Quantitative MRI of Endothelial Permeability and (Dys)function in Atherosclerosis
Summary
We have developed an accurate, non-invasive, and easy-to-use method to quantify endothelial permeability and dysfunction in the arteries using Magnetic Resonance Imaging (MRI), named qMETRIC. This technique enables assessing vascular damage and cardiovascular risk associated with atherosclerosis in preclinical models and humans.
Abstract
Cardiovascular diseases are the leading causes of death worldwide. A permeable/leaky and dysfunctional endothelium is considered the earliest marker of vascular damage and thought to drive atherosclerosis. A method to identify these changes in vivo would be desirable in the clinic. Magnetic resonance imaging (MRI)-based tools and other technologies have enabled a profound understanding of the role of the endothelium in cardiovascular diseases and risk in vivo. There is, however, a need for reproducible and simple approaches for extracting quantifiable data reflective of endothelial damage from a single imaging study. A non-invasive, easy-to-implement, and quantitative MRI workflow was developed to acquire and analyze images that allow the quantification of two imaging biomarkers of arterial endothelial damage (leakiness/permeability and dysfunction). Here, the protocol describes the application of this method in the brachiocephalic artery of atherosclerotic ApoE-/- mice using a clinical MRI scanner. First, late gadolinium enhancement (LGE) and Modified Look-Locker Inversion Recovery (MOLLI) T1 mapping protocols to quantify endothelial leakage using an albumin-binding probe are described. Second, anatomic, and quantitative blood flow sequences to measure endothelial dysfunction, in response to acetylcholine are described. Importantly, the method outlined here allows the acquisition of high-spatial-resolution 3D images with large volumetric coverage enabling accurate segmentation of vessel wall structures to improve inter- and intra-observer variability and to increase reliability and reproducibility. Additionally, it provides quantitative data without the need for high-temporal resolution for complex kinetic modeling, making it model-independent and even allowing for imaging of highly mobile vessels (coronary arteries). Therefore, the approach simplifies and expedites data analysis. Finally, this method can be implemented on different scanners, can be extended to image different arterial beds, and is clinically applicable for use in humans. This method could be used to diagnose and treat patients with atherosclerosis by adopting a precision-medicine approach.
Introduction
Cardiovascular diseases (CVDs) remain the leading cause of mortality and morbidity worldwide, accounting for nearly one-third of deaths1, and the cause of lifelong disabilities that exert a high financial cost on the healthcare systems1. Among CVDs, ischaemic heart disease and stroke are primarily caused by atherosclerotic plaques. Atherosclerosis is a multifactorial disease; however, a common hallmark is early damage of the vascular endothelial cells that lead to the formation, progression, and eventual complications of atherosclerosis. An intact vascular endothelium has fundamental vasculo-protective properties2. The endothelium regulates vascular permeability by controlling translocation of cells and molecules between the systemic circulation and the vessel wall; controls vascular tone by balancing the production of vasodilators (e.g., nitric oxide, prostacyclin) and vasoconstrictors (e.g., endothelin-1, angiotensin II); and also has anti-coagulant properties. However, both the function and permeability of the endothelial cells can deteriorate in the presence of cardiovascular risk factors (e.g., smoking, high cholesterol, diabetes, systemic inflammation, oxidative stress) and by blood flow hemodynamic patterns. A dysfunctional endothelium has reduced vasodilation in response to stressors, consequently increasing arterial stiffness. In addition, a permeable/leaky endothelium has widened tight gap junctions between adjacent cells3,4,5,6,7. Such change occurs both on the luminal endothelium and newly-formed plaque microvessels that appear fragile, leaky, and dysmorphic8. Permeable endothelial cells act as entry points for plasma-borne molecules and cells-exacerbating the risk of cardiovascular disease.
Building on this knowledge, in the past 15 years, endothelial permeability and function has emerged as a promising imaging and therapeutic target to better diagnose subjects at risk for cardiovascular disease and to assess the effects of known or novel drugs. However, direct and quantitative imaging of endothelium function is limited9,10,11,12. Currently, much of the interpretation of endothelial function in vivo is based on studies of endothelial-dependent dilation (FMD) in peripheral vessels whose function modestly correlates with atherosclerosis burden in vascular beds that cause clinical events13,14,15. Only a limited number of imaging studies have shown a direct link between endothelial dysfunction and atherosclerosis burden in vivo9,10,11,12. Conversely, more accessible MRI-based approaches have enabled imaging endothelial permeability more widely. Using the percent vessel wall signal enhancement after administration of MRI gadolinium agents has provided a semi-quantitative measurement of endothelial permeability16,17. Later, the development of dynamic contrast-enhanced (DCE) protocols has permitted an improved and more quantitative measurement of vascular endothelial permeability. Quantitative parameters such as the contrast extravasation rate (Ktrans) and microvascular volume (Vρ) derived from kinetic modeling or the area under the curve (AUC), upslope, time to peak, and peak concentration extracted from non-modeled methods correlated not only with endothelial permeability but also plaque vascularity18,19,20. However, the application of vascular DCE remains challenging despite significant technical advances because: (i) it requires both high spatial (0.5-0.7 mm2) and temporal resolution21 for accurate delineation of the vessel wall. Sampling the concentration of contrast agent in the blood to calculate the arterial input function also requires kinetic modeling, which leads to a trade-off of either limiting anatomical coverage22,23 to gain temporal resolution or vice versa24,25; (ii) data analysis may require complex pharmacokinetic modeling (e.g., Patlak vs. Tofts); (iii) provides limited image quality, poor scan-rescan reproducibility, and average inter-observer and intra-observer variability26,27. Therefore, there is still a need for reproducible and simple approaches for extracting direct and quantifiable data of endothelial permeability and (dys)function from single imaging studies that could have better clinical utility.
Here, we have developed a non-invasive, easy-to-implement, and quantitative MRI to acquire and analyze images that allows direct quantification of two markers of arterial endothelial damage (leakiness/permeability and dysfunction) using preclinical models of atherosclerosis in a single scan. The method is named Quantitative MRI of EndoThelial peRmeabIlity and dysfunCtion (qMETRIC). It involves the acquisition of late gadolinium enhancement (LGE) and Modified Look-Locker Inversion Recovery (MOLLI) T1 mapping protocols to quantify endothelial leakage, after administration of an intravascular albumin-binding probe; and acquisition of anatomic and quantitative blood flow sequences to measure endothelial dysfunction, in response to an acetylcholine bolus. We have demonstrated that qMETRIC accurately detects: the severity of atherosclerosis and the risk of complications; treatment responses; and can be adapted for use in patients5,6,7. Importantly, the method outlined here allows the acquisition of high-spatial-resolution images to enable accurate segmentation of the vessel wall to minimize inter/intra-observer bias and to increase reliability and reproducibility with large anatomical coverage. Finally, this method can be adapted for use on different scanners and can be extended to image different arterial beds (even coronary arteries28). The straightforward workflow makes this approach more accessible to the cardiovascular imaging community.
Protocol
All components of this study were carried out in accordance with the UK Animals (Scientific Procedures) Act, 1986, and with the approval of King's College London Ethical Review Panel.
The experimental workflow is summarized in Figure 1.
1. Animal preparation
- Induce atherosclerosis by feeding ApoE-/- mice a high-fat diet containing 21% fat from lard and 0.15% (wt/wt) cholesterol on average for up to 12 weeks.
- Load a 29 G needle insulin syringe with the right volume of the contrast agent (gadofosveset trisordium) to achieve a dose of 0.03 mmol/kg. Keep the injection volume between 50-150 µL.
- Place the cage on a heating pad set to 37 °C to pre-heat the animal and maintain the body temperature.
- Induce anesthesia by placing the mouse in an induction box lined with absorbent tissues. Adjust the flowmeter to 3%-5% of isoflurane at 1 L/min of O2 for about 3-5 min.
NOTE: Ensure correct anesthesia depth by identifying the slowing breathing rate, which should decrease to less than 70 breaths per minute (bpm). - Confirm anesthesia using the toe pinch method (i.e., loss of withdrawal reflex to toe pinch). Transfer the animal to a holder and insert its nose into a nose cone. Place the holder on a heating pad to maintain the animals' body temperature.
- Maintain anesthesia, delivered through the nose, by setting the anesthesia airflow in the holder to 1%-2% isoflurane at 1 L/min of O2.
- Apply vet ointment on the animal's eyes to prevent dryness while under anesthesia.
- Place the animal either prone or on its side and clean the tail with an alcohol swab. Locate one of the two tail veins. If necessary, warm up the tail with a UV lamp to make the tail veins more visible.
- Insert the 29 G insulin needle parallel to the vein with the bevel of the needle facing up. Gently inject the volume of the prefilled syringe containing gadofosveset trisodium. Ensure that there is no bleeding at the injection site after withdrawing the needle.
- Wait for 30 s for gadofosveset to circulate, and then transfer the mouse to the MRI bed.
2. Preparation of the MRI scanner (see Figure 1)
- Cover the MRI table with absorbent tissues.
- Place the MRI single-loop receiver coil on the MRI bed. Use a platform to raise the receiver coil and avoid direct contact between the receiver coil and the MRI table.
- Secure the coil to the platform using surgical tape.
- Place and secure the tubing connected to a circulating heating pump around the coil and set it to 37 °C to maintain the animal's body temperature during imaging.
- Place the anesthesia delivery tubing into the bore of the MRI scanner and tape it so that the nose cone reaches the tip of the receiver coil where the animal's head will be placed.
- Turn on the in-bore camera to monitor the animal from the console room.
- In the MRI console room, use the software interface to start a new study for the animal (patient).
3. Animal positioning in the MRI scanner and monitoring (see Figure 2)
- Transfer the anesthetized animal to the scanner room. Place the mouse in the prone position on the receiver coil and ensure that its snout fits into the nose cone to maintain anesthesia. Turn the anesthesia airflow to 1%-1.5% isoflurane at 1 L/min of O2.
- Ensure to place the animal on the MRI coil with its heart and neck regions located at the center of the receiver coil.
- Secure the nose of the mouse into the nose cone, the abdomen, and the tail of the mouse on the platform with tape.
- Place four electrodes on the anterior and the rear paws, making sure that the palm of the toes is completely open to record the electrocardiogram (ECG). Use ECG conductive gel on the mouse's paws before attaching the ECG pads to improve conductivity.
- Ensure to use tape to firmly attach the electrodes to the platform.
- Align the laser of the scanner's bed with the base (proximal end) of the heart; use the clavicle and anterior paw line as a landmark. Position the animal in the magnet's isocenter using an automatic MR table.
4. MRI image planning and acquisition
- Start a scout scan to run the standard calibrations for the MRI system.
- Set the monitoring equipment to detect the R-wave of the ECG. Adjust the thresholds for each mouse and within imaging sessions so that there is reliable triggering.
NOTE: The mouse heart frequency under deep anesthesia usually ranges between 400-600 beats per minute (bpm). - Acquire a 3D gradient echo scan (GRE) to get multiplanar pilot images (scout images) to plan the rest of the scans (see Table 1 for the MRI acquisition parameters and Figure 3 for planning).
- Identify the heart on the scout images, particularly on the coronal view, most easily by its flow artifacts.
NOTE: If the images show the mouse is not well centered over the coil or the isocenter, retract the bed and repeat positioning. - Plan a 3D contrast-enhanced MR angiography (MRA) scan (see Table 1 for scan for the MRI acquisition parameters and Figure 3 for planning) in a transverse plane extending from the base of the heart toward the neck and carotid arteries with an 8 mm field-of-view (FOV).
- Use the maximum intensity projection (MIP) images to visualize the aortic arch, brachiocephalic and carotid arteries and plan the subsequent late gadolinium enhancement (LGE), T1 mapping, and cine scans (see Figure 3 for representative images).
NOTE: If the level of the imaging volume is not correct, repeat the acquisition by moving the slices either proximally or distally. - MRI image acquisition to measure endothelial permeability.
- Use the MIP and transverse MRA images acquired before to plan a single slice 2D-Look-Locker (LL) acquisition perpendicular to the ascending aorta or carotid arteries (see Table 1 for scan for the MRI acquisition parameters and Figure 3 for representative images).
- Set the heart rate to 60 bpm when using a simulated ECG signal or set a blanking period to ensure that the inversion recovery pulse between subsequent inversion recovery pulses is 1000 ms when using the recorded ECG signal.
- Use the Look-Locker images to determine the optimal inversion time (TI) for blood signal nulling required for the LGE scan.
- LGE imaging: After 20-30 min of injection of gadofosveset, and immediately after the LL scan (described in steps 4.7.1-4.7.3) acquire an LGE scan using an inversion-recovery 3D fast gradient echo sequence (see Table 1 for the MRI acquisition parameters and Figure 3 for representative images).
- Plan a transverse 3D fast gradient echo LGE scan to cover the base of the heart (to include part of the aortic root), the brachiocephalic artery (between the aortic root to the subclavian bifurcation), and part of the carotid arteries with an 8 mm field-of-view (FOV) in the foot-head direction using the same geometry as for the MRA above (see Figure 3 for representative images).
- Set the heart rate to 60 bpm, when using a simulated ECG signal, or set a blanking period to ensure that successive inversion recovery pulses occur at every 1000 ms for the LGE scan when using the recorded ECG signal (as for step 4.7.2 above).
NOTE: This is important for consistent and heart rate independent recovery of the magnetization between successive inversion recovery pulses. - Insert the T1 obtained from the Look-Locker into the LGE sequence under Contrast > Inversion Delay.
- T1 mapping imaging: Use a 3D fast gradient echo acquisition to acquire transverse T1 mapping images 45 min after injection of gadofosveset. Plan the sequence in the same orientation and geometry as the LGE scan above (see Table 1 for the MRI acquisition parameters and Figure 3 for representative images).
- Set the heart rate to 120 bpm, when using a simulated ECG, or set a blanking period to ensure that the inversion recovery pulse between the two imaging trains occurs at every 500 ms when using the recorded ECG trace.
NOTE: The T1 mapping sequence uses two non-selective inversion pulses with inversion times between 20-2000 ms, followed by eight segmented readouts for eight individual images. The combination of the two imaging trails results in a total of sixteen images per slice with varying inversion times. The images are automatically reconstructed on the scanner using a three-parameter fit model. The equations used to generate the T1 parametric maps are:
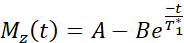
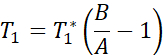
- MRI image acquisition to measure endothelial function
- Prepare a solution of diluted acetylcholine in saline. Load a 29 G needle insulin syringe with the right volume of solution to achieve (16.6 mg/kg). Keep the injection volume between 50-150 µL.
- Using the transverse MRA and corresponding MIP images, place a transverse slice across the brachiocephalic artery, between the aortic root and the subclavian bifurcation (Figure 3 for representative images).
- Use a transverse 2D gradient echo (GRE) with retrospective ECG gating to acquire temporally-resolved cine images of the brachiocephalic artery (see Table 1 for the MRI acquisition parameters Figure 3 for representative images).
- Adjust the number of maximum cardiac phases to the heart rate of each animal.
NOTE: Typically, 14 cardiac phases provide sufficient temporal resolution. - After acquiring the baseline images, enter into the MRI scanner room. While the mouse is anesthetized in the scanner, gently inject acetylcholine intraperitoneally (IP). Avoid moving the mouse on the coil.
- Wait for 6-10 min for the heart rate to stabilize and repeat the acquisition.
- At the end of the imaging procedure, return the mouse to its cage and place the cage on a heating pad for recovery.
NOTE: Mice are recovered when they regain sufficient consciousness to maintain sternal recumbency. - Export the acquired images in a digital imaging and communications in medicine (DICOM) format and use an open-platform image analysis software.
5. MRI segmentation and data analysis (see Figure 4)
- Drag and drop the Dicom files into the database of an open-platform software to load all the images.
- Use the LGE images to visualize contrast uptake in the vessel wall and calculate the area of enhancement as a surrogate marker of endothelial cell leakage.
- Select both the MRA and inversion recovery scans. Press Enter to load these images side-by-side. Click on the small icon next to the scan name and drag and drop the MRA images onto the LGE images.
- Select the option Re-sample to re-slice the MRA images using the LGE images as a reference to account for differences in slice thickness.
- Click on the small icon next to the scan name. Drag and drop the LGE images onto the MRA images (as in step 5.4 above). From the menu, choose Image Fusion to overlay the LGE and MRA images.
- From the toolbar, click on 2D Viewer, and then choose 3D Position Panel. Use the buttons to manually correct for in-plane shifts to account for potential small displacements because of animal respiration.
- Use the Closed Polygon tool located in the toolbar to manually segment the visually enhanced segment of the vessel wall. Use the co-registered MRA and LGE images to guide the segmentation.
- Segment all the LGE images that encompass the brachiocephalic artery.
NOTE: If the enhancement of the vessel wall has a diffused or patchy appearance, segment those individually in each slice. - Click on the Plugins button in the toolbar and choose ROI Tools, and then Export ROIs to export the segmented area (mm2) for each region of interest (ROI) in a spreadsheet.
- Sum the area of each slice to calculate the total area of enhancement in the brachiocephalic artery in the spreadsheet.
NOTE: The total area of enhancement can be used as a quantitative marker of endothelial permeability. - Use the T1 maps that are automatically generated on the MRI scanner computer to calculate the mean T1 value of the vessel wall that reflects the amount of uptake of gadofosveset into the vessel wall-this is another quantitative marker of endothelial permeability.
- Load the MRA and T1 map images and follow a similar approach as described above (steps 5.3-5.9) to segment the vessel wall and extract the T1 values (ms).
- In a spreadsheet, invert the T1 values and multiply by 1000 to calculate the relaxation time R1 = 1/T1 in seconds. Calculate the mean R1 for all slices covering the brachiocephalic artery in each animal.
- Load the phase contrast angiography images and velocity maps to calculate the changes in the area of the vessel and blood flow velocity, respectively, during the cardiac cycle.
- Segment both the images acquired before and after injection of acetylcholine to calculate endothelial-dependent vasoreactivity, a surrogate marker of endothelial (dys)function.
- Use the semi-automated Grow Region tool available in the ROI tab or use the Closed Polygon option available in the toolbar (as described in step 5.7) to segment the lumen area (mm2) of the brachiocephalic artery in the angiography images.
NOTE: The semi-automated tool uses pixel thresholding to cluster pixels encompassing the blood pool based on their signal intensity. - Use the Close Polygon tool to segment the corresponding blood flow velocity encoded maps to calculate the blood flow velocity (cm/s).
- Export the lumen area (mm2) and blood flow velocity (cm/s) in a spreadsheet (as described in step 5.9) and identify those that correspond to the end-diastolic (maximum area) and end-systolic (minimum area) cardiac phases.
- Use the tabulated spreadsheet to calculate the endothelium-dependent vasodilation (calculate the percentage change in the end-diastolic (ED) lumen area and blood flow velocity pre- and post-injection of acetylcholine). Use the following formulas:
area change=
flow change=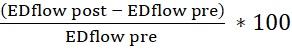
- For each animal, tabulate the corresponding data derived from the LGE images, T1 maps, and the acetylcholine test in statistical software for analysis.
Representative Results
In this report, the application of a Quantitative MRI method is demonstarted to measure EndoThelial peRmeabIlity and (dys)funCtion (qMETRIC) in the brachiocephalic artery of atherosclerotic ApoE-/- mice. This method provides direct and quantifiable data of two markers of endothelial damage – permeability and (dys)function, which can be extracted from in vivo vessel wall scans acquired within a single imaging session. First, LGE are used to measure the area of vessel wall enhancement (mm3), and T1 (or R1) maps are used to quantify the relaxation rate of the vessel wall (s-1) after administration of gadofosveset, both surrogate markers of permeability (see Figure 5 for representative results). The vessel wall R1 relaxation rate ranged from 2.42 s-1 ± 0.35 s-1 to 3.45 s-1 ± 0.54 s-1 to 3.83 s-1 ± 0.52 s-1 at 4 weeks, 8 weeks, and 12 weeks of a high-fat diet, respectively. Conversely, wild-type (R1 = 2.15 ± 0.34 s-1) and statin-treated ApoE-/- (R1 = 3.0 ± 0.65 s-1) mice showed less enhancement. In ApoE-/- mice fed with a high-fat diet for up to 12 months, the study shows with histological analysis, Evans Blue dye, and electron microscopy that endothelial permeability increases during atherosclerosis progression, which was in agreement with increased LGE vessel wall volume, increased change in vessel wall R1 relaxivity, and paradoxical vasoconstriction after acetylcholine injection5. Conversely, statin and other endothelium-targeting treatments decreased endothelial permeability and plaque size, which was reflected in smaller LGE volume, lower R1 values5,7, and improved vasodilation. Mechanistically, gadofosveset binds reversibly to serum albumin. This results in a 5-6-fold increase in the T1 relaxivity of the probe29-making it detectable by MRI with high sensitivity. Here, the study shows that bound to albumin, the uptake of the probe reflects endothelial leakiness because it correlates with the uptake of Evan's blue dye-a gold-standard ex vivo method of quantifying endothelial leakage (Figure 5) – and wider tight gap junctions5. Secondly, a simple test is demonstrated to measure endothelial (dys)function, in response to acetylcholine. In control vessels, acetylcholine causes endothelium-depended vascular relaxation leading to increased arterial area/volume and blood flow. To measure endothelial (dys)function, ECG-triggered angiography images acquired before and after administration of acetylcholine were used. The study calculates the change in the end-diastolic area (or volume) of the vessel lumen before and after the administration of acetylcholine. It was found that, unlike normal vessels that vasodilate in response to acetylcholine, atherosclerotic vessels demonstrate decreased endothelial-dependent vasodilatory function that manifests either as a reduced change in vessel area (or volume) or even paradoxical vasoconstriction of the vessel (Figure 5). Interestingly, statin treatment improved vasodilatory properties of the endothelium13.
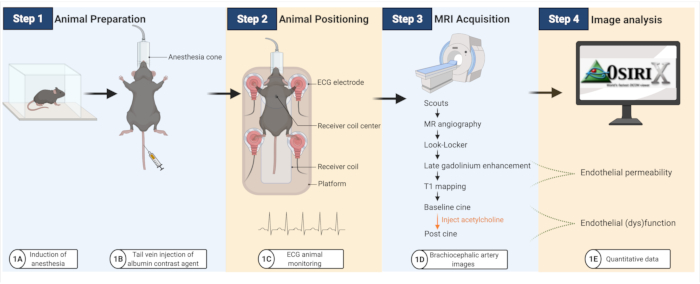
Figure 1: Workflow to image endothelial permeability and (dys)function in atherosclerotic mice. (A–B) Mice are first anesthetized and then injected with the albumin contrast agent. (C) Mice are then transferred onto an MRI coil, where ECG pads are used to monitor cardiac activity. (D–E) MRI images are acquired to quantify endothelial permeability and (dys)function that are subsequently analyzed using an open-platform software (created with BioRender.com). Please click here to view a larger version of this figure.
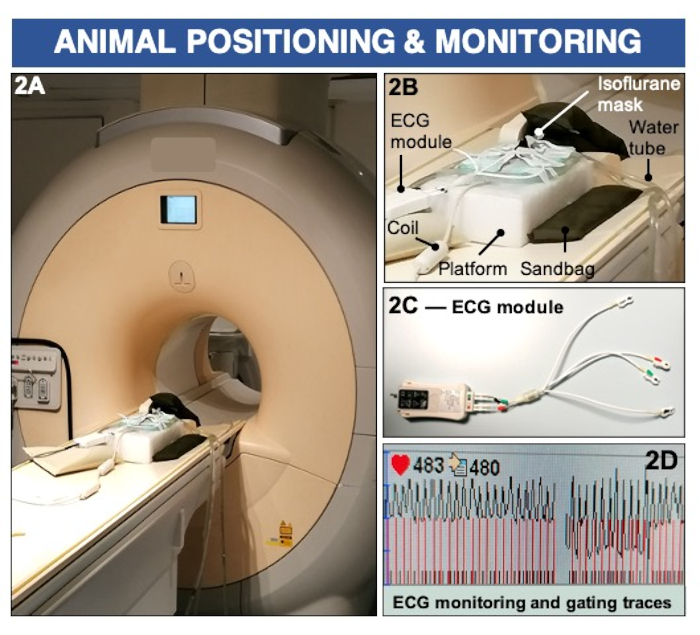
Figure 2: Animal positioning and ECG monitoring to image endothelial permeability and (dys)function using a clinical 3 Tesla MRI scanner. (A–B) The animal is positioned prone on a surface coil and maintained anesthetized using inhalable isoflurane. Sandbags are used to stabilize the imaging platform. (C–D) ECG pads are placed on the paws and connected to a clinical ECG module to record cardiac activity. Please click here to view a larger version of this figure.
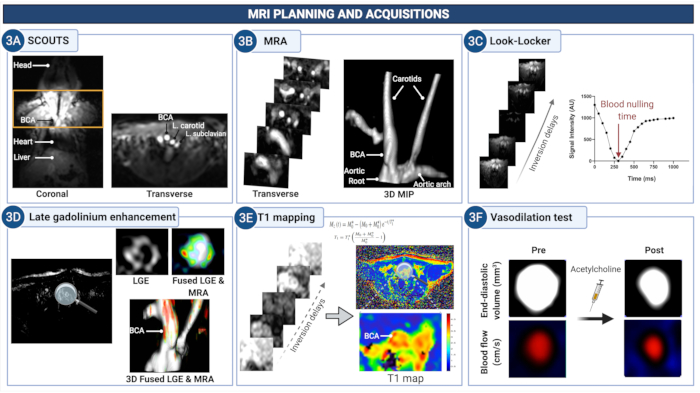
Figure 3: MRI planning and acquisition of images to quantify endothelial permeability and (dys)function in the brachiocephalic artery of atherosclerotic mice. (A) Scout images are acquired to identify the anatomical region between the aortic root and the carotid arteries. (B) The MR angiogram is used to visualize the vasculature and plan the subsequent scans. (C) Look-Locker images are acquired at the level of the brachiocephalic artery to determine the suitable time delay to null the signal from the blood in the subsequent later gadolinium enhancement images (LGE). (D) LGE images provide a visual assessment of vessel wall enhancement. (E) T1 mapping is used to calculate the vessel wall relaxation rate that is indicative of the concentration of gadolinium. (F) The endothelium-depended vasodilating properties of the vessel wall are quantified after the administration of acetylcholine. Please click here to view a larger version of this figure.
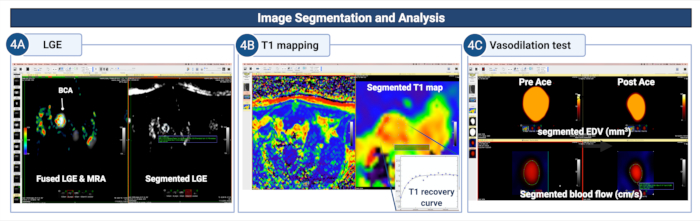
Figure 4: Image segmentation and analysis to quantify endothelial permeability and (dys)function in the brachiocephalic artery of atherosclerotic mice. (A) The vessel wall is manually segmented on the LGE images to quantify the area/volume of contrast uptake. (B) The vessel wall is segmented on the T1 mapping to calculate the vessel wall T1 relaxation rate. (C) The vessel wall segmented on the MR angiograms and blood flow encoded images is used to study the vasodilating properties of the vessel wall by calculating the changes in the changes in the end-
diastolic lumen area (or volume) and blood flow after administration of acetylcholine. Please click here to view a larger version of this figure.
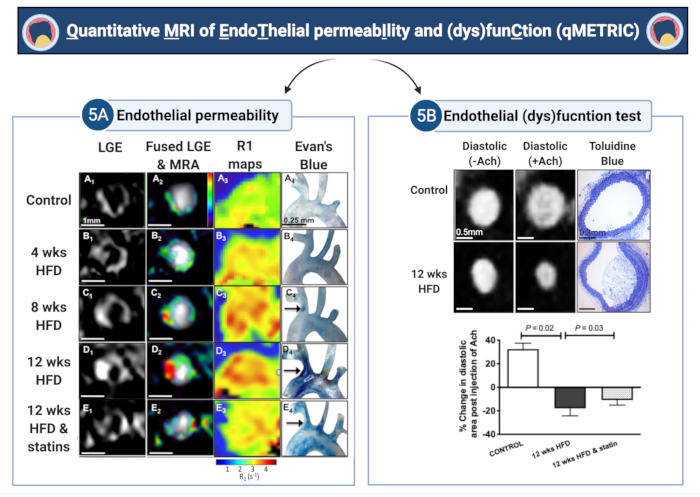
Figure 5: Quantitative imaging of endothelial permeability and (dys)function (qMETRIC) in atherosclerotic mice. (A) LGE images and R1 relaxation maps show increased uptake of the albumin-binding contrast agent within the vessel wall during atherosclerosis progression and the improvement after statin treatment. Imaging data are corroborated by the accumulation of Evan's blue dye, an albumin-binding dye, ex vivo. (B) Changes in the vasodilating properties of the vessel wall, in response to acetylcholine administration, allow quantification of endothelial-dependent vasodilation. Control vessels vasodilate, whereas atherosclerotic vessels vasoconstrict in response to acetylcholine, suggestive of endothelial damage. Treatment with statin improves endothelial damage. The terms "wks" and "HFD" in the figure represents "weeks" and "high-fat diet", respectively. This figure has been modified from Phinikaridou, A. et al.5. Please click here to view a larger version of this figure.
| Scan / Sequence | Acquisition parameters | ||
| Scout / pilot scan | 3D, fast gradient echo Transverse: FOV = 50 mm x 27 mm x 14 mm, matrix = 96 x 52, in-plane resolution = 0.5 mm x 0.5 mm, slice thickness = 0.5 mm, TR/TE = 15/6.1 ms, flip angle = 30°, averages = 1 Coronal: FOV = 200 mm x 102 mm x 14 mm, matrix = 336 x 173, in-plane resolution= 0.5 mm x 0.5 mm, slice thickness = 0.5 mm, TR/TE = 12/6 ms, flip angle = 30°, averages = 1 |
||
| MRA scan | 3D fast gradient echo, FOV = 30 mm x 30 mm x 8 mm, matrix = 200 x 200, in-plane resolution =0.15 mm x 0.15 mm, slice thickness = 0.5 mm, TR/TE = 15/6.1 ms, flip angle = 40°, averages = 1 | ||
| Look-Locker scan | 2D fast gradient echo, FOV = 30 mm x 30 mm, matrix = 80 x 80, in-plane resolution = 0.38 mm x 0.38 mm, slice thickness = 2 mm, TR/TE = 19/8.6 ms, TR between subsequent IR pulses = 1000 ms, and flip angle = 10°, averages = 1. | ||
| LGE scan | 3D fast gradient echo, FOV = 30 mm x 30 mm x 8 mm, matrix = 304 x 304, in-plane resolution = 0.1mm x 0.1 mm, measured slice thickness = 0.5 mm, slices = 32, TR/TE = 28/8 ms, TR between subsequent IR pulses = 1000 ms, and flip angle = 30°, averages = 1. | ||
| T1 mapping scan | 3D fast gradient echo , FOV = 36 mm x 22 mm x 8 mm, matrix = 192 x 102, in-plane resolution = 0.18 mm x 0.22 mm, measured slice thickness = 0.5 mm, slices = 16, TR/TE = 9.6/4.9 ms, flip angle = 10°, averages = 1. | ||
| Phase contrast angiography scan | 2D, fast gradient echo, FOV = 40 mm x 23 mm, matrix = 132 x 77, in-plane resolution = 0.3 mm x 0.3 mm x 1 mm, TR/TE = 9.8/4.9 ms, flip angle = 30°, cardiac phases = 14, averages = 6, flow velocity (foot-head direction) = 30 cm/s. | ||
TABLE 1: MRI acquisition parameters
Discussion
Determining vascular endothelial health is an attractive imaging biomarker that can potentially be used to diagnose atherosclerotic-related risk and to monitor treatment effects. The qMETRIC protocol outlined here can be used to reproducibly quantitate endothelial permeability/leakiness and (dys)function in a comprehensive, fast, and clinically applicable MRI protocol. Such an approach can provide a simpler alternative or complementary tool to existing DCE-MRI protocols for quantifying endothelial permeability. It can also provide a non-invasive tool for direct assessment of endothelial (dys)function in vascular beds, such as the coronary and carotid arteries, instead of using either invasive techniques or surrogate measurements in peripheral arteries that are less severely affected by the disease. Measuring endothelial permeability using this method allows coverage of the aorta, the aortic arch, and the brachiocephalic and carotid arteries at high spatial resolution (0.1 mm for the LGE images and 0.22 mm for T1 mapping) that is crucial for accurate segmentation of the vessel wall in rodents. Analysis of the images can be carried out using an open-source platform and requires only a simple segmentation of the vessel wall without the need for complex pharmacokinetic modeling. Importantly, this protocol can be adapted to be used in a number of different commercially available scanners and can be extended to be used in different animal models and also humans. Although this protocol describes the methodology using a clinical scanner setup, the MRI protocols can also be implemented when using high-field small animal scanners. These scanners frequently offer inversion recovery, T1 mapping, and angiography protocols that can be used or can be programmed in collaboration with the scanner manufacturers.
To obtain accurate and reproducible results, particular attention should be paid to some critical steps of the protocol. Firstly, when imaging small animals in a clinical scanner, suitable and custom-made receiver coils are necessary to maximize the signal-to-noise ratio for high image quality. The animal positioning on the coil is also crucial, avoiding separation and air-filled spaces between the animal and the coil to improve the signal-to-noise ratio. For this reason, the anatomical area of interest should be placed in the center of the coil, and then moved to the isocenter of the magnet to expose them to the magnetic field with maximum homogeneity. Secondly, a stable, strong, and accurate ECG signal is paramount for reliable imaging triggering/gating. This is important for consistent excitation of the magnetization and the timing of the image acquisition window at specific time points and for acquiring accurate time-resolved images that include the end-diastolic phase for the functional test. Small animal pad-based or needle-based electrodes are more suitable options when used at higher-field strength scanners, which are better shielded compared to clinical scanners. When these options are used at clinical field scanners, the ECG cables need to be warped together to avoid the formation of resonant circuits at the MRI Lamour frequency that may deteriorate the ECG signal during the pulse sequence. Alternatively, we propose the use of the ECG module and pads used for human scans with adjustment of the pad size to that of the mouse paw and extra stabilization of the pads with tape to improve conductivity. Thirdly, when acquiring LGE images while the contrast agent is still circulating in the bloodstream, it is crucial to choose the correct nulling time to efficiently suppress the blood pool to delineate the vessel wall. A Look-locker sequence must be run before every LGE sequence, and the inversion delay time needs to be adjusted accordingly. Fourthly, for accurate and precise T1 mapping using a modified look-locker inversion recovery (MOLLI) sequence, the proposed image acquisition scheme should be implemented to cover a range of inversion delays ranging at least from 20 ms to 2000 ms to capture the short and long T1 species. Lastly, segmentation of MRI data must be rigorous and strict criteria applied to avoid intra and/or inter-observer biases in the area/volume and T1 value calculations.
Unlike DCE-MRI, the procedure described here does not provide kinetic data of the wash-in and wash-out of the contrast agent in the vessel wall. Rather, it provides a snapshot of endothelial permeability at a specific time point after injection of the albumin-binding contrast agent, gadofosveset. However, the extracted quantitative data from these time-points highly correlated with other albumin-dyes, such as Evan’s blue dye, which is considered a gold-standard to measure endothelial permeability and increased endothelial gap-junction width. Mechanistically, both the albumin-bound and unbound-fraction of gadofosveset are small enough to pass through breaks in the endothelial junctions and lead to MRI signal enhancement. Additionally, it is possible that the unbound-fraction may also bind to intraplaque albumin after it enters the vessel wall and results in signal enhancement. It was observed that the relaxivity of the vessel wall is r1≈17 mmol/L/s, when gadofosveset is injected at a clinical dose. This value is closer to that reported for the albumin-bound fraction (r1≈25 mmol/L/s) compared to the free-fraction (r1≈6.6 mmol/L/s)5,29.
Future applications of this imaging method include basic science studies in different animal models and other arterial segments and the use of this method to assess for biological responses to existing or novel pharmaceutical agents. Studies can be performed either cross-sectionally or longitudinally to gather mechanistic and outcome data, respectively. The straightforward workflow makes this approach accessible and clinically applicable for use in humans also. Adaptation of this method for imaging human carotid and peripheral arteries is more imminent, but the application of this method for imaging the coronary arteries requires further advancements in image acquisition, reconstruction, and motion-correction that are currently being developed30,31.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We are grateful for funding to the: (1) British Heart Foundation (A.P Early Career Development Fellowship, Project grant-PG/2019/34897, and R.M.B. Project and Programme grants PG/10/044/28343, RG/12/1/29262 and RG/20/1/34802); (2) the King's BHF Centre for Research Excellence RE/18/2/34213; (3) the Wellcome EPSRC Centre for Medical Engineering (NS/A000049/1); (4) the Department of Health via the National Institute for Health Research (NIHR) Cardiovascular Health Technology Cooperative (HTC) and comprehensive Biomedical Research Centre awarded to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust; (5) Chilean Agency for Research and Development (ANID) – Millennium Science Initiative Program – NCN17_129 and FONDECYT 1180525.
Materials
| Acetylcholine | Sigma Aldrich | A6625- 100G, 16.6 mg/kg | |
| Anesthesia equipment | General Anesthetic Services | General Anesthetic Services | |
| Circulating heating pump | ThermoFisher Scientific, USA | BOM: 152510101 | |
| ECG conductive gel (Nuprep) | Waever and Company, USA | 10-30-T | |
| ECG monitoring module | Invivo, USA | REF 0700-1002 | |
| Gadofosveset trisordium (Vasovist/ Ablavar) | Lantheus Medical Imaging Inc, North Billerica, MA, USA | 0.03 mmol/kg | |
| High fat diet | Special Diets Services, Witham, UK | 21% fat from lard, 0.15% (wt/wt) cholesterol | |
| Induction box | Vet Tech Solutions LTD | ||
| Insulin syringes | BD Biosciences | 0.5 mL, 29 G | |
| OsirixX software | OsiriX Foundation, Geneva, Switzerland | Open-source platform | |
| Philips Achieva MRI Scanner (3 Tesla) | Philips Healthcare, Best, The Netherlands | Equipped with a clinical gradient system (30 mT m-1, 200 mT m-1 ms-1) | |
| Single–loop surface microscopy receiver coil | Phillips Hamburg | Diameter = 23 mm | Custom built |
Riferimenti
- Lloyd-Jones, D. M., et al. Defining and setting national goals for cardiovascular health promotion and disease reduction: The American heart association’s strategic impact goal through 2020 and beyond. Circulation. 121 (4), 586-613 (2010).
- Davignon, J., Ganz, P. Role of endothelial dysfunction in atherosclerosis. Circulation. 109 (23), 27-32 (2004).
- Ludmer, P. L., et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. New England Journal of Medicine. 315 (17), 1046-1051 (1986).
- Crauwels, H. M., Van Hove, C. E., Holvoet, P., Herman, A. G., Bult, H. Plaque-associated endothelial dysfunction in apolipoprotein E-deficient mice on a regular diet. Effect of human apolipoprotein AI. Cardiovascular Research. 59 (1), 189-199 (2003).
- Phinikaridou, A., et al. Non-invasive magnetic resonance imaging evaluation of endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Circulation. 126 (6), 707-719 (2012).
- Phinikaridou, A., et al. Increased vascular permeability measured with an albumin-binding magnetic resonance contrast agent is a surrogate marker of rupture-prone atherosclerotic plaque. Circulation; Cardiovascular Imaging. 9 (12), (2016).
- Phinikaridou, A., Andia, M. E., Passacquale, G., Ferro, A., Botnar, R. M. Noninvasive MRI monitoring of the effect of interventions on endothelial permeability in murine atherosclerosis using an albumin-binding contrast agent. Journal of the American Heart Association. 2 (5), 000402 (2013).
- Sluimer, J. C., et al. Thin-walled microvessels in human coronary atherosclerotic plaques show incomplete endothelial junctions relevance of compromised structural integrity for intraplaque microvascular leakage. Journal of the American College of Cardiology. 53 (17), 1517-1527 (2009).
- Rubenfire, M., Cao, N., Smith, D. E., Mosca, L. Carotid artery reactivity to isometric hand grip exercise identifies persons at risk and with coronary disease. Atherosclerosis. 160 (1), 241-248 (2002).
- Nguyen, P. K., Meyer, C., Engvall, J., Yang, P., McConnell, M. V. Non-invasive assessment of coronary vasodilation using cardiovascular magnetic resonance in patients at high risk for coronary artery disease. Journal of Cardiovascular Magnetic Resonance. 10, 28 (2008).
- Terashima, M., et al. Impaired coronary vasodilation by magnetic resonance angiography is associated with advanced coronary artery calcification. Journal of the American College of Cardiology; Cardiovascular Imaging. 1 (2), 167-173 (2008).
- Hays, A. G., et al. Non-invasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. Journal of the American College of Cardiology. 56 (20), 1657-1665 (2010).
- Hirooka, Y., et al. Effect of L-arginine on acetylcholine-induced endothelium-dependent vasodilation differs between the coronary and forearm vasculatures in humans. Journal of the American College of Cardiology. 24 (4), 948-955 (1994).
- Takase, B., et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. American Journal of Cardiology. 82 (12), 1535-1539 (1998).
- Al-Badri, A., Kim, J. H., Liu, C., Mehta, P. K., Quyyumi, A. A. Peripheral microvascular function reflects coronary vascular function. Arteriosclerosis Thrombosis and Vascular Biology. 39 (7), 1492-1500 (2019).
- Calcagno, C., et al. Detection of neovessels in atherosclerotic plaques of rabbits using dynamic contrast enhanced MRI and 18F-FDG PET. Arteriosclerosis Thrombosis and Vascular Biology. 28 (7), 1311-1317 (2008).
- Lobbes, M. B., et al. Atherosclerosis: contrast-enhanced MR imaging of vessel wall in rabbit model–comparison of gadofosveset and gadopentetate dimeglumine. Radiology. 250 (3), 682-691 (2009).
- Kerwin, W. S., Oikawa, M., Yuan, C., Jarvik, G. P., Hatsukami, T. S. MR imaging of adventitial vasa vasorum in carotid atherosclerosis. Magnetic Resonance Medicine. 59 (3), 507-514 (2008).
- van Hoof, R. H., et al. Vessel wall and adventitial DCE-MRI parameters demonstrate similar correlations with carotid plaque microvasculature on histology. Journal of Magnetic Resonance Imaging. 46 (4), 1053-1059 (2017).
- Calcagno, C., Mani, V., Ramachandran, S., Fayad, Z. A. Dynamic contrast enhanced (DCE) magnetic resonance imaging (MRI) of atherosclerotic plaque angiogenesis. Angiogenesis. 13 (2), 87-99 (2010).
- van Wijk, D. F., et al. Increasing spatial resolution of 3T MRI scanning improves reproducibility of carotid arterial wall dimension measurements. Magnetic Resonance Materials in Physics, Biology, and Medicine. 27 (3), 219-226 (2014).
- Li, B., et al. Turbo fast three-dimensional carotid artery black-blood MRI by combining three-dimensional MERGE sequence with compressed sensing. Magnetic Resonance Medicine. 70 (5), 1347-1352 (2013).
- Fan, Z., et al. Carotid arterial wall MRI at 3T using 3D variable-flip-angle turbo spin-echo (TSE) with flow-sensitive dephasing (FSD). Journal of Magnetic Resonance Imaging. 31 (3), 645-654 (2010).
- Li, X., Huang, W., Rooney, W. D. Signal-to-noise ratio, contrast-to-noise ratio and pharmacokinetic modeling considerations in dynamic contrast-enhanced magnetic resonance imaging. Magnetic Resonance Imaging. 30 (9), 1313-1322 (2012).
- Heisen, M., et al. The influence of temporal resolution in determining pharmacokinetic parameters from DCE-MRI data. Magnetic Resonance Medicine. 63 (3), 811-816 (2010).
- Chen, H., et al. Scan-rescan reproducibility of quantitative assessment of inflammatory carotid atherosclerotic plaque using dynamic contrast-enhanced 3T CMR in a multi-center study. Journal of Cardiovascular Magnetic Resonance. 16, 51 (2014).
- Calcagno, C., Vucic, E., Mani, V., Goldschlager, G., Fayad, Z. A. Reproducibility of black blood dynamic contrast-enhanced magnetic resonance imaging in aortic plaques of atherosclerotic rabbits. Journal of Magnetic Resonance Imaging. 32 (1), 191-198 (2010).
- Engel, L. C., et al. Non-invasive imaging of endothelial damage in patients with different HbA1c levels: A proof-of-concept study. Diabetes. 68 (2), 387-394 (2019).
- Caravan, P., et al. The interaction of MS-325 with human serum albumin and its effect on proton relaxation rates. Journal of the American Chemical Society. 124 (12), 3152-3162 (2002).
- Munoz, C., et al. Motion-corrected 3D whole-heart water-fat high-resolution late gadolinium enhancement cardiovascular magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 22 (1), 53 (2020).
- Milotta, G., et al. 3D whole-heart isotropic-resolution motion-compensated joint T1 /T2 mapping and water/fat imaging. Magnetic Resonance Medicine. 84 (6), 3009-3026 (2020).

.
