A Mouse Model of Direct Anastomosis via the Prespinal Route for Crossing Nerve Transfer Surgery
Summary
We simulated clinical surgery to establish a protocol of direct anastomosis of bilateral brachial plexus nerves via the prespinal route in mice, contributing to the study of the neural mechanisms underlying rehabilitation upon crossing nerve transfer after central and peripheral nervous system injuries.
Abstract
Crossing nerve transfer surgery has been a powerful approach for repairing injured upper extremities in patients with brachial plexus avulsion injuries. Recently, this surgery was creatively applied in the clinical treatment of brain injury and achieved substantial rehabilitation of the paralyzed arm. This functional recovery after the surgery suggests that peripheral sensorimotor intervention induces profound neuroplasticity to compensate for the loss of function after brain damage; however, the underlying neural mechanism is poorly understood. Therefore, an emergent clinical animal model is required. Here, we simulated clinical surgery to establish a protocol of direct anastomosis of bilateral brachial plexus nerves via the prespinal route in mice. Neuroanatomical, electrophysiological, and behavioral experiments helped identify that the transferred nerves of these mice successfully reinnervated the impaired forelimb and contributed to accelerating motor recovery after brain injury. Therefore, the mouse model revealed the neural mechanisms underlying rehabilitation upon crossing nerve transfer after central and peripheral nervous system injuries.
Introduction
The brachial plexus (BP) consists of five nerves with different spinal segments (C5-T1) responsible for sensation and movement in the arm, hand, and fingers. After exit of these five BP nerves from the spinal cord, they merge to form three nerve trunks: the superior (formed by the merging of C5 and C6), medial (from C7), and inferior (branches of C8 and T1). Severe injuries, especially due to traffic accidents, often lead to avulsion of the BP nerve roots, and such dysfunction has a devastating effect on patients1. As a powerful clinical approach, crossing nerve transfer surgery has been performed to repair avulsion injuries to the BP by reconnecting the injured nerve ends to the healthy side of the BP2,3. This surgery results in functional improvements of injured hands and direct reorganization of the sensorimotor cortex in both hemispheres in patients4. Animal studies have revealed that drastic reorganization in the cortical circuits was induced after crossing nerve transfer5. Because peripheral sensorimotor modification can reactivate the dormant plasticity of the mature brain, crossing nerve transfer surgery also exhibits great potential in repairing brain injuries6.
Recently, we confirmed the possibility of the creative use of crossing nerve transfer as a new peripheral nerve change strategy for problems with the central nervous system. A type of crossing nerve transfer surgery, contralateral cervical seventh nerve transfer (CC7), was applied to achieve significant functional recovery of the paralyzed arm by transferring the C7 nerve from the nonparalyzed side to the paralyzed side in the patient after brain injury7. A unique feature of this surgical operation is that the sensory and motor signals of the paralyzed upper extremity communicated to the contralesional hemisphere through the "left-right crossover" displaced nerve. Notably, the functional recovery caused by CC7 surgery is not limited to the function innervated by the C7 nerve itself8. In addition, CC7 surgery can be used not only to treat children with cerebral palsy but also to achieve rehabilitation in middle-aged and elderly stroke patients. Therefore, there are sufficient reasons to believe that crossing nerve transfer can stimulate neuroplasticity to accelerate motor recovery from brain damage by modulating the peripheral sensorimotor system.
Although crossing nerve transfer surgery has achieved substantial rehabilitation in the clinical treatment of both brachial plexus injuries (BPI) and brain injuries, the neural mechanisms underlying this surgery remain poorly understood. The lack of a suitable animal model featuring clinical features has restricted the study of internal mechanisms. Traditionally, in the clinic, the C7 nerve root contralateral to the lesion is transferred to the injured side through a nerve graft (e.g., ulnar nerve, sural nerve, or saphenous nerve) and connected with the affected brachial plexus (e.g., median nerve, C7 root, or lower trunk)2,3,9. A relatively new modification of this surgery involves the unaffected C7 root being directly transferred to the affected C7 nerve via the prespinal route without any gap, suggesting an optimal solution7. Currently, mice exhibit an advantage in cell-type specificity and genetic strain diversity and are more suitable to study neurophysiological mechanisms. Hence, clinical surgery was simulated to establish a protocol for direct anastomosis of bilateral C7 nerve roots via the prespinal route in mice and contribute to the study of the neural mechanisms underlying rehabilitation upon crossing nerve transfer.
Protocol
All the animal experiments were approved by the Institutional Care of Experimental Animals Committee of Fudan University and the Chinese Academy of Science in conformity with the National Institute of Health guidelines. Eight-week-old adult male C57BL/6N mice were used.
1. Preoperative setup
- Ensure an appropriate stock of autoclaved sterilized surgical instruments, equipments, analgesic medications, and anesthetic medication.
- Ensure adequate working space on an operating table.
- Prepare the operating table using a diaper-covered customized surgical foam board as a bed for the mouse. Fix a warming pad to the foam board with medical tape covered with sterile gauze.
- Create retractors by bending an acupuncture needle using vascular forceps, folding it in half, and then bending the tip of the folded acupuncture needle into a hook. Fix a rubber strip at the end of the acupuncture needle, and use a thumbtack to fix the end of the rubber strip to the foam board.
- Calibrate the stereomicroscope; choose a stereomicroscope with an adequate focus distance. Cover the zoom/focus buttons with sterilized aluminum foil to allow the surgeon to adjust them during the operation. The sterilized aluminum foil was placed on the zoom/focus buttons allowing the surgeon the use it with sterile gloves.
2. Mouse anesthesia and preparation
- Weigh the mouse and anesthetize corresponding to the body weight (isoflurane 3%). Ensure that the mouse does not respond when the interdigital spaces of its paw are pinched to confirm the depth of anesthesia. Adequate depth of anesthesia should be maintained throughout the procedure (1% isoflurane).
- Apply ophthalmic ointment bilaterally to the eyes to prevent irritation or drying of the cornea during surgery.
- Prepare the surgical site by shaving the fur on the neck and chest with an automatic clipper. Remove and clean the loose hair.
- Place the mouse in a supine position on the warming pad covered with sterile gauze. Maintain the temperature of the mouse at 37 °C during the whole operation. Fix the mouse with medical tape to cause the forelimbs to abduct horizontally and prevent the hind limbs and tail from moving. A sterilized disposable surgical drape with appropriate opening was placed on the mice.
3. Operative procedure
- Inject tramadol as the pre-emptive analgesia (20 mg/kg, i.p.). Mark the transverse incision on the superior edge of the clavicle. Use three cycles of alternating scrubs of iodophor disinfection solution and ethanol to disinfect the surgical site. Confirm the depth of anesthesia with a toe pinch before surgery.
- Working under a microscope, make a 4 mm transverse incision along the mark using a sterile scalpel. Enlarge the incision during the procedure as necessary.
- Bluntly dissect through the subcutaneous fascia and identify the inferior border of the submandibular gland. Pull the submandibular gland upward to expose the supraclavicular fossa and sternum.
NOTE: There might be small-caliber blood vessels in this area. Electrocautery can be used to stop bleeding. - Make a partial median sternotomy incision (~4 mm) by incising the sternum from head to tail along the middle line. Protect the pleura, heart, and blood vessels during sternotomy.
- Identify the sternohyoid muscle. Pull the sternum gently with two small customized retractors made of acupuncture needles and identify the sternohyoid muscle, over the trachea and esophagus. Retract this muscle to expose the carotid artery, internal jugular vein, phrenic nerve, vagus nerve, trachea, and esophagus.
NOTE: Gently retract the sternum to avoid open pneumothorax. Unlike in humans, the esophagus of the mouse is not behind the trachea but adjacent to the trachea on the left side. - Identify the left brachial plexus. At the lateral edge of the left internal jugular vein, pull the fascia and adipose tissue outward to expose the brachial plexus. Look for the superior trunk, composed of the C5 and C6 nerves, which has three branches. Identify the middle trunk composed of the C7 nerve and the inferior trunk composed of the C8 and T1 nerves, along the upper trunk up to the tail of the mouse.
NOTE: There are longitudinal blood vessels on the surface of the brachial plexus. Use electrocautery to prevent bleeding. When separating the left brachial plexus, protect the chylous canal to avoid a chylous fistula. - Harvest the left C7 nerve. Dissect the anterior division and posterior division of the middle trunk (C7 nerve) distally to the division-to-cord level under the clavicle and block the C7 nerve with 0.1 mL of 2% lidocaine by local infusion into nerve trunk. Resect the C7 nerve by vannas spring scissors at its merger points with the lateral cord and posterior cord. Trim the C7 nerve so that the length of each division is similar.
NOTE: The anterior and posterior divisions of the C7 nerve and the anterior and posterior divisions of upper and lower trunks run for a long distance before confluence, so the C7 nerve should be freed sufficiently before resection. In fact, the C7 nerve is not always divided into two divisions; sometimes, it is divided into three divisions or even into four in rare cases. - Remove the left C6 lamina ventralis. Carefully protect the phrenic nerve and severe the anterior scalene muscle at the level of the C6 segment to expose the C7 nerve root. Cut small branches of the C7 nerve innervating the paraspinal muscle with microforceps. Pull out the C7 nerve gently and excise the C6 lamina ventralis carefully.
NOTE: There is a bony prominence between the medial side of the left carotid artery and the lateral side of the esophagus. This bony prominence is the lamina ventralis of the 6th cervical vertebrae. The longitudinal muscle of the lateral edge of the C6 lamina ventralis is the anterior scalene muscle, and the phrenic nerve runs on the surface of the anterior scalene muscle. - Harvest the right C7 nerve. Severe the anterior scalene muscle on the right side, similar to the left side, and transect the right C7 nerve root close to the intervertebral foramen. Dissect the right C7 nerve from its division level.
NOTE: Carefully cut the right C7 nerve to prevent damage to the blood vessels under the nerve. - Transfer the left C7 nerve.
- Remove the muscular longus colli beside the vertebral bodies partially on both sides. Bluntly separate and expand the space between the trachea-esophagus and vertebral body.
- Send a half-fold 5-0 nylon sutures from the right side of the vertebral body to the left side through the prespinal route.
- Hitch the left C7 nerve with an infusion tube and guide the nerve to the right side via the prespinal route.
- Retract the trachea and esophagus gently and coapt the anterior and posterior divisions of the left C7 nerve to the right C7 nerve root without tension using 12-0 Nylon sutures. Suture the epineurium around the nerves with 4-5 stitches to coaptate the nerves strongly.
NOTE: It is crucial to choose a plastic infusion tube of appropriate thickness. Too thin of a tube could damage the nerve, and too thick of a tube could damage the trachea and esophagus. In addition, the space between the trachea-esophagus and the vertebral body is a "V"-shaped space, and cutting part of the muscular longus colli can shorten the transfer pathway.
4. Wound closure
- Irrigate the wound with sterile normal saline and dry it with sterile gauze.
- Suture the sternum and close the skin using 5-0 monofilament sutures.
5. Postoperative care
- Wait for the mouse to wake from anesthesia. Transfer the mouse to a clean cage without bedding material but warmed with a warming blanket. Observe the mouse until it is ambulatory. Use tramadol (20mg/kg, i.p.) as the postoperative analgesia.
- Place the mice in a recovery cage and monitor it till recovery. Restore the water and diet of the mice after the operation. Monitor the mice postoperatively for signs of impairment or infection every day, including malnourishment, hunched posture, and ruffled fur. At two weeks after surgery, suture removal should occur.
NOTE: Apply erythromycin ointment to the wound surface every day for three consecutive days. - If any complications, such as wound edema is observed, that should be immediately resolved.
6. Behavioral analysis
NOTE: All behavioral testing and analysis were done by an observer blinded to the experimental groups.
- Cylinder test
NOTE: The cylinder test evaluates the use of forelimbs during spontaneous vertical exploration within a cylinder at 4 and 8 weeks after surgery21.- Place the mice in a transparent cylinder (diameter 9 cm, height 15 cm) on an elevated frame.
- To facilitate observation and recording, fix a mirror at a 45° angle below the cylinder.
- Record spontaneous rearing of each mouse observed with the help of the mirror for 10 min.
- Manually determine the length of time for which the (i) right paw, (ii) left paw, or (iii) both paws made contact with the glass walls. Count a total of 20 movements during each session. Exclude mice that are not active during the test from the analysis.
- Score the test performance as:
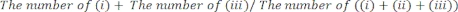
- Grid-walk test
NOTE: The grid-walk test assesses the accurate placement of the forepaws on the rungs of a grid during spontaneous exploration at 4 and 8 weeks after surgery.22.- Place the mice on a wire grid (20 cm x 24 cm) with 25 mm square holes and allow them to freely explore for 10 min while recording their performance with a video camera.
- Score a foot slip in the case of either of the following:
- Look for instances when the paw completely misses a rung (in which case the limb falls between the rungs and the animal loses balance).
- Look for instances when the paw is correctly placed on a rung but slips off while bearing body weight.
- Express the test result as foot slip of right forelimb / total foot slip. Although neither the cylinder test nor the grid-walk test requires training, obtain baseline scores by testing each animal once before surgery.
Representative Results
Unilateral brain injury often causes permanent dysfunction of the contralateral limb due to the limitations of compensative neural plasticity in adults10,11. Previously, we reported that CC7 surgery could be used to treat hemiplegic upper limbs in adult patients after brain injury7. To evaluate the effectiveness of the protocol for direct anastomosis bilateral C7 nerves via the prespinal route, we performed the crossing nerve transfer surgery in mice following unilateral traumatic brain injury (TBI). Figure 1 describes the TBI procedures and verifies the damage range and effect. First, an electric cortical contusion impactor (eCCI) was used to damage the cerebral cortex of the left hemisphere (anteroposterior = +1.0 mm to -2.0 mm, mediolateral = 0.5 mm to 3.5 mm) in adult mice to result in unilateral brain injury. After 2 weeks, anatomical structures confirmed that this TBI protocol almost destroyed the sensorimotor cortex, an important location for initiating movements. These mice with unilateral TBI exhibited significant motor defects of the right forelimb.
Figure 2 describes the CC7 procedures. The path diagram of CC7 surgery revealed that path A, representing the prespinal route, was the shortest approach compared to the others. The length of path A is even lower than the length of the harvested C7 nerve on the left side (nonparalyzed side). This finding provided the anatomical basis for the choice of the prespinal route to complete nerve transfer surgery. CC7 surgery was performed in direct anastomosis via the prespinal route at two weeks post-TBI. The cervical 7 (C7) nerve on the nonparalyzed side was directly transferred to the paralyzed side instead of making its original brain connections. Figure 3 shows the results of electron microscopy that revealed that the transferred C7 nerve had successfully regenerated. The myelin sheath thickness of the transferred C7 nerve gradually increased, starting at 4 weeks post-CC7 surgery, and was almost comparable to that in the control group at 8 weeks post-CC7 surgery. Figure 4 identifies muscle reinnervation of the transferred C7 nerve using electromyographic recordings. Electrically stimulating the proximal end of C7 nerve anastomosis stably induced action potentials in multiple muscles of the affected forelimb at 4 weeks postoperatively, in agreement with the electron microscopy results. Figure 5 shows that the transferred C7 nerve contains motor fibers from the ventral horn and sensory fibers from the dorsal root ganglia of the spinal cord C7 segment on the healthy side through cholera toxin subunit B (CTB) retrograde labeling.
Figure 6 shows that the mouse model also exhibited significant motor recovery after unilateral TBI, consistent with the results of the clinical studies. To verify the effect of CC7 surgery on the recovery of injured motor function after TBI, a TBI + Sham group and a Control + Sham group were established. The mice in the TBI + Sham group and the TBI + CC7 group received the same procedures for TBI injury simultaneously, while the mice in the Control + Sham group received only sham surgery. While the mice in the TBI + CC7 group received nerve transfer surgery, mice in the TBI + sham group and the Control + Sham group underwent bilateral cervical 7 (C7) nerve resection. In cylinder tests, the TBI + CC7 group showed a significantly higher usage rate of the impaired forelimb than the TBI group at both 4 and 8 weeks post-CC7 surgery (p < 0.01). In grid-walking tests, the TBI + CC7 group showed a lower error rate than the TBI group at 4 weeks post-CC7 surgery. Moreover, the error rate of the TBI + CC7 group was significantly lower than that in the TBI group at 8 weeks post-CC7 surgery (p < 0.05). These behavioral results showed that CC7 surgery could improve the motor function of the affected limb in TBI mice. Together, these results suggest that the transferred C7 nerve rebuilt by CC7 surgery via the prespinal route was successfully regenerated and reinnervated the impaired forelimb, contributing to motor restoration in adult mice with unilateral TBI.
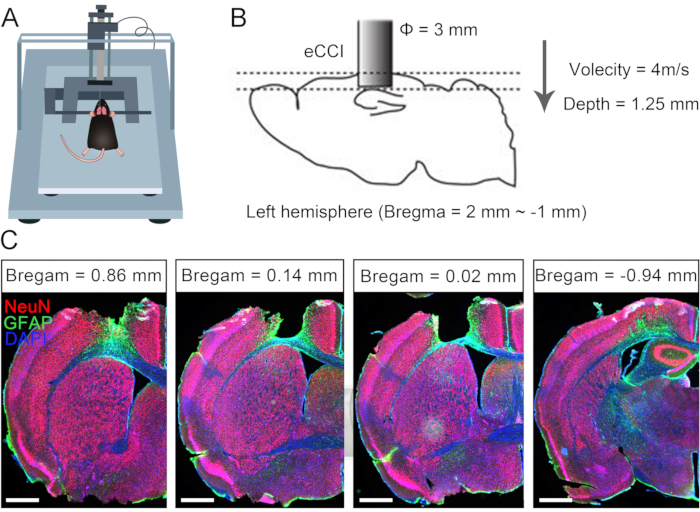
Figure 1: Characterization of unilateral traumatic brain injury. (A) Schematic showing the mouse position in eCCI. (B) The parameters and damage range of eCCI. (C) Representative coronal section showing the lesioned cortex (2 weeks after TBI, scale bar = 500 µm). Abbreviation: eCCI = electric cortical contusion impactor. Please click here to view a larger version of this figure.
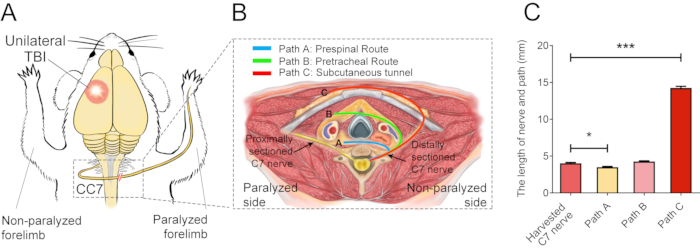
Figure 2: The surgical elementary diagram. (A) Schematic diagram showing the experimental design for performing the contralateral C7 nerve transfer in TBI mice. The red circle shows the position of the trauma. The red double-slash within the dashed rectangle shows the sutured nerve. (B) A cross-section shows three alternative routes of the contralateral C7 nerve transfer in the mice. Path A, the blue line depicts the prespinal route of the transferred nerve; Path B, the green line, depicts the pretracheal route of the transferred nerve; Path C, the red line, depicts the subcutaneous tunnel of the transferred nerve. (C) The graph shows the length of the routes and the harvested C7 nerve in (B). The length of path A (3.3 ± 0.10 mm) was significantly lower than the length of the harvested C7 nerve (4.05 ± 0.11 mm; * p < 0.05, one-way ANOVA, n = 20 in each group). The length of path C (14.15 ± 0.20 mm) was significantly greater than that of the harvested C7 nerve (*** p < 0.001, one-way ANOVA, n = 20 in each group). The length of path B was 4.2 ± 0.08 mm (n=20). Please click here to view a larger version of this figure.
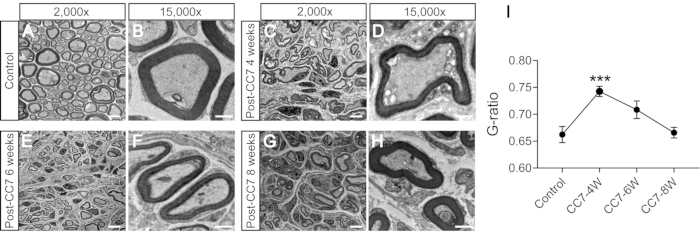
Figure 3: The electron microscopy analysis of a cross-section of the nerve. (A,B) Images of the nerve in control mice. Scale bar = 5 µm (A) and 1 µm (B). (C,D) Images of the regenerated nerve one month after surgery. Scale bar = 5 µm (C) and 1 µm (D). (E, F) Images of the regenerated nerve at one point five months after surgery. Scale bar = 5 µm (E) and 1 µm (F). (G, H) Image of the regenerated nerve at two months after surgery. Scale bar = 5 µm (G) and 1 µm (H). Magnification of A, C, E, and G, 2,000x; magnification of B, D, F, and H, 15,000x. (I) The G-ratio (the ratio of the inner to the outer diameter of the myelin sheath) is lower in control group samples than in 4-weeks samples and equal to samples at 6-8 weeks post-surgery (***: p < 0.001; comparison at different group axons with t-test; n = 3 mice in each group). Abbreviations: CC7= contralateral cervical seventh nerve transfer; CC7-XW = X weeks post-surgery. Please click here to view a larger version of this figure.
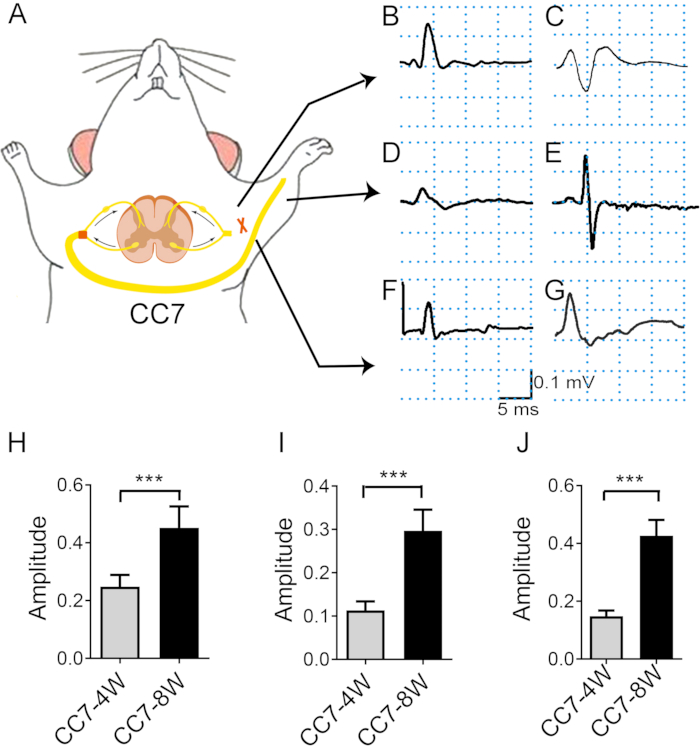
Figure 4: Electromyography analysis after the contralateral C7 nerve transfer indicates the rate of nerve regeneration. (A) Schematic diagram showing the electronic transfer stimulation and in vivo electromyography recording. The stimulation intensity was the same throughout the test (2 mA). The stimulation site is the C7 nerve proximal to the anastomosis. (B, C) Photographs showing action potential recorded at the pectoralis major at two weeks (B) and four weeks (C) after surgery. (D, E) EMG was recorded in extensor digitorum 4 weeks (D) and 8 weeks (E) post-surgery. (F) At three weeks, CMAPs emerged in the triceps brachii. (G) At four and eight weeks, CMAPs of triceps brachii increased. (H) The mean amplitude of pectoralis major reached ~0.25 mV ± 0.16 mV at 4 weeks versus 0.45 mV ± 0.03 mV at 8 weeks, showing a significant difference between the two time points (*** p < 0.001, t-test, n = 6 in each group). (I) The mean amplitude of triceps brachii reached ~0.15 mV ± 0.01 mV at 4 weeks versus 0.46 mV ± 0.02 mV at 8 weeks, showing a significant difference between the two time points (***: p < 0.001, t-test, n = 6 in each group). (J) The mean amplitude of extensor digitorum reached ~0.11 mV ± 0.01 mV at 4 weeks versus 0.29 mV ± 0.02 mV at 8 weeks, showing a significant difference between the two time points (***: p < 0.001, t-test, n = 6 in each group). Abbreviations: EMG = electromyography; CMAP = compound muscle action potential. Please click here to view a larger version of this figure.
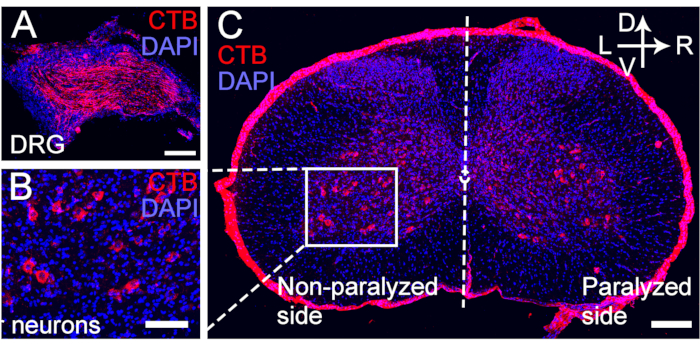
Figure 5: CTB retrograde labeling of motor and sensory neurons of the transferred C7 nerve. (A–C) CTB was injected at the distal end of the C7 nerve anastomosis at 4 weeks post CC7 surgery. (A)The sensory neurons were labeled for the DRG. (B, C) The motor neurons of the transferred C7 nerve were labeled for the spinal anterior horn. Magnification, 20x. Scale bar = 200 µm (A, B); 100 µm (C). Abbreviations: CTB = cholera toxin subunit B; DRG = dorsal root ganglion; DAPI = 4′,6-diamidino-2-phenylindole. Please click here to view a larger version of this figure.
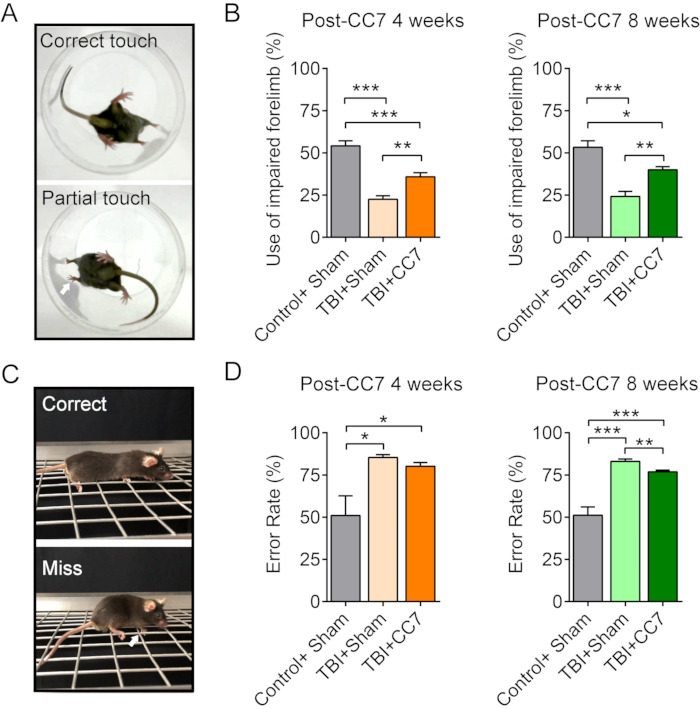
Figure 6: Behavioral changes after CC7 surgery. (A) The images show the cylinder test of the mice. (B) Summary graph showing the effect of CC7 transfer at 4 weeks and 8 weeks after surgery on the TBI mice (n = 6 mice). p = 0.001; unpaired t-test. The average usage of the impaired forelimb was 54.17% ± 3.01% in Control + Sham group versus 22.5% ± 2.14% in TBI + Sham group; 35.83% ± 2.39% in TBI + CC7 group at 4 weeks post-CC7 surgery, indicating a significant difference (one way ANOVA; p < 0.05, n = 6 in each group). At 8 weeks after CC7 transfer, the usage was 53.33% ± 3.80%, 24.17% ± 3.01%, and 40.00% ± 1.83% in Control + Sham group, TBI + Sham group, and TBI + CC7 groups, respectively, a significant difference (*p < 0.05, one way ANOVA, n = 6 in each group). (C) The images display the grid walk test. (D) The graph shows that the mean error rates of the impaired forelimb in TBI + Sham group were 85.41% ± 1.59% (n = 6) equaling to the TBI + CC7 group 80.17% ± 2.19% (n = 6), and both were more than the Control + Sham group (50.99% ± 11.69%). At 8 weeks after surgery, the error rate in TBI + CC7 group was 76.87 ± 1.07% (n = 6), which is significantly lower than that of the TBI + Sham group (83.06% ± 1.41%; p < 0.05, one-way ANOVA, n = 6 in each group). Abbreviations: CC7= contralateral cervical seventh nerve transfer; TBI = traumatic brain injury. Please click here to view a larger version of this figure.
Discussion
In the clinic, crossing nerve transfer surgery has been used to treat patients with brachial plexus avulsion injury and after brain damage, such as stroke and TBI7,9,12. Notably, brain damage is a severe neurological condition that can lead to several complications, including epilepsy, cerebral hernia, and infection13. Not all patients with unilateral brain injury are suitable for CC7 surgery. In general, CC7 surgery has been performed in patients with central hemiplegia at the chronic stage (6 months post injury) to avoid the influence of brain edema as much as possible. Patients with cognitive impairment and quadriplegia after brain injuries are excluded from treatment for CC7 surgery.
Most studies have reported using a subcutaneous approach and sural or ulnar nerve graft anastomosis to transfer the contralateral C7 nerve root14,15. However, nerve regeneration by such methods requires six months, which can hinder the motor recovery process and even potentially influence brain plasticity14. In previous studies, contralateral C7 transfer was performed in rats, and the bilateral C7 nerve was used via 4 strands of the interpositional autografted sural nerve. However, there have been no reports of C7 nerve transfer via the prespinal route in mice. We performed CC7 surgery of the modified prespinal route in mice and verified the velocity of functional recovery after C7 nerve transfer. In this study, contralateral C7 nerve transfer via the prespinal route improved paralyzed limb function one month after surgery, reflecting a shorter recovery time of the nerve grafted animal model. Therefore, this model could precisely simulate clinical situations and lay the foundation for further experiments.
How to dissect the nerve root and reduce risk are essential issues for C7 transfer. Unlike in humans, the brachial plexus of the mouse is located in the chest below the clavicle5,16. Therefore, the access strategy had to be altered to allow for the observation of the root of the C7 nerve and spine17. Sternotomy is a safe and effective operative approach and is commonly applied in mouse experiments in cardiothoracic surgery18,19. The C6 lamina ventrali is also an obstacle to transferring nerves. Thus, sternotomy surgery was performed to dissect the C7 nerve root and sever the C6 lamina ventrali to shorten the transfer distance.
Although the prespinal route can significantly increase the success rate of direct anastomosis of nerve transfer surgery, not all mice can be anastomosed directly. This is mainly due to the anatomical differences in these mice. The middle trunk (C7 nerve) merges with the upper or lower trunk at a location very close to the intervertebral foramen. Thus, the length of the C7 nerves available for harvesting is insufficient. Currently, the only approach is nerve transplantation or replacement of mice. This model is typically employed in 8-week-old mice (20-25 g), as the mice are mature and C7 nerves are of adequate size to be handled. Although this surgical protocol is also applicable to young mice, the difficulty of the operation will increase significantly in younger mice.
The forelimb motor function of mice in the TBI + CC7 group was significantly increased at one month and two months, suggesting that the transferred C7 nerve contributed to the recovery of the impaired forelimb. Remyelination is critical for functional neural recovery. A previous study showed that the myelin sheaths of injured nerves regenerated after one month, consistent with these results20. Here, the transferred nerve gradually matured, which was consistent with the behavioral test. Electromyography was used to further test the rate of functional recovery after nerve transfer. The results demonstrated that the transferred nerve innervated the affected muscle 4 weeks after the operation. Notably, this study is the first to determine the time point of reinnervation with a direct anastomosis after crossing nerve transfer surgery.
In summary, we simulated clinical surgery to establish a protocol for direct anastomosis of bilateral brachial plexus nerves via the prespinal route in mice and confirmed the function of the displaced nerve. The mouse model contributed to the elucidation of the neural mechanisms underlying rehabilitation upon crossing nerve transfer after central and peripheral nervous system injuries.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82071406, 81902296, and 81873766).
Materials
| 1 mL syringe | KDL | K-20200808 | |
| 12-0 nylon sutures | Chenghe | 20082 | |
| 5-0 silk braided | MERSILK,ETHICON | QK312 | |
| 75% ethanol | GENERAL-REAGENT | P1762077 | |
| Acupuncture needle | Chengzhen | 190420 | Use for making retractors |
| Automatic clipper | Codos | CHC-332 | |
| C57BL/6N mice | SLAC laboratory (Shanghai) | C57BL/6Slac | |
| Electrocautery | Gutta Cutter | SD-GG01 | |
| Erythromycin ointment | Baiyunshan | H1007 | |
| Iodophor disinfection solution | Lionser | 20190220 | |
| Medical tape | Transpore,3M | 1527C-0 | |
| Micro needle holder | Chenghe | X006-202003 | |
| Micro-forceps | Chenghe | B001-201908 | |
| Micro-scissors | 66VT | 1911-2S276 | |
| Operating microscope | OLYMPUS | SZX7 | |
| Ophthalmic scissor | Chenghe | X041D1251 | |
| Pentobarbital sodium | Sigma | 20170608 | |
| Plastic infusion tube | KDL | C-20191225 | |
| Sterile normal saline | KL | L121021109 | |
| Vascular forceps | Jinzhong | J31020 | |
| Warming pad | RWD | 69027 |
Riferimenti
- Aszmann, O. C., et al. Bionic reconstruction to restore hand function after brachial plexus injury: a case series of three patients. Lancet. 385 (9983), 2183-2189 (2015).
- Gu, Y., Xu, J., Chen, L., Wang, H., Hu, S. Long term outcome of contralateral C7 transfer: a report of 32 cases. Chinese Medical Journal. 115 (6), 866-868 (2002).
- Gu, Y. D., et al. Long-term functional results of contralateral C7 transfer. Journal of Reconstructive Microsurgery. 14 (1), 57-59 (1998).
- Feng, J. T., et al. Brain functional network abnormality extends beyond the sensorimotor network in brachial plexus injury patients. Brain Imaging and Behavior. 10 (4), 1198-1205 (2016).
- Stephenson, J. B. t., Li, R., Yan, J. G., Hyde, J., Matloub, H. Transhemispheric cortical plasticity following contralateral C7 nerve transfer: a rat functional magnetic resonance imaging survival study. The Journal of Hand Surgery. 38 (3), 478-487 (2013).
- Hübener, M., Bonhoeffer, T. Neuronal plasticity: beyond the critical period. Cell. 159 (4), 727-737 (2014).
- Zheng, M. X., et al. Trial of contralateral seventh cervical nerve transfer for spastic arm paralysis. The New England Journal of Medicine. 378 (1), 22-34 (2018).
- Spinner, R. J., Shin, A. Y., Bishop, A. T. Rewiring to regain function in patients with spastic hemiplegia. The New England Journal of Medicine. 378 (1), 83-84 (2018).
- Hua, X. Y., et al. Contralateral peripheral neurotization for hemiplegic upper extremity after central neurologic injury. Neurosurgery. 76 (2), 187-195 (2015).
- Robertson, C. S., et al. Effect of erythropoietin and transfusion threshold on neurological recovery after traumatic brain injury: a randomized clinical trial. Journal of the American Medical Association. 312 (1), 36-47 (2014).
- Skolnick, B. E., et al. A clinical trial of progesterone for severe traumatic brain injury. The New England Journal of Medicine. 371 (26), 2467-2476 (2014).
- Wang, G. B., et al. Contralateral C7 to C7 nerve root transfer in reconstruction for treatment of total brachial plexus palsy: anatomical basis and preliminary clinical results. Journal of Neurosurgery. Spine. 29 (5), 491-499 (2018).
- Wilson, L., et al. The chronic and evolving neurological consequences of traumatic brain injury. The Lancet. Neurology. 16 (10), 813-825 (2017).
- Hua, X. Y., et al. Enhancement of contralesional motor control promotes locomotor recovery after unilateral brain lesion. Scientific Reports. 6, 18784 (2016).
- Hua, X. Y., et al. Interhemispheric functional reorganization after cross nerve transfer: via cortical or subcortical connectivity. Brain Research. 1471, 93-101 (2012).
- Pan, F., Wei, H. F., Chen, L., Gu, Y. D. Different functional reorganization of motor cortex after transfer of the contralateral C7 to different recipient nerves in young rats with total brachial plexus root avulsion. Neuroscience Letters. 531 (2), 188-192 (2012).
- Yamashita, H., et al. Restoration of contralateral representation in the mouse somatosensory cortex after crossing nerve transfer. PLoS One. 7 (4), 35676 (2012).
- Tavakoli, R., Nemska, S., Jamshidi, P., Gassmann, M., Frossard, N. Technique of minimally invasive transverse aortic constriction in mice for induction of left ventricular hypertrophy. Journal of Visualized Experiments: JoVE. (127), e56231 (2017).
- Melhem, M., et al. A Hydrogel construct and fibrin-based glue approach to deliver therapeutics in a murine myocardial infarction model. Journal of Visualized Experiments: JoVE. (100), e52562 (2015).
- Liu, B., et al. Myelin sheath structure and regeneration in peripheral nerve injury repair. Proceedings of the National Academy of Sciences of the United States of America. 116 (44), 22347-22352 (2019).
- Overman, J. J., et al. A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke. Proceedings of the National Academy of Sciences of the United States of America. 109 (33), 2230-2239 (2012).
- Yoshikawa, A., Nakamachi, T., Shibato, J., Rakwal, R., Shioda, S. Comprehensive analysis of neonatal versus adult unilateral decortication in a mouse model using behavioral, neuroanatomical, and DNA microarray approaches. International Journal of Molecular Sciences. 15 (12), 22492-22517 (2014).

