Humanized Mediator Release Assay as a Read-Out for Allergen Potency
Summary
Here we present the mediator release assay, using a rat basophilic leukemia cell line transfected with the human IgE receptor, to simulate the degranulation of effector cells typically observed in type 1 allergic reactions. This method investigates the biological activity of allergens in a highly sensitive, reproducible, and tailorable manner.
Abstract
Mediator release assays analyze in vitro immunoglobulin E (IgE)-mediated degranulation and secretion of mediators by effector cells, such as mast cells and basophils, upon stimulation with serial dilutions of putative allergens. Therefore, these assays represent an essential tool that mimics the in vivo degranulation process, which occurs upon allergen exposure in sensitized patients or in skin prick tests. Additionally, these assays are usually employed to investigate the allergenic potential of proteins and the reactivity of patients’ sera’s reactivity. Herein, we describe a simple 2-day protocol using an immortalized rat basophil leukemia cell line transfected and humanized with the human high-affinity IgE plasma-membrane receptor (FcεRI). This variant of the mediator release assay is a robust, sensitive, and reproducible in vitro cell-based system without the need to immobilize the antigen to solid matrices. The protocol consists of the following steps: (1) complement inactivation of human sera, (2) harvesting, seeding, and passive sensitization of the cells, (3) stimulation with antigen to cause mediator release, and (4) measuring of β-hexosaminidase activity as a surrogate for the released inflammatory mediators, such as histamine. The assay represents a useful tool to assess the capacity of the allergen-IgE cross-linking to trigger cell degranulation and can be implemented to standardize allergen extracts, to compare patients’ reactivity to minor or major allergens and to allergenic extracts (pollen, cat dander, etc.), to investigate the potency of allergen homologs, isoforms, and fold-variants (e.g., hypoallergenicity), as well as the effects of ligands on the allergenic activity. A more recent application includes the use of the assay to monitor the treatment efficacy in the course of allergen immunotherapy.
Introduction
Type I hypersensitivity reactions, characterized by Immunoglobulin E (IgE) production specific for a respective antigen, affect nearly a third of the world's population. These reactions are associated with several allergic manifestations such as asthma and rhinoconjunctivitis and can even lead to systemic life-threatening reactions1. Unlike in vivo tests, immunochemical approaches, such as the enzyme-linked immunosorbent assay (ELISA), are solely suitable for investigating the target binding of antibodies but do not address the functional aspect of proteins that can cause immediate hypersensitivity reactions. The immobilization of the allergens on solid supports (e.g., ELISA plates) could cause changes in their structural integrity and the destruction of allergy relevant epitopes2. Even skin prick tests (SPT), the most common tool to confirm sensitization against certain allergens, have their limits concerning the detection of symptomatic IgE-mediated food allergy or allergen availability3,4. In order to find an ethical, highly specific, sensitive, and cost-effective method for testing the biological potency of allergens to cause a type I hypersensitivity reaction, the so-called mediator release assays have been established.
The principle of these assays relies on events following the sensitization phase and the accompanying ability of IgE to bind to the α-chain of the high-affinity receptors expressed on the surface of effector cells, such as mast cells and basophils. IgE is mainly produced by plasma cells in the mucosal-associated lymphoid tissue. Although it is the least abundant immunoglobulin (around 0.05% in non-atopic individuals) in the blood, it possesses an extraordinarily high biological activity being the main cause for allergic symptoms. The half-life of IgE can increase from 2-3 days to several weeks and even months when bound to its receptors on effector cells. Subsequent binding of an antigen to the variable region of two receptor-bound IgE molecules leads to their cross-linking followed by the induction of a downstream signaling cascade in the effector cell leading to degranulation and the release of several pro-inflammatory mediators causing vasodilation, such as histamine, serine proteases (e.g., tryptase), and prostaglandins5,6,7. The secretion of cytokines such as interleukin 4 (IL-4) and IL-13 are responsible for the maintenance of the inflammatory T helper 2 (Th2) response and the class-switching of B cells to IgE-producing plasma cells5,8,9. On the other hand, released thromboxane causes bronchoconstriction, and leukotrienes stimulate smooth muscle contraction as well as vascular leakage, and play a crucial role in airway inflammation leading to asthma or allergic rhinitis10,11.
Research tools for analyzing most of the aforementioned mediators have been established, although with some major disadvantages. Tryptase assays are suitable clinical approaches for the measurement of systemic anaphylaxis through mast cell activation but their sensitivity and specificity in allergy diagnoses is too inaccurate compared to gold standard methods such as SPT. On the other hand, cysteinyl leukotriene assays are not capable of diagnosing allergies to β-lactams or nonsteroidal anti-inflammatory drugs12. Protocols for the measurement of histamine as a major mediator released in allergic reactions were already established in the 1960s. Once released in the peripheral blood, histamine is immediately degraded by histamine methyltransferases resulting in a plasma half-life of only a few minutes, making its analysis quite challenging13. Aside from its instability, the monitoring of histamine was shown to have a low specificity and sensitivity for drug allergies as well as commercial food proteins and wasp venoms12.
In vitro models with effector cell lines have been introduced as an alternative to the labor-intensive procedures of isolation and cultivation of basophils from allergic patients to perform release assays. Therefore, the rat basophilic leukemia- (RBL-) based assay using the RBL-2H3 cell line has been established3. Since this cell line is not capable of binding human IgE, it was first transfected with the α-, β-, and γ-chain of the human IgE plasma-membrane receptor (FcεRI). Several clones have been generated and tested for expression levels and homogeneity of the human α-chain, of which the clone RBL-30/25 emerged as the most promising candidate for in vitro testing. The signaling cascade induced upon receptor activation of the transfected clone was tested via calcium mobilization assays. As an indicator for degranulation and surrogate for histamine release, the lysosomal enzyme β-hexosaminidase was measured, which has the significant advantage of higher stability14. The mediator release using RBL-30/25 cells reaches up to 100% and is, therefore, used to test sera derived from allergic patients. The assay was tested for the mediator release after challenging sensitized cells with commercial allergen extracts. This led to the finding that there is a tremendous variation in the composition (of up to 60-fold regarding the total protein content) of allergen extracts derived from different manufacturers and used for diagnostic (e.g., SPT) or therapeutic approaches3,15,16.
Herein, we provide a detailed description of the RBL protocol to perform the mediator release assay using serum from allergic donors. During passive sensitization, IgE in the serum is captured by the high affinity FcεR1 receptor expressed on the surface of the basophilic cells. Upon antigen-stimulation, bound IgEs specific for the antigen are cross-linked, triggering cell degranulation and the release of the mediator β-hexosaminidase. The activity of β-hexosaminidase is subsequently measured using a suitable substrate. For the assay, huRBL-2H3 cells were used, and termed huRBL in the following protocol. The protocol describes a standard antigen dilution series with 8 steps diluted 1:10 ranging from 1 µg/mL to 0.1 pg/mL of allergen.
Protocol
Ethical approval to use sera derived from birch pollen allergic patients was obtained from the Dutch ethical committee (approval number: NL65758.018.18).
1. Safety procedures
- Work under sterile conditions using a biological safety class 2 workbench during the first day of the experiment (Biosafety Level 2). Follow the safety guidelines of the institution for the usage of human serum.
2. Complement inactivation of human sera
- Harvest a dense culture of P3X63Ag8.653 cells (termed Ag8 cells henceforth), from the cell culture flask and transfer them into a centrifugation tube.
- Use the following culture medium for these cells: Modified Eagles´s Minimum Essential Medium with reduced serum concentration, 1% Penicillin-Streptomycin (100 units Pen., 0.1 mg/mL Strep.), 5% heat-inactivated fetal calf/bovine serum (FCSi).
- Centrifuge Ag8 cells for 5 min at 250 x g at room temperature.
- Re-suspend the cell pellet to a final concentration of approximately 1 x 106 cells/mL in huRBL medium (Minimum Essential Medium Eagle with Alpha Modification, 4 mM L-Glutamine, 5% FCSi, 1% G418 (100% stock: 10 g/125 mL dH2O).
NOTE: Maintain Ag8 cells by passaging for future use as well. - Dilute human sera 1:10 in Ag8 cell suspension. Final serum dilution in the assay will be 1:20.
NOTE: For sera with low specific IgE a 1:5 (1:10 final dilution) can be used. - Incubate for 1 h at 37 °C and 5%-7% CO2.
3. Harvesting and seeding of huRBL cells
- Aspirate the medium from a T-75 cell culture flask carefully without touching the huRBL cells (huRBL cells are adherent). Ensure that the cells are around 50%-90% confluent.
NOTE: Depending on the cell confluence, the cell content of a dense T-75 cell culture flask is usually enough for one to two 96-well plates. - Wash the cells twice by adding 10 mL of Dulbecco's phosphate-buffered saline (DPBS). Add DPBS to the opposite side of the flask and not directly onto the cells.
- Aspirate DPBS and add 5 mL of pre-warmed 1x trypsin-EDTA (0.05%/0.02% EDTA diluted in DPBS) for cell detachment.
- Incubate the flask for 5 min at 37 °C.
- Detach cells by carefully tapping the flask.
- Transfer the cell suspension into a 15 mL centrifugation tube and fill up with huRBL medium or DPBS to dilute the trypsin-EDTA.
- Centrifuge the cells at 250 x g for 5 min at room temperature.
- Aspirate the supernatant and resuspend the pellet in 5 mL of huRBL medium for cell counting.
- Count the cells and dilute them in huRBL medium to obtain a final concentration of 2 x 106 cells/mL.
- Use a sterile 96-well plate and add 50 µL of huRBL cell suspension per well, which is equivalent to 1 x 105 cells/well.
4. Passive sensitization of huRBL cells
- Centrifuge the pre-incubated Ag8/serum suspension for 5 min at 250 x g.
- Transfer 50 µL of the centrifuged Ag8/serum suspension to each well containing huRBL cells without disturbing the Ag8 cell pellet.
- Include the no antigen control, which are sensitized, but unstimulated cells (do not add antigen), serving as an indication for the bottom signal plateau/background. Background and maximum lysis control wells do not need to be sensitized with serum. Add 50 µL of huRBL medium to control wells instead.
- Cover the plate with the lid and incubate overnight at 37 °C and 5%-7% CO2.
5. Antigen-stimulated degranulation and mediator release
- Prepare the antigen dilution in 1x Tyrode's buffer (9.5 g/L Tyrode's salts, 0.1% bovine serum albumin (BSA), 0.5 g/L Sodium hydrogen carbonate (NaHCO3) in dH2O) in advance. A final amount of 100 µL per well is needed.
NOTE: Not every allergen, either purified from natural sources or recombinantly produced, might be stable in 1x Tyrode's buffer. Therefore, perform stability tests in 1x Tyrode's buffer prior to the assay procedure. Alternatively, dilute 1x Tyrode's buffer in deuterium oxide (D2O) to increase the signal-to-noise ratio of the assay. - Make 8 dilutions of the antigen of interest of a 1:10 dilution series in reaction tubes starting with either 10 or 1 µg/mL of protein.
NOTE: Always test the dilution series beforehand. Alternatively, adapt the 1:10 dilution series (e.g., 1:5, 1:20, or 1:30) in order to cover the full release curve. In addition, the starting concentration can vary depending on the experimental setup. - To wash huRBL cells plated on the 96-well plate, remove the sera-containing cell medium first by carefully aspirating, inverting, and tapping the plate on absorbent paper.
- Wash cells with 200 µL of 1x Tyrodes's buffer per well. Treat all wells similarly.
NOTE: Add the washing solution slowly to the cells in order to not disturb them. - Leave it for approximately 30 s and repeat the washing step three times in total.
- After adding the washing solution for the final time, leave the solution in the wells until ready to continue with adding the antigen dilution.
NOTE: Avoid exposing cells to air for too long. - Transfer 100 µL of antigen solution to each well containing the pre-sensitized huRBL cells.
NOTE: If analyzing several different parameters, transfer the individual samples of the dilution series into an additional non-binding 96-well plate (use the same layout as on the huRBL plate) and transfer them afterward with a multichannel pipette directly on the huRBL cell plate. This way, exposing the cells to air for too long can be avoided, which might result in poor assay performance (lower/no signal). - Cover control wells (maximum lysis and non-sensitized background cells) with 100 µL of 1x Tyrode's buffer. Do not stimulate these control wells with the antigen.
- Additionally, add 100 µL of 1x Tyrode's buffer to the sensitized no-antigen wells of the dilution series, which is needed to take antigen-independent spontaneous release of sensitized cells into account during data analysis.
- Incubate huRBL cells for 1 h at 37 °C and 5%-7% CO2.
6. Fluorescence measurement of β-hexosaminidase activity
- Treat the wells of the maximum lysis control with 10 µL of 10% Triton X-100 per well and mix properly in order to lyse the cells completely and obtain the 100% release of β-hexosaminidase.
- Add 50 µL of substrate solution into a new non-binding 96-well plate. Substrate solution for one 96-well plate: 5 mL of 0.1 M citric assay buffer, pH 4.5; and 80 µL of 10 mM 4-methylumbelliferyl N-acetyl-β-D-glucosaminide.
- Transfer 50 µL of cell supernatant of all wells to the new plate containing the substrate solution.
NOTE: Pipette the supernatant carefully off the huRBL plate in order to not disrupt the huRBL cells. - Incubate the plate with substrate solution and cell supernatant for 1 h at 37 °C to allow conversion of the fluorogenic substrate.
NOTE: Keep the huRBL plate for cell viability assay. - Add 100 µL of stopping solution (15 g/L glycine, 11.7 g/L NaCl dissolved in dH2O, pH 10.7) per well.
- Measure the fluorescence at 360 nm excitation and 465 nm emission using a plate reader.
7. Data analysis
- For basic calculations of percentage release, use any spreadsheet software.
- For the background subtraction/baseline removal, subtract the average of the background wells from all other wells.
- Calculate the mean of maximum lysis wells and express data of the dilution series in percentage. This way one can express data as a percentage of cell release normalized to the maximum enzyme release caused by cell lysis.
- Complete dose-response mediator release curves are represented best as XY graphs with the antigen concentration on a log on the X-axis and percentage of mediator release on the Y-axis.
- Add the values of the no-antigen control as a dashed line to indicate the background or the bottom plateau.
NOTE: Several similarly treated sera can be compared using this normalization strategy. For direct comparison, it is further recommended to calculate the half maximum release, which is the antigen concentration (in ng/mL) necessary for half maximal release defined as the average of the maximal and minimal values per curve. The antigen concentration to stimulate half maximal release is calculated by interpolation of the half maximal release value into a logarithmic regression line.
Representative Results
The mediator release assay, based on huRBL cells (Figure 1A and B), results in a bell-shaped dose-response curve (Figure 1C). For simplified data representation, the antigen concentration necessary for the half maximum mediator release can be calculated using linear regression (Figure 1D). A cell viability assay is performed to exclude cytotoxic effects derived from either the sensitizing serum or the antigen used for stimulation (Figure 1E). The assay can be used to test the reactivity of different sera to a certain antigen. In our case, 4 out of 5 sera, derived from birch pollen allergic patients, responded to Bet v 1 stimulation. Serum #1 showed the highest mediator release (Figure 2). Serum #5 did not respond to Bet v 1 stimulation and, thus, might react to other birch pollen allergens (e.g., Bet v 2, profilin). These data indicate that Bet v 1 is a potent allergen responsible for IgE-mediated allergic symptoms. By using the huRBL assay, the cross-reactivity of IgE to homologous allergens can be assessed (Figure 3). Here, both birch pollen allergic patients responded well to Bet v 1, whereas only patient #2 responded also to Cor a 1, the Bet v 1-homologous food allergen found in hazelnuts. Based on these data, patient #2 most likely has higher Cor a 1-cross-reactive IgE levels than patient #1, resulting in oral allergy symptoms upon hazelnut consumption. Even the assessment of the hypoallergenic nature of mutant variants of allergens (decreased potency) can be analyzed and compared to their wild-type counterpart (Figure 4). In the provided example, the release curve of the fold variant shifted towards a higher antigen concentration compared to the wild-type allergen, resulting in a significantly higher concentration of antigen necessary to provoke half maximal release (Figure 4B). These data imply that the generated mutant/fold variant is less allergenic compared to the wild-type protein. This reduced potency to trigger IgE-mediated degranulation highlights the hypoallergenic character of the fold variant. Based on this assay, the fold variant is an interesting candidate for allergen-specific immunotherapy since it might cause reduced IgE-associated side effects during the treatment.
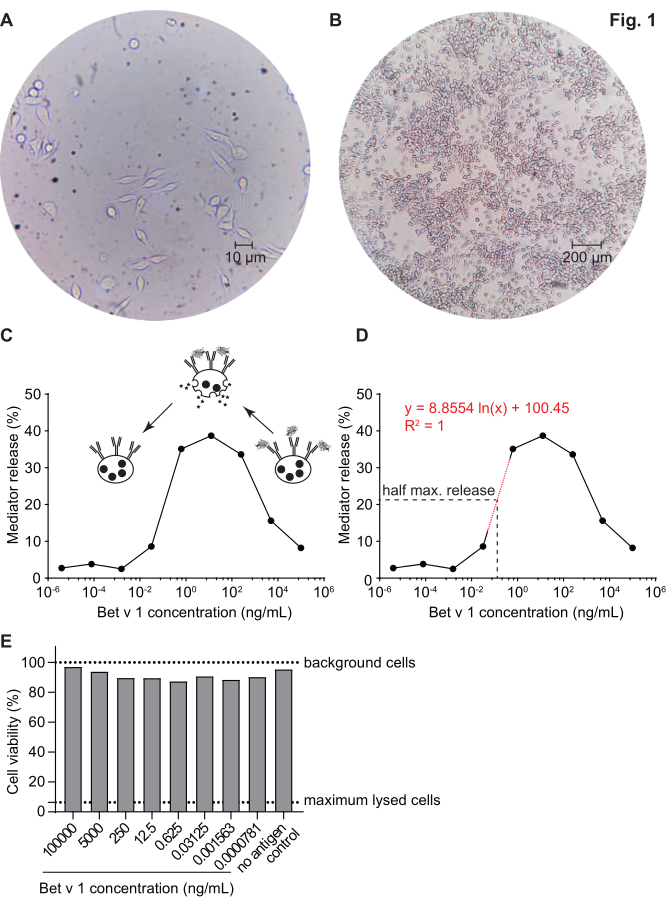
Figure 1: Humanized RBL cells and a representative bell-shaped curve of IgE-allergen cross-linking-induced β-hexosaminidase release. RBL cells are adherent to the culture flasks, which gives them a rod-like shape as they are trying to attach themselves (A). An ideal level of confluence for cells to be harvested is no more than 90% (B). Cells are shown under magnification of 40x and 10x, respectively. Cells that were sensitized with human serum of a birch pollen allergic individual reacting upon challenge with recombinant Bet v 1 (rBet v 1), the major birch pollen allergen (C). As surrogate for mediator release, the β-hexosaminidase activity is measured in cell supernatants. The bell-shaped curve results from a monovalent occupation of antigen epitopes on IgE due to the excess of allergen, which competitively inhibits the allergen-IgE cross-linking at high antigen concentrations. Another explanation for the low release at high allergen concentrations is the inhibition of intracellular pathways in presence of excess antigen. For determination of the allergen concentration necessary to obtain half maximal release, a logarithmic regression line based on the experimental values representing the linear part of the slope of the mediator release curve was used (D). The red dotted line represents the logarithmic regression line used for calculation. The formula of the regression line is shown in red. The half maximal release is defined as: half maximal release = (minimum release value + maximum release value)/2. In the example, the calculated half maximal release was 20.6%. The representative human serum used in this experiment was diluted 1:20 for incubation with huRBL cells, and the antigen concentration used for stimulation ranged from 100 µg/mL to 0.004 pg/mL of Bet v 1. A cell viability assay, in this case a MTT assay, was performed with the remaining cells after antigen stimulation to assess the influence of the sensitizing serum as well as of the antigen dilution on cell viability and cell count (E). Untreated background cells and lysed cells (maximum lysis) are shown as dotted line. Please click here to view a larger version of this figure.
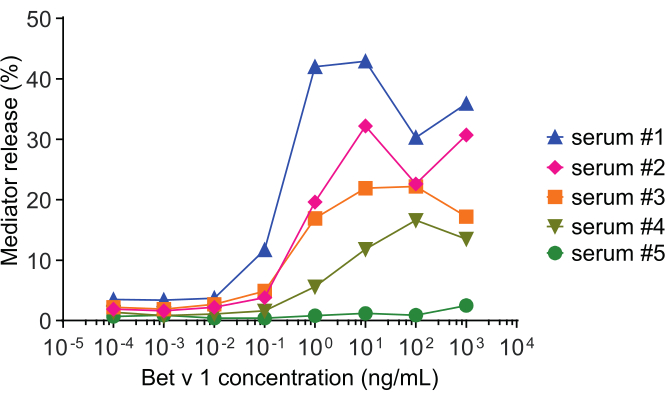
Figure 2: Representative curves of β-hexosaminidase percent release of five different human sera. The same antigen concentration range of rBet v 1 was incubated with huRBL cells that were sensitized with sera of different birch pollen sensitized individuals. There is a clear difference of percent release between the different patients corresponding to the severity of their symptoms. Notice that patient #5 is non-reactive to the major birch pollen allergen Bet v 1. All five human sera used to obtain these mediator release curves were diluted equally 1:20 for incubation with huRBL cells. Please click here to view a larger version of this figure.
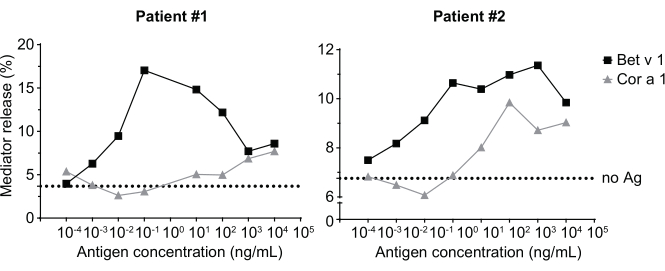
Figure 3: Cross-reactivity of IgE derived from sera of birch pollen sensitized patients with the Bet v 1 homologous hazelnut allergen Cor a 1. Two representative sera of patients sensitized to birch pollen strongly react to Bet v 1 as well as to a lesser degree to the homologous allergen Cor a 1. Patient 2 is shows a significant reaction to Cor a 1, and thus will likely exhibit oral allergy symptoms upon hazelnut consumption, compared to patient 1 where the mediator release is almost negligible. The dotted line represents the no-antigen control, which are cells sensitized with the human sera but not stimulated with an allergen, and, thus, serves as indication for the bottom signal plateau/background. Please click here to view a larger version of this figure.
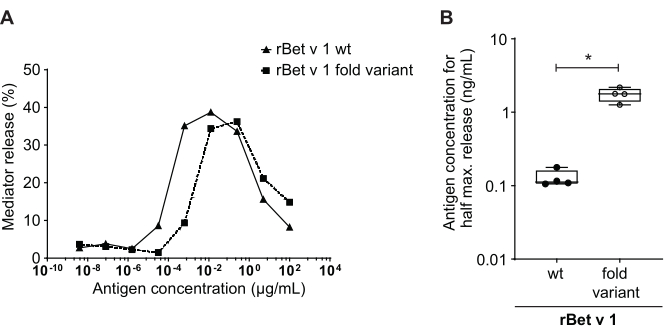
Figure 4: Comparison of percent release between rBet v 1 wild type and a hypoallergenic fold-variant. The same serum of a birch pollen-sensitized individual was incubated with rBet v 1 wild type (wt) and a hypoallergenic fold-variant of the major birch pollen allergen (A). Even though mediator release is seen in both antigens, there is a clear shift toward higher antigen concentrations when comparing the fold-variant to wild type rBet v 1 for the same percent release. A standard way of comparing the difference in percent release of different antigens is calculating the concentration of antigen needed to attain half maximum release (B). This is usually performed in biological replicates (testing of the same antigen range for each allergen in different human sera). Usually, in order to draw any significant conclusions, the mediator release is performed with sera from at least 8 to 10 different patients. Here the results of four different sera are plotted as an example. A paired t-test was used for statistical analysis. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001; ****p ≤ 0.0001. Please click here to view a larger version of this figure.
| Potential questions and troubleshooting | Solution |
| Assay-to-assay variability due to altered cell responsiveness | Ensure that the cell passage cycle number does not exceed 20 to 30 passages. Make frozen stocks at early passages for future experiments. |
| Rather rely on biological replicates (use of different sera) than technical ones. | |
| Sera contains low levels of specific IgE | A lower final serum dilution can be used (i. e. 1:10) instead of 1:20. Conversely, sera containing high levels of specific IgE can be further diluted (1:30 or 1:40). |
| Not enough cells to perform the assay | Make sure the confluency in a T-75 flask is around 50-90%. Passage more flasks. |
| Cytotoxic effects of sera, i. e. due to incomplete complement-inactivation | Perform a cell viability assay in addition to the mediator release assay. Increase Ag8 concentration to avoid incomplete complement-inactivation. |
| Low signal | Improve signal-to-noise ratio of the assay by diluting the 1x Tyrode´s buffer in deuterium oxide (D2O) instead of dH2O, or by using a sera with higher levels of specific IgE for the allergen of interest. |
| Allergen is not stable in Tyrode’s buffer (e. g. precipitation) | Make stability tests in 1x Tyrode’s buffer prior to the assay procedure. Substitution of Tyrode’s buffer is not recommended. |
| Problems finding the right starting concentration for the respective allergen | Adaption of dilution series to cover the full release curve (more dilution steps, 1:20 dilution instead of 1:10). |
| Poor assay performance indicated by low/no signal | Avoid cytotoxic effects from either sera or antigen stimulation (e.g. enzymatic allergens). Wash and soak the cells carefully. Avoid exposure to air for too long and prevent cells from drying out. |
| How do I know if the bottom signal plateau is reached? | Add "no antigen" controls to your plate. These are sensitized cell, which were only stimulated with 1x Tyrode´s buffer but without an allergen. |
| Do I need a positive control in addition to the maximum lysis wells? | As additional positive control a serum and antigen combination known to cause degranulation can be used or an anti-FcεR1 antibody. |
| How many wells do I need? | That depends on your titration series, the number of antigens and sera you want to analyze. Plan the layout for the 96-well plates according to how many sera/antigens you are going to test. Do not forget to add the "no antigen controls", the background cells (non-sensitized, non-stimulated) as well as the maximum lysis wells. |
| How many sera should I test? And do I need replicates? | Although the assay is quite robust, there is some assay-to-assay variability due to altered cell responsiveness. Therefore, it is recommended to rather rely on biological replicates (using different sera) than on technical replicates. A minimum of eight different sera is sufficient to analyze allergens. However, as shown in Fig. 4B, significant results can already be obtained using less sera. |
Table 1: Troubleshooting.
Discussion
The herein described huRBL cell-based mediator release assay is a robust method that can easily be performed and implemented in any laboratory. The only requirement is that cells need to be cultivated under sterile conditions. The assay is used to assess the likelihood of an allergen or allergenic source to evoke patients' IgE-crosslinking and basophil degranulation17. The assay can easily be adapted to any allergen or allergenic source as long as the patient´s serum with a high level of specific IgE, recognizing the allergen of interest, is available. It is recommended to perform a cell viability assay in addition to the mediator release assay in order to account for any potential cytotoxic effects that might result in poor assay performance. This might be due to incomplete complement-inactivation of the sera or other serum-derived cytotoxic effects. Even the antigen itself, for instance, because of proteolytic/enzymatic activity, can harm the huRBL cells. We are usually using a cell viability assay with MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide) to evaluate potential cytotoxic effects. The assay can easily be performed with the huRBL cells left after the cell supernatant was collected and transferred (see step 6.3. of the protocol). Compared to other immunochemical methods, such as ELISAs and western blotting, for determining the allergenic potential of either individual allergens or complex extracts based on allergen-IgE binding, this assay can detect not only the binding of IgE to an allergen but can also measure the functionality of both, human IgE and the allergen, to provoke IgE-mediated basophil degranulation18. Thus, it can aid in studying the severity of allergic symptoms ex vivo using patients' sera. The assay is reported to be more consistent and efficient than the classical passive cutaneous anaphylaxis tests since the assay utilizes the RBL-2H3 cells, which are relatively easy to handle and produce less variability in results compared to primary cells, such as mast cells or human basophils19,20. In addition to this, the assay provides a good representation of the biological activity of allergens and can accurately estimate the total allergen content in a given complex sample3. For troubleshooting certain steps in the protocol please refer to Table 1.
Regarding the applicability of this version of the mediator release assay, it has mostly been used for research purposes but also for the standardization of allergenic extracts based on their biological activity. This includes the analysis of different batches of SPT solutions, provocation test solution, as well as extracts used for allergen-specific immunotherapy; as shown for pollen, cat dander, house dust mite, and peanut extracts, as well as bee venom3,17,21. The technique can be applied especially in diagnosing food allergies, as it can detect even minimal amounts of allergenic constituents in complex food products such as peanuts, milk, wheat, and eggs22. In this respect, is has also been reported as a valuable tool for the assessment of allergenicity of animal food allergens, such as tropomyosins, and can aid in distinguishing potent allergens from non-allergens23. As a research tool, the assay is used to study the impact of food processing as well as to evaluate the influence of ligand binding to allergens and its effect on allergenicity24,25. For instance, the binding of Bet v 1 to ligands was shown not to affect the allergen-IgE cross-linking, although it caused an increase in its thermal and proteolytic stability25. The assay can be used to compare patient's reactivity to minor and major allergens, as well as to investigate the cross-reactivity of allergen homologues and isoforms, as shown in our example using Bet v 1 and the homologous food allergen Cor a 1 (Figure 3). Regarding allergen isoforms, the mediator release assay was used to identify the major allergen Amb a 1.01 as the most potent IgE-reactive isoform in ragweed pollen (Ambrosia artemisiifolia). In comparison, the other two identified isoforms in ragweed pollen extracts, Amb a 1.02 and Amb a 1.03, showed reduced reactivity to patients' IgE26.
In recent years, the assay has been applied to study potential anti-allergic compounds and novel hypoallergenic variants of allergens, aiding in the identification of suitable candidates for allergen-specific immunotherapy27,28. Another novel approach is to use the assay to monitor treatment efficacy in the course of allergen-specific immunotherapy. In this regard, our research group developed a huRBL assay inhibition system, which correlated well with the reduction of the patient's symptom score during allergen-specific immunotherapy29. The assay has also been proposed to study the immunosuppressive effects of TGFβ1 on allergen-induced IgE-mediated degranulation30.
The limitations of the assay are that even though the huRBL cells possess some features of mast cells or basophils, they do not completely mimic the natural function of these effector cells. For example, mast cells widely express the pattern recognition receptor Toll-like receptor 4 (TLR4), necessary for pathogen recognition, whereas it is completely deficient in the RBL-2H3 cells31. Due to this difference in functionality, the assay does not fully mimic the real-life situation, which needs to be kept in mind when interpreting the data. In addition, since the huRBL cells are cancerous basophilic cells, changes in culture conditions and prolonged culturing can lead to phenotypic differences leading to altered results among different laboratories20. Another aspect is the choice of allergen concentration that has to be taken into account when adapting this method since high allergen concentrations might result in non-IgE mediated degranulation due to the presence of high amounts of proteases or endotoxins18. Other limitations are the dependency on human sera with relatively high specific IgE levels (RAST class 5-6), and the need for cell culture systems, which remains an obstacle that needs to be overcome in order to implement the technique in the daily clinical routine.
Apart from these limitations, the huRBL assay represents a valuable research tool for the diagnosis and treatment of allergic diseases and can be used in a wide range of applications.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Prof. Dr. Stefan Vieths of the Department of Molecular Allergology, Paul-Ehrlich-Institut, Langen, Germany, for providing the humanized/FcεRI-transfected RBL cells and for giving his consent to write this research methodology paper. We want to thank Prof. Dr. Fatima Ferreira for providing excellent feedback. We further would like to thank Prof. Dr. Ronald van Ree and Dr. Jaap Akkerdaas of the Department of Experimental Immunology, Amsterdam University Medical Centers, location AMC, Amsterdam, The Netherlands, for giving their consent to publish representative data provided in this methods paper, which were generated in the course of the project BM4SIT – innovations for allergy (www.BM4SIT.eu). The work of the authors has been supported by the Austrian Science Fund (Project P32189), by the University of Salzburg priority program Allergy-Cancer-BioNano Research Centre, by the doctoral program Immunity in Cancer and Allergy-ICA funded by the Austrian Science Fund (FWF W01213), and by the BM4SIT project (grant number 601763) from the European Union's Seventh Framework Program FP7.
Materials
| 4-Methylumbelliferyl N-acetyl-β-D-glucosaminide | Sigma | M2133 | |
| 96-well plate for huRBL cells (Nunc MicroWell 96-Well, Nunclon Delta-treated, flat-bottom microplate) | ThermoFisher Scientific | 167008 | |
| 96-well plate for substrate solution and cell supernatant (Greiner Bio-One non-treated 96-well microplates) | Fisher Scientific | 655101 | |
| Bovine serum albumin (BSA) | Sigma | 10735078001 | |
| Citric acid | Applichem | 131018 | |
| Dulbecco's phosphate-buffered saline (DPBS without calcium and magnesium) | Sigma | D8537 | |
| G418 | Sigma | A1720 | |
| Glycine | Applichem | A3707 | |
| Heat-inactivated fetal calf/bovine serum (FCSi) | Sigma | F0804 | |
| L-Glutamine (200 mM) | Sigma | G7513 | |
| Minimum Essential Medium Eagle with Alpha Modification, with ribonucleosides, deoxyribonucleosides and sodium bicarbonate, without L-glutamine, liquid, sterile-filtered, suitable for cell culture | Sigma | M8042 | |
| Opti-MEM reduced serum medium, GlutaMAX supplement | Gibco/ThermoFisher Scientific | 51985034 | |
| Penicillin-Streptomycin (10K units Pen. 10 mg/mL Strep.) | Sigma | P4333 | |
| Sodium chloride (NaCl) | Applichem | A2942 | |
| Sodium hydrogen carbonate (NaHCO3) | Applichem | 131638 | |
| Triton X-100 | Sigma | X100 | |
| Trypsin-EDTA | Sigma | 59418C | |
| Tyrode’s salt | Sigma | T2145 |
Riferimenti
- Curin, M., et al. Next-generation of allergen-specific immunotherapies: molecular approaches. Current Allergy and Asthma Reports. 18 (7), 39 (2018).
- Okamoto-Uchida, Y., et al. Different results of IgE binding- and crosslinking-Based allergy tests caused by allergen immobilization. Biological and Pharmaceutical Bulletin. 39 (10), 1662-1666 (2016).
- Vogel, L., Lüttkopf, D., Hatahet, L., Haustein, D., Vieths, S. Development of a functional in vitro assay as a novel tool for the standardization of allergen extracts in the human system. Allergy. 60 (8), 1021-1028 (2005).
- Ocmant, A., et al. Basophil activation tests for the diagnosis of food allergy in children. Clinical and Experimental Allergy. 39 (8), 1234-1245 (2009).
- Platts-Mills, T. A. The role of immunoglobulin E in allergy and asthma. American Journal of Respiratory and Critical Care Medicine. 164 (8), 1-5 (2001).
- Galli, S. J., Tsai, M. IgE and mast cells in allergic disease. Nature Medicine. 18 (5), 693-704 (2012).
- Lawrence, M. G., et al. Half-life of IgE in serum and skin: Consequences for anti-IgE therapy in patients with allergic disease. The Journal of Allergy and Clinical Immunology. 139 (2), 422-428 (2017).
- Draber, P., Halova, I., Polakovicova, I., Kawakami, T. Signal transduction and chemotaxis in mast cells. European Journal of Pharmacology. 778, 11-23 (2016).
- Peebles, R. S. Prostaglandins in asthma and allergic diseases. Pharmacology & Therapeutics. 193, 1-19 (2019).
- Cyphert, J. M., et al. Allergic inflammation induces a persistent mechanistic switch in thromboxane-mediated airway constriction in the mouse. American Journal of Physiology. Lung Cellular and Molecular Physiology. 302 (1), 140-151 (2012).
- Méndez-Enríquez, E., Hallgren, J. Mast cells and their progenitors in allergic asthma. Frontiers Immunology. 10, 821 (2019).
- Demoly, P., Lebel, B., Arnoux, B. Allergen-induced mediator release tests. Allergy. 58 (7), 553-558 (2003).
- Yamaga, S., et al. Decreased intracellular histamine concentration and basophil activation in anaphylaxis. Allergology International. 69 (1), 78-83 (2020).
- Huang, L., et al. A rapid and sensitive assay based on particle analysis for cell degranulation detection in basophils and mast cells. Pharmacological Research. 111, 374-383 (2016).
- González-Pérez, R., Poza-Guedes, P., Barrios Del Pino, Y., Matheu, V., Sánchez-Machín, I. Evaluation of major mite allergens from European standardized commercial extracts for in vivo diagnosis: addressing the need for precision medicine. Clinical and Translational Allergy. 9, 14 (2019).
- Focke, M., Marth, K., Valenta, R. Molecular composition and biological activity of commercial birch pollen allergen extracts. European Journal of Clinical Investigation. 39 (5), 429-436 (2009).
- Hoffmann, A., Vieths, S., Haustein, D. Biologic allergen assay for in vivo test allergens with an in vitro model of the murine type I reaction. The Journal of Allergy and Clinical Immunology. 99 (2), 227-232 (1997).
- Sun, N., Zhou, C., Zhou, X., Sun, L., Che, H. Use of a rat basophil leukemia (RBL) cell-based immunological assay for allergen identification, clinical diagnosis of allergy, and identification of anti-allergy agents for use in immunotherapy. Journal of Immunotoxicology. 12 (2), 199-205 (2015).
- Kaul, S., Hoffmann, A. Mediator release assay of rat basophil leukemia cells as alternative for passive cutaneous anaphylaxis testing (PCA) in laboratory animals. Altex. 18 (1), 55-58 (2001).
- Passante, E., Frankish, N. The RBL-2H3 cell line: its provenance and suitability as a model for the mast cell. Inflammation Research. 58 (11), 737-745 (2009).
- Kaul, S., et al. Mediator release assays based on human or murine immunoglobulin E in allergen standardization. Clinical and Experimental Allergy. 37 (1), 141-150 (2007).
- Huang, J., et al. Application of in vitro and in vivo models in the study of food allergy. Food Science and Human Wellness. 7 (4), 235-243 (2018).
- Klueber, J., et al. Homologous tropomyosins from vertebrate and invertebrate: Recombinant calibrator proteins in functional biological assays for tropomyosin allergenicity assessment of novel animal foods. Clinical and Experimental Allergy. 50 (1), 105-116 (2020).
- Zhang, T., et al. Different thermal processing effects on peanut allergenicity. Journal of the Science of Food and Agriculture. 99 (5), 2321-2328 (2019).
- Soh, W. T., et al. Multiple roles of Bet v 1 ligands in allergen stabilization and modulation of endosomal protease activity. Allergy. 74 (12), 2382-2393 (2019).
- Wolf, M., et al. Amb a 1 isoforms: Unequal siblings with distinct immunological features. Allergy. 72 (12), 1874-1882 (2017).
- Eichhorn, S., et al. Rational design, structure-activity relationship, and immunogenicity of hypoallergenic Pru p 3 variants. Molecular Nutrition & Food Research. 63 (18), 1900336 (2019).
- Abd Rani, N. Z., et al. Mechanistic studies of the antiallergic activity of phyllanthus amarus Schum. and Thonn. and its compounds. Molecules. 26 (3), (2021).
- Huber, S., et al. Does clinical outcome of birch pollen immunotherapy relate to induction of blocking antibodies preventing IgE from allergen binding? A pilot study monitoring responses during first year of AIT. Clinical and Translational Allergy. 8 (1), 39 (2018).
- Araujo, G. R., et al. TGFβ1 mimetic peptide modulates immune response to grass pollen allergens in mice. Allergy. 75 (4), 882-891 (2020).
- Passante, E., Frankish, N. Deficiencies in elements involved in TLR4-receptor signalling in RBL-2H3 cells. Inflammation Research. 59, 185-186 (2010).

