manipulator, move the glass capillary toward the heart of the larvae, penetrate the skin, and aim for the pericardial cavity. Carefully insert the capillary in the middle of the common cardinal vein (CCV) and the sinus venosus (SV), located around 100-200 µm from the heart atrium16, (Figure 1B and Figure 2C). The angle of the needle is approximately 20-30° in relation to surface of the plate. If the rim of the plate is too high, remove it to allow easier access for the capillary.
NOTE: This is a critical step, as a very careful penetration is required; fast movements can perturb the heart or cause blood clothing in this area. If the blood flow is perturbed, a different sample should be used. Since the 3D manipulator keeps a fixed position, avoid movements of the stage in order not to interfere with the heart tissue. - Once the capillary is placed into the SV, change to a 40x, 1.2 NA water immersion objective. Using the red and green 488/561 dichromatic mirrors, find the nuclear mCherry signal in β-cells, and focus on the islet.
NOTE: Individual nuclei should be clearly visible and low green fluorescence should be present (Figure 1D). - Set up a simultaneous acquisition for GCaMP6s and mCherry fluorescence with the following parameters in the Smart Setup Menu: GFP (GCaMP6s), excitation: 488 nm, emission: 498-555 nm, false-color: green (Select GFP); mCherry, excitation: 561 nm (mCherry), emission: 570-640 nm, false-color: red (Select mCherry). Transmitted light, false-color: grays.
- Adjust the plane of imaging by adjusting the focal plane along the z-axis of the islet. Find a plane that contains the majority of β-cells.
- Adjust the gain of the nuclear mCherry signal for a uniform identification of each individual cell and covering around 70% of the color histogram.
- Adjust the gain of the GCaMP6s signal for covering at least 25% of the color histogram, as the GCaMP6s increases its brightness up to 4-fold.
- Adjust the gain of the transmitted light for a uniform signal of the sample covering around 70% of the color histogram.
NOTE: The gray channel is used to visualize and ensure proper blood flow of the islet. - In the Acquisition Mode, set the image resolution to 512 x 512 pixels, zoom to 5, line step to 3, scan speed to 13, and averaging line to 2-3. Select the option Time-series, and set the Duration to 500 cycles, with acquisition rate of 150 ms per frame.
NOTE: The first 50 frames of the time-series correspond to the baseline fluorescence before the glucose injection. It has been observed that some β-cells show basal activity in vivo. A responding β-cell will show an increase in green fluorescence intensity with the glucose injection over time. Most β-cells show a response to the 25 mM glucose injection in vivo. - Start the imaging. After the first 50 frames (7.5 s), inject the glucose using the micro-pump.
NOTE: The injections might produce a slight movement that can shift the focal plane of the islet. During the movie recording, gut-movements might change the focal plane of the islet. - Keep an eye on the image acquisition and manually adjust the x, y, and z coordinates to keep the same focal plane along the movie.
- After the glucose injection, the system records the islet response in terms of Ca2+ influx as an increase in the GCaMP fluorescence. After each injection, let the larvae rest for at least 5 min before any further stimulation.
NOTE: Ensure that the nuclei of the cells remain stable during the process. If the islet is moving extensively during acquisition or if the islet had moved out of the focal plane, the sample cannot be used for further analysis. - Repeat the injection three times. Save the videos as TIF, CZI, or LSM formats (recommended). After recording the Ca2+ responses, remove the capillary from the heart very slowly. Move to the next sample.
NOTE: The zebrafish larvae can be recovered into a Petri dish with fresh E2 containing PTU.
4. Quantification of GCaMP fluorescence traces for individual β-cells
NOTE: If the animal presents too much of movement, the movie can be stabilized using the FIJI plug-in Descriptor-based series registration (2d/3d + t)17.
- Load the image to be analyzed by dragging and dropping into FIJI. In the window Bio-format Import Options, click on OK.
NOTE: For formats not supported by FIJI, convert the videos into tiff format for analysis. - To extract the GCaMP fluorescent intensity, click on the menu Analyze > Set Measurements. Tick the box Integrated density and click on OK.
- Open the ROI Manager.
- In the FIJI menu, select Polygon selections located in the toolbar.
- Manually draw a polygon covering an area slightly larger than the cell nucleus and including some of the cytoplasmic area of the cell to ensure proper single-cell quantification of the Ca2+ response.
- Make sure that the position of the ROI is consistent over the frames; if necessary, adjust the ROI.
- Add the selected ROI to the ROI Manager by clicking on the Add [t] button.
- To quantify the signal of the GCaMP, separate the channels of the image. Click on the FIJI menu Image > Colors > Split Channels and select Green Channel.
- In the ROI Manager, select all the areas and click on the menu More > Multi Measure. Save the results.
NOTE: If the cell moves out of the polygon area due to the glucose injection or animal movement, extract the cell fluorescence before and after the movement by manually adjusting the polygon area. - To perform the single-cell analysis, execute the following steps.
- Remove the background florescence. For this purpose, consider the background fluoresce as the minimum value registered over the movie for each cell (FMIN). The minimum is subtracted from the entire time series (FT – FMIN) for each cell.
- Normalize the fluorescence values across the movie. To achieve this, divide each value by the highest intensity value over the recordings for each cell. For this purpose, calculate the difference between FMIN and FMAX, i.e., (FMAX – FMIN). Divide (FT – FMIN) / (FMAX – FMIN).
NOTE: This step allows comparison of Ca2+ response among different cells and among islets from different animals, as they will emit varying levels of fluorescent intensities depending on the focal plane and the cell's or islet's accessibility to imaging.
- After acquisition of single-cell fluorescence values, use a program (e.g., Excel or R) to process the fluorescent values measured from step 4 and automatically plot the fluorescent traces. For performing the analysis in this protocol (step 6), the Supplemental Table 1 (Singlecelltrace.xlsx) was used.
Representative Results
Using this protocol, the glucose response of individual β-cells in their native environment was characterized. For this purpose, the zebrafish larva is mounted on a glass-bottom Petri dish. Using a 3D manipulator, a glass capillary was inserted into the circulation, targeting the SV (Figure 1 and Figure 2). This permits the injection of specific volumes of solutions with a defined concentration. Simultaneously, the glucose-induced influx of Ca2+ for all the β-cells in the imaging plane was recorded. The fluorescent intensity values were extracted using FIJI17. Finally, the time of response was evaluated for each β-cell in vivo (Figure 3 and Supplemental Movie 1). To this end, the changes in the GCaMP6s signal can be plotted for each individual β-cell as shown in Figure 3A,3B and Supplemental Movie 1. The fluorescent normalization allows to compare the cell's time of response (Figure 3B). The time of response T25 (s) was defined as the period elapsed from glucose injection to the increase in fluorescence by 25% above the baseline. The β-cells show a coordinated glucose-induced influx of Ca2+ within 5 s after the glucose injection (Figure 3A). With a time resolution of 150 ms per frame, the individual time of response for each cell can be estimated (Figure 3C).
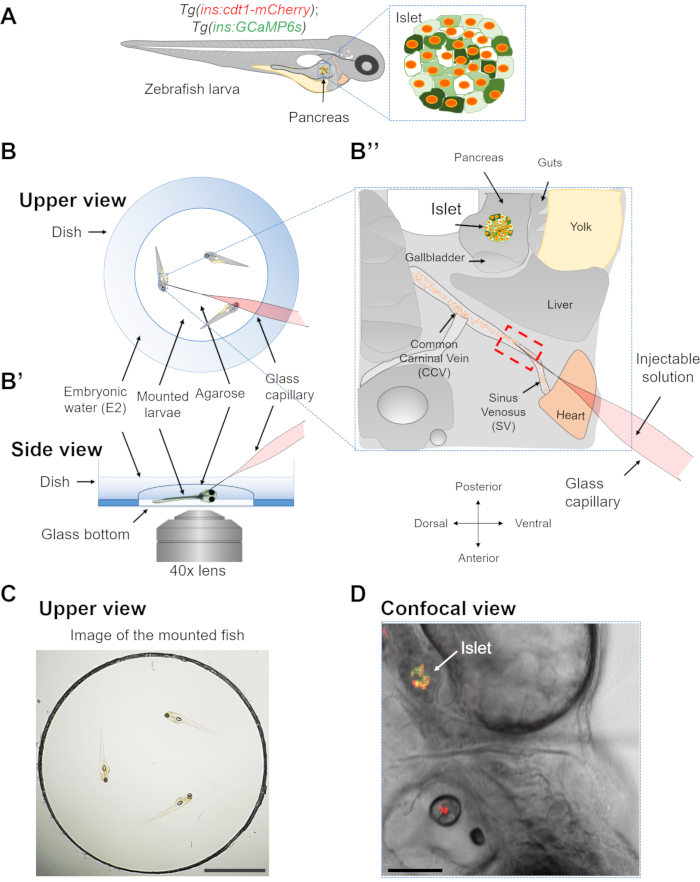
Figure 1: Mounting zebrafish larvae for imaging. (A) Cartoon representing a transgenic zebrafish larva expressing the genetically-encoded Ca2+ indicator GCaMP6s (green) and the nuclear marker cdt1-mCherry (red) under the insulin promoter. (B) Schematic representing the upper view of a mounted zebrafish larva in a glass-bottom dish and with a glass capillary inserted into the sinus venosus (SV) of the heart. (B') Side view of the schematic showing the same fish with the side of the pancreas in direct contact with the glass bottom of the dish. (B'') Cartoon representing the transgenic fish as imaged with a 10x objective. The red box depicts the area where the glass capillary should be inserted. (C) Photograph of mounted larvae in a glass-bottom dish. Scale bar = 0.5 cm. (D) Confocal and bright-filed image of a zebrafish larvae showing the fluorescently labeled islet. Scale bar = 200 µm Please click here to view a larger version of this figure.
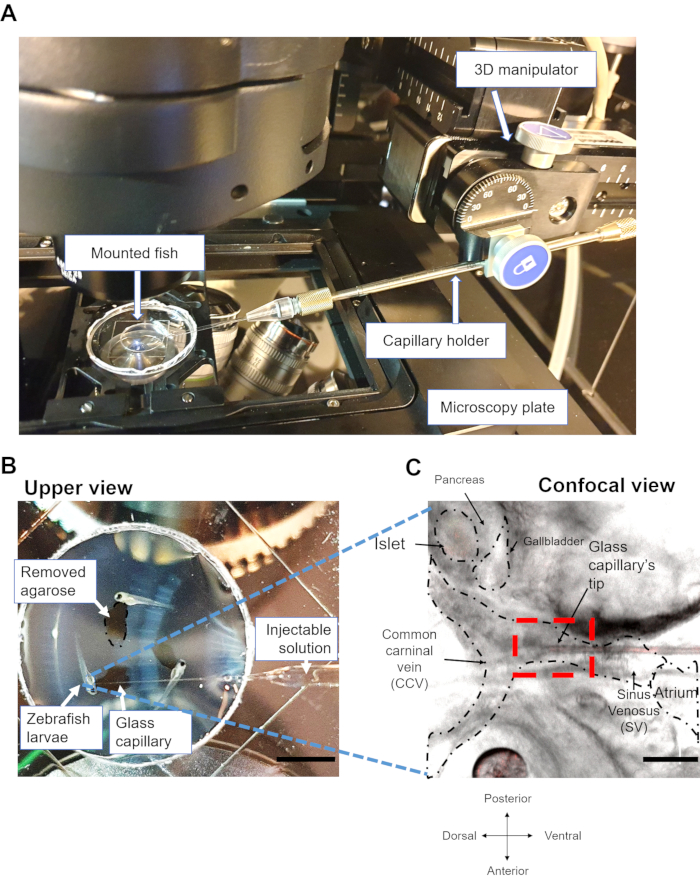
Figure 2: Setup of imaging and glucose-stimulation. (A) The image shows zebrafish larvae mounted in the glass-bottom dish, which is held inside the microscope's plate holder. The photograph shows the 3D manipulator, the capillary holder, and the mounted fish. (B) Photograph of the upper view showing the mounted zebrafish larva in a glass-bottom dish with an inserted glass capillary. Scale bar = 0.5 cm. (C) Confocal image of a zebrafish larva showing the tip of the capillary inserted in between the common cardinal vein (CCV) and the sinus venosus (SV).The dotted lines depict the CCV and SV. The red square indicates the area where the needle has to be inserted. Scale bar = 100 µm. Please click here to view a larger version of this figure.
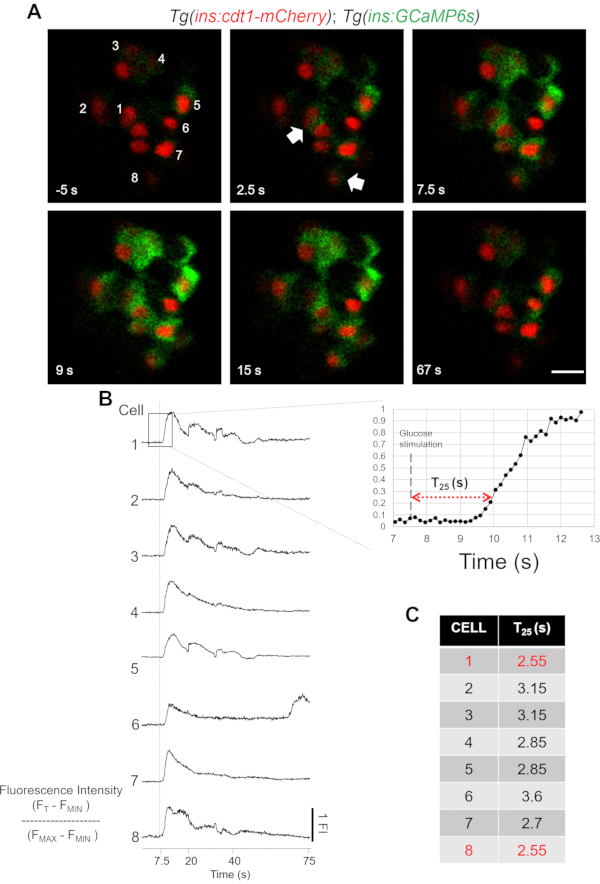
Figure 3: In vivo imaging of glucose-stimulated Ca2+ influx in β-cells. (A) Snapshots from the time-lapse recording of the primary islet at 150 ms per frame. The timestamp indicates the relative time to glucose injection occurring at 0 s. The white arrows show β-cells that respond first to the glucose; the subsequent frames show the response of the rest of β-cells. The numbers indicate individual cells corresponding to the quantifications shown in (B). Scale bar = 10 µm. (B) Representation of normalized fluorescence traces corresponding to individual β-cell across the entire imaging session. The time of response T25 (s) is defined as the period elapsed from the time of glucose injection to a 25% increase in fluorescence intensity above the baseline. (C) The time of response for individual cells was measured and plotted in the table. Please click here to view a larger version of this figure.
Supplemental Movie 1: In vivo imaging of glucose-stimulated influx of Ca2+ in zebrafish β-cells. In vivo imaging of zebrafish β-cells expressing GCaMP6s and cdt1-mCherry under the insulin promoter upon stimulation with 25 mM glucose injection delivered at time = 7.5 s. Please click here to download this Movie.
Supplemental Table 1. Ready-to-use spread sheet for plotting the fluorescence traces of individual cells. Copy and paste, into the spread sheet, the RawFluorescence values obtained in the step 4.4. The results can be visualized in the sheet Review. Please click here to download this Table.
Discussion
In this protocol, the Ca2+ dynamics of β-cells in their native microenvironment with single-cell resolution was explored. This is possible by stimulating the zebrafish β-cells with a glucose injection in the circulation while recording their Ca2+ dynamics using GCaMP6s.
The protocol provides three main advantages. First, researchers have demonstrated that zebrafish β-cells show a coordinated response to glucose stimulation in vivo7,13,18. In contrast, it was observed that in cell culture, the response of β-cells is often uncoordinated19. Thus, in vivo imaging is essential for preserving the physiological and coordinated response of the β-cells. Second, the protocol preserves the native microenvironment where the islet is densely innervated and perfused with blood. In particular, blood perfusion allows fast β-cell response to glucose. The average in vivo time of response takes place on an average 4 s after the glucose injection (SD = ± 4.40 s, n = 40). This is faster than previous in vitro studies of isolated mouse islets showing an average time of response of 301.2 s (SD = ± 42.1 s) after stimulatory glucose is added (10 mM glucose)20. Similarly, ex vivo-cultured zebrafish islets take 1-2 min to show Ca2+ influx upon addition of stimulatory glucose (10 mM)5,6,13,19. This highlights a key difference between the in vitro models to study islet biology and the in vivo zebrafish model in which blood flow is preserved. Third, other protocols for in vivo stimulation of β-cells in zebrafish larvae use bathing with glucose solutions8,9. However, the bathing method does not allow to know the exact amount of glucose entering the circulation. In addition, it requires relatively long times of bathing to elicit a response (up to 3 min)9. A recent report used bathing with high glucose concentrations to elicit the Ca2+-influx of β-cells. Strikingly, this approach stimulated Ca2+-influx in vivo even in the complete absence of islet vascularization, suggesting that the bathing method may rely on a tissue-diffusion mechanism for glucose delivery to the islet8.
The major limitation of the protocol is the restriction to one confocal z-plane of the islet to ensure high-speed imaging. A Z-stack covering the whole islet leads to slower imaging speeds and loss of time resolution. These limitations could be overcome using faster imaging technologies such as a resonant scanner, Z-Piezo, or light sheet microscopy, which should enable capturing whole-islet Ca2+ dynamics. Indeed Zhao et al. reported the usage of a 2-photon light-sheet microscope to resolve whole-islet Ca2+ transients in 3D9. Therefore, this protocol could be combined with higher-speed microscopy in order to resolve whole-islet Ca2+ dynamics.
The critical step of our protocol is to properly insert the capillary into the inflow tract of the zebrafish larva. This can be a limiting step, as any rough movement or misplacement of the capillary can affect the stimulation.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Nikolay Ninov received funding from the Center for Regenerative Therapies Dresden at TU Dresden and the German Center for Diabetes Research (DZD), as well as research grants from the German Research Foundation (DFG) and the International Research Training Group (IRTG 2251), Immunological and Cellular Strategies in Metabolic Disease. We are grateful to the Light Microscopy Facility at the CRTD for the support in all the imaging techniques. We thank the Fish Facility at the CRTD for all the fish technical assistance and support.
Materials
| 35 mm diameter glass-bottom dishes | Mattek | P35G-1.5-14-C | We use this glass-bottom dish to mount the zebrafish larvae and perform confocal microscopy |
| Blue-Green filter cube | ZEISS | 489038-9901-000 | Filter Set 38 HE |
| Confocal Microscope | ZEISS | LSM 980 | |
| D-(+)-Glucose | Sigma-Aldrich | G7528 | |
| Excel (2016) | https://www.office.com/ | ||
| Femtojet | Eppendorf | 5252000013 | This equipment is a pneumatic micropump, which allows precise volume delivery and is accompanied by a capillary holder. We use the micropump Femtojet (injection pressure between 500-1000 hPa; compensation pressure = 0 hPa; and delivery time = 1 second). |
| Femtotips, glass capillaries ready to be used. | Eppendorf | 5242952008 | This are ready to use glass capillaries that can substitute the pulled-capillaries. |
| FIJI, using ImageJ Version: 1.51c | https://fiji.sc/ | ||
| Fluorescence lamp | ZEISS | 423013-9010-000 | Illuminator HXP 120 V |
| Glass capillaries 3.5" | Drummonds Scientific Company | 3-000-203-G/X | We use these glass capillaries to prepare the injection capillaries by pulling them with a capillary -puller |
| Injectman | Eppendorf | 5192000019 | This equipment allows for 3D manipulation of the capillary holder |
| Low melting agarose | Biozym | Art. -Nr.: 840101 | We use the agarose to mount the zebrafish onto the glass-bottom dish |
| Microloader tip for glass microcapillaries 0.5 – 20 µL, 100 mm | Eppendorf | 5242956003 | Long tips for loading the glass capillaries with the solutions |
| Micro-tweezers | Dumont Swiss made | 0102-4-PO | We use the micro-tweezers to move the zebrafish larvae during the mounting and to cut off the tip of the glass capillary (eg. Dumont, size 4). |
| Mineral oil | Sigma-Aldrich | M5904 | We use the mineral oil to calibrate the drop size to inject |
| P-1000 Next Generation Pipette Puller | Science Products | P-1000 | We use this capillary puller to prepare the glass capillary using the Drumond capillaries. We use the P-1000 capillary puller with the following parameters: Heat: 650, Pull: 20, Vel: 160, Time: 200, Pressure: 500. |
| PTU (1-phenyl-2-thiourea ) | Sigma-Aldrich | P7629 | We use this compound to inhibit pigmentation during zebrafish development |
| Red Filter Cube | ZEISS | 000000-1114-462 | Filter set 45 HQ TexasRed |
| Stereo microscope | ZEISS | 495015-0001-000 | SteREO Discovery.V8 |
| Syringe filters, sterile. Pore size 0.2 µm | Pall Corporation | 4612 | We use these to filter all the solutions and prevent the capillary needle clothing |
| Transgenic Zebrafish line:Tg(ins:cdt1-mCherry;cryaa:CFP); Tg(ins:GCaMP6s;cryaa:mCherry) | |||
| tricaine methanesulfonate (MS222) | Sigma-Aldrich | E10521 | We use this compound to anesthetize the fish larvae |
Riferimenti
- Speier, ., et al. Noninvasive high-resolution in vivo imaging of cell biology in the anterior chamber of the mouse eye. Nature Protocol. 3 (8), 1278-1286 (2008).
- Jacob, S., et al. In vivo Ca(2+) dynamics in single pancreatic beta cells. Federation of American Society of Experimental Biology Journal. 34 (1), 945-959 (2020).
- Chen, C., et al. Alterations in beta-cell calcium dynamics and efficacy outweigh islet mass adaptation in compensation of insulin resistance and prediabetes onset. Diabetes. 65 (9), 2676-2685 (2016).
- Cohrs, C. M., et al. Vessel network architecture of adult human islets promotes distinct cell-cell interactions in situ and is altered after transplantation. Endocrinology. 158 (5), 1373-1385 (2017).
- Janjuha, S., Pal Singh, S., Ninov, N. Analysis of beta-cell function using single-cell resolution calcium imaging in zebrafish islets. Journal of Visualized Experiments: JoVE. (137), (2018).
- Singh, S. P., et al. Different developmental histories of beta-cells generate functional and proliferative heterogeneity during islet growth. Nature Communications. 8 (1), 664 (2017).
- Lorincz, R., et al. In vivo monitoring of intracellular Ca2+ dynamics in the pancreatic beta-cells of zebrafish embryos. Islets. 10 (6), 221-238 (2018).
- Mullapudi, S. T., et al. Disruption of the pancreatic vasculature in zebrafish affects islet architecture and function. Development. 146 (21), (2019).
- Zhao, J., et al. In vivo imaging of beta-cell function reveals glucose-mediated heterogeneity of beta-cell functional development. eLife. 8, 41540 (2019).
- Yang, Y. H. C., Kawakami, K., Stainier, D. Y. A new mode of pancreatic islet innervation revealed by live imaging in zebrafish. eLife. 7, 34519 (2018).
- Eames, S. C., Philipson, L. H., Prince, V. E., Kinkel, M. D. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 7 (2), 205-213 (2010).
- Nath, A. K., et al. PTPMT1 inhibition lowers glucose through succinate dehydrogenase phosphorylation. Cell Reports. 10 (5), 694-701 (2015).
- Salem, V., et al. Leader β-cells coordinate Ca2+ dynamics across pancreatic islets in vivo. Nature Metabolism. 1 (6), 615-629 (2019).
- Ninov, N., et al. Metabolic regulation of cellular plasticity in the pancreas. Current Biology. 23 (13), 1242-1250 (2013).
- Nüsslein-Volhard, C., Dahm, R. . Zebrafish: A Practical Approach. , (2002).
- Isogai, S., Horiguchi, M., Weinstein, B. M. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Biologia dello sviluppo. 230 (2), 278-301 (2001).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Delgadillo-Silva, L. F., et al. Modelling pancreatic beta-cell inflammation in zebrafish identifies the natural product wedelolactone for human islet protection. Disease Models & Mechanisms. 12 (1), 036004 (2019).
- Emfinger, C. H., et al. Beta-cell excitability and excitability-driven diabetes in adult zebrafish islets. Physiological Reports. 7 (11), 14101 (2019).
- Zhang, M., Goforth, P., Bertram, R., Sherman, A., Satin, L. The Ca2+ dynamics of isolated mouse beta-cells and islets: implications for mathematical models. Biophysical Journal. 84 (5), 2852-2870 (2003).

.
