Three-Dimensional Imaging of Organoids to Study Primary Ciliogenesis During ex vivo Organogenesis
Summary
Stem cell-derived organoids facilitate the analysis of molecular and cellular processes that regulate stem cell self-renewal and differentiation during organogenesis in mammalian tissues. Here we present a protocol for the analysis of the biology of the primary cilium in mouse mammary organoids.
Abstract
Organoids are stem cell-derived three-dimensional structures that reproduce ex vivo the complex architecture and physiology of organs. Thus, organoids represent useful models to study the mechanisms that control stem cell self-renewal and differentiation in mammals, including primary ciliogenesis and ciliary signaling. Primary ciliogenesis is the dynamic process of assembling the primary cilium, a key cell signaling center that controls stem cell self-renewal and/or differentiation in various tissues. Here we present a comprehensive protocol for the immunofluorescence staining of cell lineage and primary cilia markers, in whole-mount mouse mammary organoids, for light sheet microscopy. We describe the microscopy imaging method and an image processing technique for the quantitative analysis of primary cilium assembly and length in organoids. This protocol enables a precise analysis of primary cilia in complex three-dimensional structures at the single cell level. This method is applicable for immunofluorescence staining and imaging of primary cilia and ciliary signaling in mammary organoids derived from normal and genetically modified stem cells, from healthy and pathological tissues, to study the biology of the primary cilium in health and disease.
Introduction
Development of multicellular organisms and the maintenance of homeostasis in their adult tissues reside in a fine-tuned regulation between self-renewal and differentiation of stem cells, which orchestrate in time and space normal tissue development and regeneration1. Subversion of this regulation causes developmental anomalies and cancers2. Thus, understanding the molecular and cellular mechanisms that orchestrate stem cell self-renewal and differentiation is of key interest in developmental and cancer biology.
Recent development of ex vivo organogenesis methods, in which tissue stem cells generate three-dimensional organoids have transformed our capabilities to study the dynamics of stem cells during mammalian organogenesis and maintenance of tissue homeostasis in a dish3. Organoids represent a good alternative to cumbersome genetically modified animal models to study these processes. Protocols for the development of organoids from tissue stem cells of many organs have now been developed3, including small intestine and colon, stomach, liver, pancreas, prostate, and mammary gland3. Additionally, the development of somatic genome-editing techniques in organoid-forming stem cells now enables to quickly interrogate the molecular and cellular mechanisms that control their biology4,5.
The primary cilium is a microtubule-based structure that is assembled at the surface of stem and/or differentiated cells of various tissues6. It is generally non-motile and is assembled as a single structure per cell7. Primary ciliogenesis is the dynamic process of assembling the primary cilium7. At the cell surface, the cilium acts as a cell signaling platform8. Thus, the primary cilium is thought to act as a key regulator of stem cell self-renewal and/or differentiation in many tissues, including the brain9,10, the mammary gland4,11, the adipose tissue12, and the olfactory epithelium13, among others. Primary ciliogenesis and/or ciliary signaling are dynamically regulated in distinct cell lineages and at different developmental stages4,13,14, but the underlying mechanisms remain to be largely determined.
Ex vivo organogenesis shows promise for the development of basic knowledge on the molecular and cellular mechanisms that control stem cell biology, including primary ciliogenesis and ciliary signaling. However, it relies on the ability to properly image whole mount organoids at the single cell level and at sub-cellular scales. We recently used a mouse mammary stem cell-derived organoid model to show that primary cilia positively control mouse mammary stem cell organoid-forming capacity4. Here we present a comprehensive protocol for the immunofluorescence staining of whole mount mouse mammary organoids (Figure 1A,B), which enables the analysis of primary cilia through light sheet microscopy during ex vivo organogenesis in three-dimension. Alternative methods were recently published for the immunofluorescence staining and imaging of organoids through confocal microscopy15,16. This protocol focuses instead on the preparation and imaging of organoids through light sheet microscopy.
Protocol
NOTE: The protocol below is recommended for the staining of organoids that were grown in 5 wells of a 96 well plate and pooled together (> 100 organoids). Organoids were derived from mouse mammary stem cells. Donor mice were housed and handled in accordance with protocols approved by the Animal Care Committee of the University of Rennes (France).
1. Reagents
- To prepare the fixative solution, dilute 125 µL of 16% paraformaldehyde (PFA) aqueous commercial solution in 375 µL of phosphate-buffered saline (PBS) to generate a 4% PFA solution.
CAUTION: Manipulate PFA that is a toxic substance under a chemical hood. - To prepare the permeabilization buffer, dilute 1.5 µL of Triton X100 in 500 µL of PBS, to produce a 0.3% Triton X100 solution.
- To prepare the blocking buffer, dilute 1.5 µL of Tween-20 and 75 µL of normal goat serum in PBS, to generate a 5% goat serum-0.1% Tween 20 solution.
- To prepare the light sheet mounting medium, dissolve 1 g of ultrapure low melting point agarose in 100 mL of dH20 or PBS at 65 °C. Prepare 1 mL aliquots and store at room temperature.
2. Organoids recovery
- Transfer organoids from the culture wells to a low binding polymer 1.7 mL tube, after pipetting them up and down 3 times in the wells, with an FBS-coated tip for which the end was cut (minimal diameter of the extremity 1.5 mm).
NOTE: The coating prevents organoids from sticking to the tip. The low-binding polymer material enables to reduce the attachment of organoids to the side of the tube during the entire staining procedure. - Fill the tube with PBS, and spin down at 350 x g for 3 min. Remove the supernatant.
3. Fixation, permeabilization and blocking
- Resuspend the organoids in 500 µL of 4% PFA. Incubate for 30 min at room temperature. Spin down at 350 x g for 30 s. Remove the supernatant.
NOTE: From this step onwards, resuspend organoids in the different buffers by simply adding the buffers on the pellet of organoids without touching them. Perform all washing steps at room temperature. - Wash the organoids with 1 mL of PBS for 3 min. Spin down at 350 x g for 30 s. Remove the supernatant.
PAUSE: Fixed organoids can be kept in PBS at 4 °C for at least a week. - Permeabilize the organoids by resuspending them in 500 µL of PBS-Triton X100 0.3% and incubate for 30 min at room temperature. Spin down the organoids at 350 g for 30 s.
- Wash the organoids by resuspending them with 1 mL of PBS and incubate for 3 min. Spin down at 350 x g for 30 s. Remove the supernatant. Repeat once.
- Optional: Resuspend the organoids in 500 µL of ice-cold methanol. Incubate at – 20 °C for 10 min. This step may be required for the staining of specific centrosomal markers.
- Optional (if step 5 was performed): Wash the organoids with 1 mL of PBS for 3 min. Spin down at 350 x g for 30 s. Remove the supernatant. Repeat once.
- Block non-specific antibody binding sites in organoids by resuspending them with 500 µL of blocking buffer (PBS, 5% goat serum, 0.1% Tween 20). Incubate 1 h and 30 min at room temperature. Spin down at 350 g for 30 s. Remove the supernatant.
- Wash the organoids by resuspending them with 1 mL of PBS and incubate for 3 min. Spin down at 350 x g for 30 s. Remove the supernatant.
4. Labelling
- Resuspend the organoids with 200 µL of blocking buffer with diluted primary antibodies and incubate overnight at 4 °C with mild shaking (60 rpm on a horizontal shaker). Place the tubes with a 45° angle with the horizontal plan of the shaker. It will maintain the organoids at the bottom of the tubes in the staining buffer.
- Wash the organoids by resuspending them with 1 mL of PBS and incubate for 5 min. Spin down at 350 x g for 30 s. Remove the supernatant. Repeat twice.
NOTE: Some organoids in the last step may stick to the side of the tube, resulting in organoid loss during aspiration of the supernatant after centrifugation, adding 0.2% (w/v) bovine serum albumin (BSA) to the PBS in the washing steps may reduce organoid loss. - Resuspend the organoids with 200 µL of blocking buffer with secondary antibodies and incubate for 1 h and 30 min with mild shaking (60 rpm on a horizontal shaker). Place the tubes with a 45° angle with the horizontal plan of the shaker. It will maintain the organoids at the bottom of the tubes in the staining buffer.
NOTE: Hoechst (or other nuclear dyes, such as DAPI or DRAQ5) can be added to the buffer with secondary antibodies. - Wash the organoids by resuspending them with 1 mL of PBS and incubate for 5 min. Spin down at 350 x g for 30 s. Remove the supernatant. Repeat twice.
NOTE: Some organoids in the last step may stick to the side of the tube, resulting in organoid loss during aspiration of the supernatant after centrifugation, adding 0.2% (w/v) BSA to the PBS in the washing steps may reduce organoid loss.
5. Preparation of the agarose sample for imaging
- Melt light sheet mounting medium by incubating it at 65 °C. Once the medium has melted, incubate it at 37 °C for 5 min.
- Resuspend the organoids in 100 µL of mounting medium using a 200 µL tip, with the extremity of the tip cut (minimal size of the extremity: 1.5 mm), by pipetting up and down twice.
- Suck the mounting medium with the organoids in a glass capillary (green capillary, inner diameter: 1.5 mm) using a plunger. Incubate the capillary at room temperature for 5 min for the mounting medium to solidify.
PAUSE: Capillary can be stored in PBS at 4 °C for a week before imaging.
6. Imaging
- Using a light sheet microscope (e.g., ZEISS Lightsheet Z.1), image the organoids with 20x or 40x water immersion objectives.
- Place the glass capillary in the observation chamber. Locate the capillary with the front camera and place the tip of the glass capillary at the upper limit of the detection objective.
- Select the sample and set the optimal focus using the tip of the glass capillary in brightfield illumination. Push out the agarose sample slowly from the capillary and locate organoids within the solidified agarose.
- Keep the organoids to be imaged close to the tip of the capillary, to reduce movements of the agarose sample in the PBS. Rotate the capillary to set the optimal sample orientation and define the desired zoom.
- Select acquisition mode. Define the channels to image, set proper orientations of the light sheet, set the laser power for each channel (to reduce photobleaching, use low laser power), set the size of the image and the desired illumination side(s). Example of imaging parameters: Image size 1920 x 1920, illumination: dual side.
- Define the Z-stack to image by setting the Z-extremities of the stack using the Z-stack module and set the Z-step size to optimal.
- Process the output file using the image processing module of the microscope software to navigate in the sample, obtain a Z-projection and 3D-representation.
- Turn the sample and acquire a new image from a different angle or image other organoids.
- Analyze images with an interactive microscopy image analysis software (e.g., Imaris) enabling (i) the visualization of the sample in 3D (ii) the segmentation of objects (iii) identification and quantitative analysis of objects.
Representative Results
Ex vivo organogenesis methods are transforming our capabilities to study mammalian tissue development and maintenance of tissue homeostasis in a dish. The analysis of molecular and cellular mechanisms that regulate these processes, including primary ciliogenesis and ciliary signaling, relies on the ability to image organoids in three-dimension.
The protocol described above enables the staining of whole-mount mammary organoids. They arise from mammary stem cell-enriched basal cells that are FACS-purified from adult female mice (Figure 1A)4. The staining procedure enables the visualization of cell lineage, centrosome, and primary cilium markers in organoids in less than 24 h (Figure 1B). The strength of the immunofluorescence staining and imaging procedures that we describe for the visualization of the three-dimensional architecture of organoids is exemplified here through the staining and visualization of integrin α6, also known as CD49f (Figure 1C). Additionally, the staining of primary cilia (through the staining of Arl13b, a primary cilium marker), of centrosomes (through a γtubulin staining), and of mammary-stem cell enriched basal cells (through the staining of Slug, an epithelial-mesenchymal transition marker) illustrates the ability to visualize whole-mount organoids at the cellular and sub-cellular scales (Figure 1C).
The method described here enables semi-automated segmentation of objects and image analysis (Figure 1C, Animated Figure 1). Using an interactive microscopy image analysis software, the total cell number, the number of cells from distinct cell lineages, and the number and size of primary cilia can be quantified with accuracy.
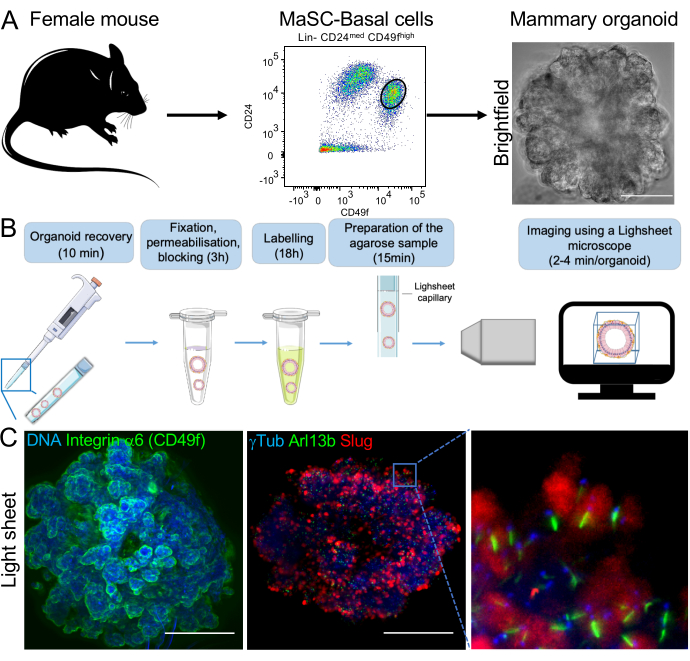
Figure 1: Overview of the immunofluorescence staining and imaging procedures for the analysis of mammary organoids. (A) Mammary glands from adult C57BL6/J female mice are dissociated and mammary stem cell-enriched basal cells are FACS-purified (CD49fhigh;CD24med phenotype) and plated in three-dimension according to Guen et al., 20174. Mammary organoids ranging from 100-300 µm in size are observed 7-14 days after plating by brightfield microscopy. Scale bar: 100 µm. (B) Organoids are recovered, fixed, permeabilized and stained prior to be embedded in agarose in a glass capillary. The procedure takes less than 24 h. Agarose samples containing the organoids are imaged through light sheet microscopy. Imaging of an organoid takes 2-4 min. (C) Mammary organoids were stained for the indicated proteins and imaged following the procedure described above. Hoechst was used as a nuclear dye. All scale bars 100 µm. Please click here to view a larger version of this figure.
Animated Figure 1: Three-dimensional analysis of an organoid at the cellular and sub-cellular scales. Slug-expressing basal cells (Slug staining: red), primary cilia (Arl13b staining: green) and centrosomes (gTubulin staining: magenta) were observed in a mouse mammary organoid through post-acquisition image processing using an interactive image analysis software enabling semi-automated segmentation. Hoechst was used as a nuclear dye (blue). Please click here to download this File.
Discussion
The detailed protocol presented here enables the staining and imaging of mouse mammary organoids that grow in semi-solid medium. This protocol is presumably applicable to the staining of organoids mimicking the architecture of various tissues that grow in semi-solid and solid media. For organoids that grow in 100% Matrigel with medium on top, the recovery and fixation steps slightly differ. The culture medium must be removed from the culture well. After a quick PBS wash, the fixative solution (4% PFA) may be directly added in the culture well on the Matrigel-containing organoids. Organoids in Matrigel can be incubated with the fixative solution for 30-60 min at room temperature. Fixed organoids are subsequently transferred to the staining tube after resuspending them in the fixative solution. The fixative solution and the residual Matrigel must be completely removed after centrifugation and prior to the permeabilization step. Organoid permeabilization is a critical step in the protocol. Co-staining of primary cilium and centrosome markers generally requires Triton X-100 and methanol incubation steps. The co-staining of other markers such as cell-cell junction markers or other cytoskeletal proteins may be affected by these permeabilization steps and other strategies may have to be used.
Loss of organoids is an issue during the entire staining procedure. To reduce organoid loss, it is critical to coat the tip that is used to transfer the organoids from the culture well to the staining tube. It is also critical to use a low-binding polymer tube as the staining tube. These measures will prevent organoids from sticking to the tip or the tube during the transferring and washing steps, respectively. Organoids may still be lost during the washing steps after the incubations with antibodies. Adding 0.2% (w/v) BSA to the PBS in the washing steps may reduce organoid sticking to the side of the tube and organoid loss. To ensure enough recovery of organoids for imaging at the end of the procedure, we recommend starting the experiment with more than 100 organoids.
The protocol presented here enables whole-mount immunofluorescence staining and preparation of organoids for light sheet microscopy. Alternative methods for the staining and preparation of organoids for confocal microscopy were recently published15,16. The light sheet microscopy technology enables faster imaging of organoids than confocal microscopy and offers the possibility to image organoids at the subcellular scale while keeping the overview of entire 3D structures. Visualization of entire structures facilitates the analysis of molecular and cellular mechanisms that occur in a heterogeneous manner in organoids, such as primary ciliogenesis. In the protocol presented here, organoids are embedded in agarose in a glass capillary and can be rotated along the Y-axis in order to facilitate their imaging through the best-suited angle of observation. Proper embedding of the organoids in agarose for imaging is a critical step of the protocol. In the last PBS wash following the incubation with the secondary antibodies, as much PBS as possible must be removed from the staining tube, without sucking the organoids. Reducing the amount of PBS in the staining tube prior to organoid resuspension in the mounting medium will reduce dilution of the agarose solution and ensure proper solidification of the agarose sample in the capillary.
Proper positioning of the agarose sample containing the organoids during imaging is another critical step. It is important to keep the organoids to be imaged in the agarose sample out but close to the tip of the glass capillary. The agarose sample far from the glass capillary tends to move in the PBS in the observation chamber. However, the agarose close to the glass capillary remain immobilized for proper imaging of the organoids. If a movement close to the capillary is still affecting proper imaging, cutting the extremity of the agarose sample that is floating in the observation chamber may solve the problem. While the glass capillary may be re-used for multiple samples, we do not recommend the re-use of the plunger. We found that the agarose sample tend to slowly escape from the glass capillary when a plunger is re-used, affecting significantly the possibility to image organoids.
The protocol presented here does not include optical clearing of organoids. We found that organoids can be efficiently imaged up to 100-150 µm in depth using our protocol. Imaging of larger whole organoids may require the acquisition of two images from each side of the same organoid. The images can be stitched during the image processing step. Nevertheless, an optical clearing step, as described by others15, is presumably compatible with the protocol as well. It must be performed after the labelling step and before agarose-embedding of the samples. While optical clearing is not strictly necessary for organoid imaging using a light sheet microscope, optical clearing is especially valuable when imaging organoids with a confocal microscope. Rotation of samples is not possible with this type of microscope.
Light sheet microscopes enable faster image acquisition than standard confocal microscopes and thus enable the analysis of many organoids in a short period of time. The study of multiple organoids enables robust statistical analysis when quantitative measurements are performed during post-acquisition image processing. Advanced confocal microscopes may, however, offer higher image resolution than light sheet microscopes. Both types of microscopy require time consuming post-acquisition image processing for the analysis of organoids in three-dimension. This limitation should be considered. The output file in light sheet microscopy is very large (e.g. 3-8 GB per image) and management of large datasets can be an issue. The protocol presented here enables three-dimensional analysis of fixed organoids. The fixation and agarose-embedding steps may of course represent a limitation for some applications that require the analysis of very dynamic events in the same organoid over an extended period of time. Live-imaging of organoids, which express fluorescent reporters, may be more appropriate for such analysis. In this setting, organoids would have to be embedded and imaged in a specific gel and in a medium at the appropriate temperature, which preserve the viability and normal physiology of organoids. The light sheet illumination method that reduces photobleaching and phototoxicity and enables faster imaging of samples, in comparison to standard illumination with a confocal microscope, is especially valuable for live-imaging.
In conclusion, the protocol presented here enables the immunofluorescence staining of whole mount mammary organoids that arise from normal and genetically modified mouse mammary stem cells with or without diverse pharmacological perturbations. Thus, it allows an in-depth analysis of the molecular and cellular processes, including primary ciliogenesis and ciliary signaling, that regulate stem-cell self-renewal and differentiation during the course of ex vivo organogenesis. This protocol may also be applicable to the staining of breast cancer organoids that arise from transformed cells of cancerous tissues. Therefore, this method should contribute to the development of our knowledge on the biology of the cilium during mammary organoid formation in healthy and pathological conditions. This protocol is presumably applicable to the staining of organoids derived from stem cells of different tissues, with minor modifications. Thus, it may enable the analysis of the biology of the primary cilium in many tissues in health and disease.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Xavier Pinson for help in development of light sheet microscopy; the Biosit biotechnology center, including MRic, Arche, the Flow cytometry core facilities, and SFR Santé F. Bonamy, including the MicroPICell core facility, for technical support. This work was supported by Fondation ARC, Cancéropôle Grand Ouest, Université de Rennes 1, Fondation de France. M.D. was supported by a Graduate Fellowship from the University of Rennes. V.J.G. was supported by a Postdoctoral Fellowship from Fondation ARC.
Materials
| Anti-mouse IgG1 647 | Thermo-Fisher | A21240 | |
| Anti-mouse IgG2A 488 | Thermo-Fisher | A21131 | |
| Anti-rabbit 546 | Thermo-Fisher | A11035 | |
| Arl13b | NeuroMab | 73-287 | |
| EMS 16% Paraformaldehyde Aqueous Solution, EM Grade | Electron Microscopy Sciences | 15710 | |
| FBS | Thermo-Fisher | 10270106 | |
| gtubulin | Sigma-Aldrich | T5326 | |
| Hoechst 33342 | Thermo-Fisher | 62249 | |
| Integrin a6 | Biolegend | 313616 | |
| Light Sheet Capillary | Zeiss | 701908 | |
| Light Sheet plunger | Zeiss | 701998 | |
| Low binding Microcentrifuge tubes | BioScience | 27210 | |
| Normal Goat Serum Blocking Solution | Vector labs | S-1000 | |
| PBS | Sigma-Aldrich | p3587 | |
| Slug | Cell Signaling Technology | 9585 | |
| Triton-X100 | Sigma-Aldrich | T9284 | |
| Tween-20 | Euromedex | 9005-64-5 | |
| UltraPure Low Melting Point Agarose | Thermo-Fisher | 16520050 |
Riferimenti
- Visvader, J. E., Clevers, H. Tissue-specific designs of stem cell hierarchies. Nature Cell Biology. 18 (4), 349-355 (2016).
- Batlle, E., Clevers, H. Cancer stem cells revisited. Nature Medicine. 23 (10), 1124-1134 (2017).
- Clevers, H. Modeling Development and Disease with Organoids. Cell. 165 (7), 1586-1597 (2016).
- Guen, V. J., et al. EMT programs promote basal mammary stem cell and tumor-initiating cell stemness by inducing primary ciliogenesis and Hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America. , (2017).
- Hendriks, D., Clevers, H., Artegiani, B. CRISPR-Cas Tools and Their Application in Genetic Engineering of Human Stem Cells and Organoids. Cell Stem Cell. 27 (5), 705-731 (2020).
- Satir, P., Pedersen, L. B., Christensen, S. T. The primary cilium at a glance. Journal of Cell Science. 123, 499-503 (2010).
- Guen, V. J., Prigent, C. Targeting Primary Ciliogenesis with Small-Molecule Inhibitors. Cell Chemical Biology. 27 (10), 1224-1228 (2020).
- Goetz, S. C., Anderson, K. V. The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics. 11 (5), 331-344 (2010).
- Han, Y. G., et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nature Neuroscience. 11 (3), 277-284 (2008).
- Tong, C. K., et al. Primary cilia are required in a unique subpopulation of neural progenitors. Proceedings of the National Academy of Sciences of the United States of America. 111 (34), 12438-12443 (2014).
- Wilson, M. M., Weinberg, R. A., Lees, J. A., Guen, V. J. Emerging Mechanisms by which EMT Programs Control Stemness. Trends in Cancer. 6 (9), 775-780 (2020).
- Hilgendorf, K. I., et al. Omega-3 Fatty Acids Activate Ciliary FFAR4 to Control Adipogenesis. Cell. 179 (6), 1289-1305 (2019).
- Joiner, A. M., et al. Primary Cilia on Horizontal Basal Cells Regulate Regeneration of the Olfactory Epithelium. Journal of Neuroscience. 35 (40), 13761-13772 (2015).
- Bangs, F. K., Schrode, N., Hadjantonakis, A. K., Anderson, K. V. Lineage specificity of primary cilia in the mouse embryo. Nature Cell Biology. 17 (2), 113-122 (2015).
- van Ineveld, R. L., Ariese, H. C. R., Wehrens, E. J., Dekkers, J. F., Rios, A. C. Single-Cell Resolution Three-Dimensional Imaging of Intact Organoids. Journal of Visualized Experiments. (160), e60709 (2020).
- Pleguezuelos-Manzano, C., et al. Establishment and Culture of Human Intestinal Organoids Derived from Adult Stem Cells. Current Protocols in Immunology. 130 (1), 106 (2020).

.
