Locomotor Assessment of 6-Hydroxydopamine-induced Adult Zebrafish-based Parkinson’s Disease Model
Summary
The present protocol describes the intracerebroventricular (ICV) injection of adult zebrafish with neurotoxic 6-hydroxydopamine (6-OHDA) at the ventral diencephalon (Dn) and the assessment of the impairment and subsequent recovery of swimming behavior postlesion by using the open tank test, which is accompanied by analysis using a video tracking software.
Abstract
The limitations of current treatments in delaying dopaminergic neuronal loss in Parkinson's disease (PD) raise the need for alternative therapies that can restore these neurons. Much effort is currently directed toward a better understanding of neuroregeneration using preclinical in vivo models. This regenerative capability for self-repair is, however, inefficient in mammals. Non-mammalian animals like zebrafish have thus emerged as an excellent neuroregenerative model due to its capability to continuously self-renew and have a close brain homology to humans. As part of the effort in elucidating cellular events involved in neuroregeneration in vivo, we have established the 6-hydroxydopamine (6-OHDA)-induced adult zebrafish-based PD model. This was achieved through the optimized intracerebroventricular (ICV) microinjection of 99.96 mM 6-OHDA to specifically ablate dopaminergic neurons (DpN) in the ventral diencephalon (Dn) of zebrafish brain. Immunofluorescence indicated more than 85% of DpN ablation at day three postlesion and full restoration of DpN at lesioned site 30 days postlesion. The present study determined the impairment and subsequent recovery of zebrafish swimming behavior following lesion by using the open field test through which two parameters, distance traveled (cm) and mean speed (cm/s), were quantified. The locomotion was assessed by analyzing the recordings of individual fish of each group (n = 6) using video tracking software. The findings showed a significant (p < 0.0001) reduction in speed (cm/s) and distance traveled (cm) of lesioned zebrafish 3 days postlesion when compared to sham. The lesioned zebrafish exhibited full recovery of swimming behavior 30 days postlesion. The present findings suggest that 6-OHDA lesioned adult zebrafish is an excellent model with reproducible quality to facilitate the study of neuroregeneration in PD. Future studies on the mechanisms underlying neuroregeneration as well as intrinsic and extrinsic factors that modulate the process may provide important insight into new cell replacement treatment strategies against PD.
Introduction
Parkinson's disease (PD), a disease distinctively characterized by muscle rigidity, resting tremor, and bradykinesia, is the fastest growing neurological disease in the world1,2. The risk and prevalence of PD increase rapidly with age especially in individuals aged 50 years and above3. The etiology and pathogenesis of PD hitherto remain poorly understood. This has often left the early-onset of PD undiagnosed. At present, the lack of dopamine and the loss of dopaminergic neurons (DpN) in PD patients are strongly linked to the manifestation of motor symptoms4. Capitalizing on this relationship, several treatments have been designed either to act directly as dopamine replacement (i.e., levodopa) or to compensate for the loss of DpN (i.e., deep brain stimulation). Although these treatments bring about symptomatic benefits, they do not modify the deteriorating course of the disease5. In view of this significant weakness, cell replacement therapy has been proposed. The efficacy of this approach is, however, inconsistent given the challenges of graft preparation, cell growth control, and phenotype instability. Cell replacement therapy, which had raised ethical concerns, also poses the risk of inducing brain tumors and unwanted immune reactions6,7.
The limitations of current therapeutic strategies have led to a greater emphasis on the regeneration of DpN as a potential approach in treating PD. Regeneration of DpN or neuroregeneration has emerged as one of the promising breakthroughs in the management of PD, not only due to its potential as a new therapeutic method but also as means to understand the mechanism of the disease8,9. This approach focuses on the restoration of neuronal function through differentiation, migration, and integration of existing progenitor cells into the lesioned circuitry10. In order to further explore neuroregeneration, various in vivo studies have been undertaken. It was found that vertebrates such as mammals, amphibians, and reptiles generate new brain cells following injury11,12. Among the vertebrates, mammalian animals are more sought after given their genetic resemblance to human beings. Mammals, however, exhibit limited and poor reparative capacity in the central nervous system (CNS) that can last through adulthood following a brain lesion13. In general, mammals are unsuited as animal models for understanding neuroregeneration given that the low number of neurons produced will not be sufficient to restore damaged neural circuits observed in PD. As such, the teleost-based model, specifically in zebrafish, is greatly favored for its high proliferative rate, capability to continuously self-renew, and close brain homology with humans14,15.
Zebrafish is most commonly used to study disordered movement in PD16. The zebrafish-based PD model is usually induced by neurotoxins, which include 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 6-hydroxydopamine (6-OHDA)17. Although effective in inducing specific loss of DpN and decrease of dopamine levels, MPTP-based models do not closely mimic the conditions of PD as the DpN loss is not restricted solely to the CNS18. The inability of 6-OHDA to cross the blood-brain barrier restricted its effects on cellular and functional changes within the brain when it is administered intracranially as opposed to intramuscularly19. Peripheral administration of 6-OHDA caused a global reduction of dopamine levels throughout the nervous system20. While administration of 6-OHDA into the cerebrospinal fluid caused ablation of DpN throughout the CNS21, which does not mimic the condition as seen in PD whereby the loss of DpN occurs specifically at the substantia nigra of the human brain. ICV administration of 6-OHDA, on the contrary, specifically induced significant ablation of DpN in the area of ventral Dn in the zebrafish brain, which closely resembled substantia nigra22. Interestingly, recovery of DpN was reported 30 days post 6-OHDA-induced lesion and these neurons survived over the course of life23,24. The functional recovery of DpN was demonstrated through a locomotor assessment of distance traveled (cm) and mean speed (cm/s) using the 6-OHDA-induced adult zebrafish-based PD model22.
Protocol
The present study has been approved by the Committee on Animal Research and Ethics (CARE), Universiti Technologi MARA (UiTM) [Reference No: UiTM CARE 346/2021, dated 7 May 2021].
NOTE: The published protocols22,25,26 for standard husbandry and maintenance of the 6-OHDA-lesioned adult zebrafish PD model were utilized. Experiments were conducted with adult male zebrafish (Danio rerio) aged more than five months old with a standardized length of 3.2-3.7 cm.
1. Zebrafish maintenance and pre-ICV microinjection preparations
- Maintain the fish in an aerated water tank under a controlled temperature of 28 ± 1.0 °C. For zebrafish husbandry and maintenance, use distilled water mineralized with commercial sea salt (1 g/L) throughout the experiment27.
- House a maximum of 25 fish per 45 L tank or one fish per 1.8 L water and expose them to a schedule of 14 h light and 10 h dark photoperiod. Feed the fish at least twice per day with food pellets supplemented with freeze-dried worms.
- Prepare a concentrated stock solution of tricaine methanesulfonate (MS-222) by dissolving 2.5 g of MS-222 and 5 g of sodium bicarbonate in 250 mL of distilled water. Dilute 2 mL of stock solution to produce 200 mL of working anesthesia solution.
- Prepare 99.96 mM of 6-OHDA by first dissolving 0.2 mg of ascorbic acid in 1 mL of 0.9% w/v sterile-filtered NaCl. Filter the solution with 0.2-micron filter before adding 25 mg of 6-OHDA in powder form into the solution. Prepare the solution fresh before each injection and store it in dark at 4 °C.
CAUTION: Wear appropriate personal protective equipment (i.e., gloves, laboratory coat, and face mask) and practice good laboratory practices when handling the chemicals. All handlings of the chemicals should be done within a biosafety cabinet.
2. Anaesthetisation and ICV injection of zebrafish
- Fast the fish for 24 h to avoid regurgitation during anesthesia. Anaesthetise the fish by immersing it into a container containing 0.01% w/v of MS-222 solution for approximately 1 min or until all visible muscular movement ceases.
- Position the anesthetized fish on a water-soaked sponge placed under a stereomicroscope and wet the fish regularly.
- Identify the position for injection based on the intersection between the metopic suture (MS), coronal suture (CS), and sagittal suture (SS) that connects the frontal and parietal skull of the zebrafish brain.
- Make a small hole of 1.0 mm2 area using a sharp 27 G needle in the skull guided by the specific anatomical position on the zebrafish skull (Figure 1A,B).
- Lower the microcapillary injector at a 60° angle until it reaches a depth of 1,200 µm from the cranial roof of the zebrafish skull (Figure 1C). Press the Z limit to fix the position.
- Set the initial injection pressure to 4000 hPa and compensation pressure to 10 hPa. Set the duration of injection to 0.3 s. Lower the intensity of injection with each subsequent injection.
- Inject 0.5 µL of 99.96 mM neurotoxin 6-OHDA (or 0.9% w/v saline for sham control group) and let the microcapillary rest for 20 s. Continue to wet the fish with distilled water throughout the injection process to prevent drying out.
- Slowly remove the microcapillary and resuscitate the fish under running distilled water. Place the fish in an isolated recovery tank and remove any distractions that can potentially disturb the recovery process.
- Flush the microcapillary before the next injection to clear the blockage and ensure that the intensity of injection is sufficient to yield the desired volume of 0.5 µL of 6-OHDA.
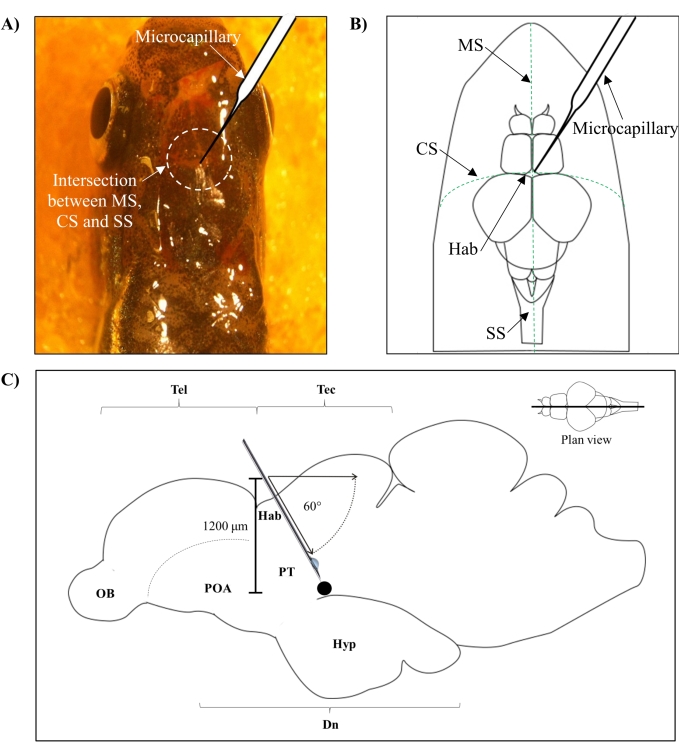
Figure 1: Injection site of neurotoxin, 6-OHDA. (A) The point of microcapillary entry is guided by the intersection between the metopic suture (MS), coronal suture (CS), and sagittal suture (SS) that connects the frontal and parietal skull of the zebrafish brain (plan view). (B) A schematic drawing (plan view) of the zebrafish skull and brain shows the microcapillary, which is lowered directly above the habenula (Hab), and its point of entry at the intersection between hemispheres. (C) A schematic drawing (sagittal section) of the zebrafish brain shows the angle of injection and depth of penetration. The black dot represents the lesioned site that is situated above the targeted area, the ventral diencephalon. Abbreviations: 6-OHDA: 6-hydroxydopamine, CS: coronal suture, Dn: diencephalon, Hab: habenula, Hyp: hypothalamus, MS: metopic suture, OB: olfactory bulb, POA: preoptic area, PT: posterior tuberculum, SS: sagittal suture, Tec: tectum, and Tel: telencephalon. Please click here to view a larger version of this figure.
3. Locomotor assessment
NOTE: Locomotor assessment of zebrafish (n = six / group; sham vs lesioned) was assessed individually via the open tank test using established protocols28,29 at day three and day 30 post-6-OHDA lesion.
- Video recording
- Place the experimental tank (length 20 cm, width 11.5 cm, height 13 cm) with its walls covered with white paper on a raised platform (Figure 2A).
- Illuminate the tank from the bottom using a light source. Fill the tank with distilled water (80%-90% full) and maintain the temperature at 28 ± 1.0 °C. Measure the temperature using a thermometer and regulate it using a commercial aquarium heater.
- After a minimum of 2 min of acclimatization, record the fish swimming behavior from a plan view on the 2-dimensional (2D) plane of the experimental arena using a video camera for 5 min (Figure 2B). To avoid inconsistency in the swimming behavior of the earlier and the last batch of recordings, do not exceed the acclimatization by 10 min30.
- Analyze the videos using video tracking software with the open-tank protocol for the acquisition of distance traveled (cm) and mean speed (cm/s) of each subject.
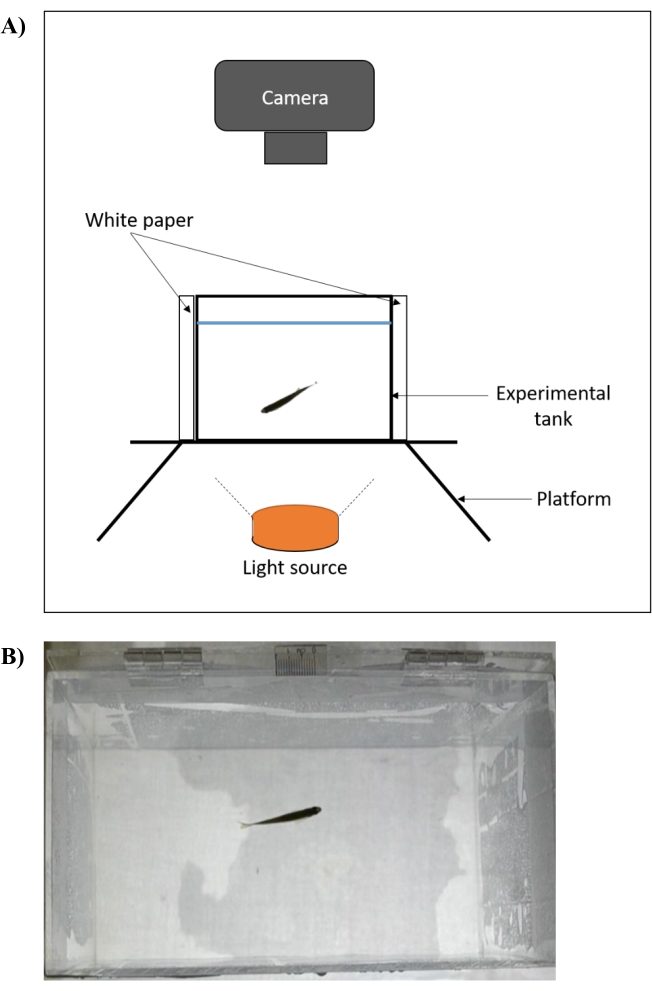
Figure 2: Experimental setup of an open tank test for assessment of zebrafish locomotor behavior. (A) The experimental tank (front view) is placed on a raised platform that is illuminated from below. The four walls of the tank are covered with white paper and the recordings are captured axially. The temperature is measured using a thermometer and regulated at 28 ± 1.0 °C using a commercial aquarium heater. (B) Screenshot (plan view) of video recording that is captured using the setup. Please click here to view a larger version of this figure.
- Data analysis
- Double click on the icon to open the video tracking software. Click on the File tab and select Create New Empty Experiment. This will allow the user to customize the experiment parameters according to the aims of the investigation.
- Click on the Protocol tab, select Video Sources, and click on Add New Video Source. Click on the available drop-down list and select the Video File option. This will prompt the file browsing pop-up from which the video recordings of interest can be selected.
- Click on the Apparatus subtab and select the Rectangular icon to set up the apparatus. Drag the rectangular icon to cover the whole experimental arena. Set the scale bar accordingly and input the numerical value of the scale measurement used in the length of the ruler section. The present experiment used a 10 mm scale for the open tank test.
- Set the animal color by selecting The Animals are Darker than The Apparatus Background. Leave the other available options in Tracking to the preset default settings.
- In the Zones subtab, click on the previously drawn apparatus. This zone is set as the standard zone of which the position is the same for all tests.
- Select the following options under test scheduling and test data report: Test Duration, Total Distance Traveled, and Average Speed. Other available tests on the list are optional and dependent on the researcher's investigative interest.
- In the Experiment tab, assign the animals according to their test group by typing in the group name under the Name section and the number of animals per group in the Number of Animals section.
- Switch to Tests tab to run the experiment. Click on the Start Test icon and wait until all the videos are analyzed.
- In the Risultati tab, click on the View the Report icon to view the locomotor data in text report form.
Representative Results
The present experiment assessed the changes in adult zebrafish swimming behavior following ICV microinjection with 6-OHDA. The reason for using 6-OHDA as the neurotoxin of choice was due to its inability to cross the blood-brain barrier, which produced specific and targeted ablation of DpN in the area of interest-ventral diencephalon (Dn)16. The DpN subpopulation here holds anatomical resemblance to the DpN subpopulation in the human's substantia nigra pars compacta31.
As per our previous work22, the cellular effect of 6-OHDA ICV microinjection against DpN of adult zebrafish was confirmed through immunohistostaining of DpN marker-tyrosine hydroxylase (TH). The main brain region of interest was the Dn, made up of the preoptic area (POA), posterior tuberculum (PT), and hypothalamus (Hyp). It was found that 99.96 mM 6-OHDA resulted in a 100% survival rate of the adult zebrafish with the lowest number of TH-immunoreactive (TH-ir) in Dn. It was also found that more than 85% (p < 0.01) of TH-ir DpN in the Dn was ablated on day three postlesion. The number of TH-ir DpN then increased by more than 50% at day 14 postlesion before achieving full regeneration 30 days postlesion (Figure 3). This data supports the regenerative capabilities of DpN subpopulation in Dn of adult zebrafish following ablation32.
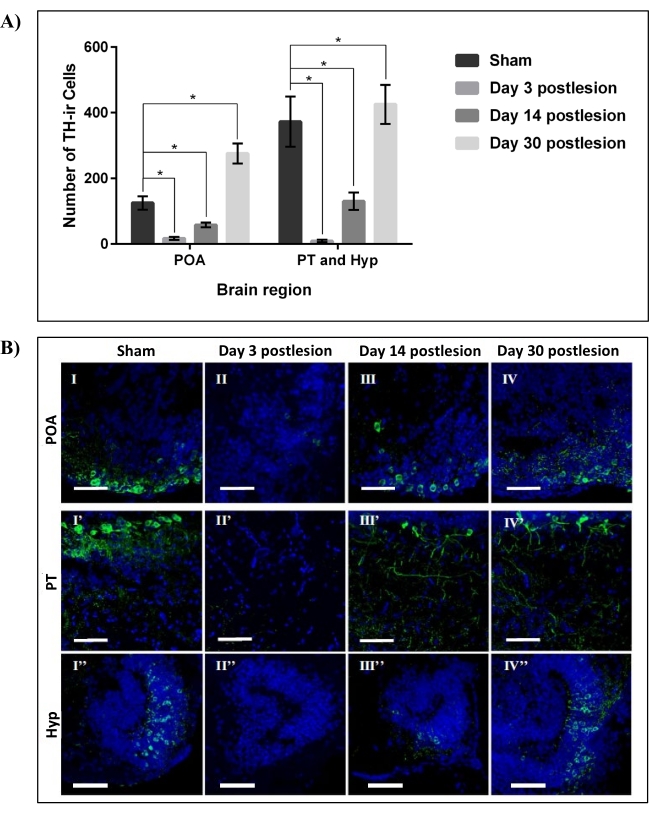
Figure 3: Regeneration of DpN in the Dn region of zebrafish lesioned by 99.96 mM 6-OHDA. (A) The number of TH-ir DpN in three main areas of the Dn region, POA, PT, and Hyp, over four data points: sham, 3, 14, and 30 days post-lesioning by 99.96 mM 6-OHDA neurotoxin. Each bar represents mean ± SD of n = 6 independent experiments; *p < 0.05. (B) Representative confocal microscope images of sagittally sectioned zebrafish brain of sham (I, I', and I''), 3 days post-lesioning (II, II', and II''), 14 days post-lesioning (III, III', and III''), and 30 days post-lesioning (IV, IV', and IV'') stained with TH (DpN; green) and DAPI (nuclei; blue). Scale bar = 50 µm. Abbreviations- DAPI: 4′, 6-diamidino-2-phenylindole, 6-OHDA: 6-hydroxydopamine, Dn: diencephalon, DpN: dopaminergic neurons, Hyp: hypothalamus, POA: preoptic area, PT: posterior tuberculum, SD: standard deviation, and TH-ir: tyrosine hydroxylase immunoreactive. Adapted from Vijayanathan et al.22. Please click here to view a larger version of this figure.
We then performed locomotor assessment using the open tank test to investigate changes in distance traveled (cm) and mean speed (cm/s) of adult zebrafish following ICV microinjection of 6-OHDA and sham. Experimental fish were then assessed on day three postlesion (least number of TH-ir DpN observed) and day 30 postlesion (fully restored DpN reported at lesion site). Analysis of zebrafish swimming behavior using a video tracking software indicated that both the mean speed (cm/s) and distance traveled (cm) of the lesioned group on day three postlesion were significantly reduced (p < 0.001) to <45% when compared to sham (Figure 4). The lesioned group exhibited recovery of motor function 30 days postlesion with no significant difference of both the mean speed (cm/s) and distance traveled (cm) when compared to sham.
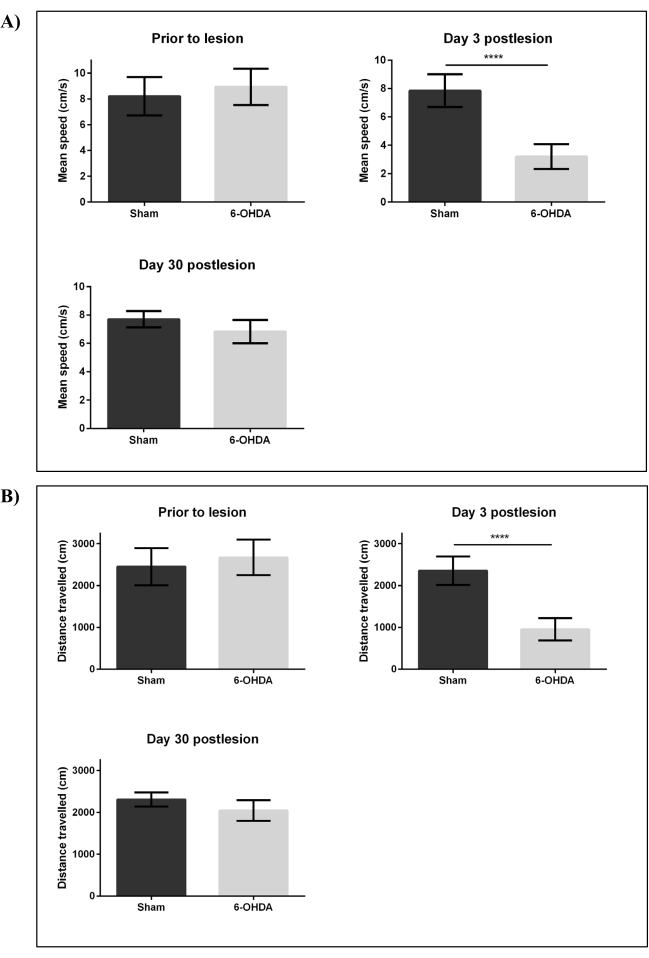
Figure 4: Changes in swimming behavior following intracerebroventricular injection by 6-OHDA. Swimming behavior of adult zebrafish was assessed before lesioning, on day three and day 30 postlesion by 99.96 mM 6-OHDA. Parameters that were assessed included: (A) mean speed (cm/s) and (B) distance traveled (cm). Each bar represents mean ± SD of six fish; ****p < 0.0001 (Student t-test). Abbreviations: 6-OHDA: 6-hydroxydopamine, SD: standard deviation. Please click here to view a larger version of this figure.
Discussion
The present work successfully demonstrated the locomotor assessment of the established 6-OHDA-induced, adult zebrafish-based PD model. The entire experiment involved three major steps: pre-ICV microinjection preparations, ICV microinjection of zebrafish, and locomotor assessment. To ensure the healthy recovery of adult zebrafish following the ICV microinjection procedure and good experimental outcome, some good practices for each step have been recommended in the present study.
Pre-ICV microinjection preparation: Animal selection was best performed the day before the experiment. The gender was identified and the length of the fish was measured. Male adult zebrafish with a standardized length of 3.2-3.7 cm were placed in a separate experimental tank. Additionally, the fish should undergo 24 h fasting period to avoid regurgitation during anaesthesia33. A fish tank (with its four walls covered with white paper) with a standing water tank set up should be prepared before the experiment to lessen the exterior stress and help the recovery process of the zebrafish. All chemicals were prepared fresh before the start of each experiment as they could deteriorate rapidly over time and become unstable at room temperature34,35.
Microinjection of 6-OHDA:Clean and gentle handling of zebrafish should be performed throughout the process to prevent the introduction of unnecessary injury and infection to the fish. The fish should be placed on top of a wet sponge and kept in a moist condition to avoid drying out36. A small incision was made using a sterile needle with firm and appropriate force to avoid extra pressure that may crack the zebrafish skull. This incision should allow entry of the microcapillary into the brain cavity. The microcapillary was then lowered until a depth of 1,200 µm from the entry point, which is in between two hemispheres-the telencephalon and tectum (Figure 1B,C). The entry point was chosen between these hemispheres to prevent any additional laceration of neurons37. This technique involved the use of a microinjector, the pressure and timing of the delivery should be calibrated to ensure delivery of 0.5 µL of neurotoxin. This calibration can be performed by measuring the size of the droplet formed on the filter paper38. Our practice usually involved the following set up of programmable parameters whereby the intensity of injection was lowered with each subsequent injection (injection pressure: 4000 hPa, duration of injection: 0.3 s, and compensation pressure: 10 hPa). In order to avoid the neurotoxin from leaking out of the brain cavity, a 20 s interval was applied between injection and withdrawal of the microcapillary35. Due to the small size of the capillary, the microcapillary may be blocked after each injection. As such, the microcapillary should be essentially flushed prior to the next injection so as to clear the blockage and ensure that the intensity of injection is sufficient to yield the desired volume of 0.5 µL of 6-OHDA. The fish would then be transferred into a recovery tank maintained at 28 ± 1.0 °C. If the fish fails to recover within 30 s, flush out its gill and mouth with distilled water until full recovery of muscle movements occurs.
Locomotor Assessment: To ensure good locomotor assessment on 6-OHDA-induced adult zebrafish, the behavioral study should be conducted within the same time frame for each time point. Each behavioral study should allow for a minimum of 2 min acclimatization period and should be performed within 4 h39. The present experiment was carried out in the early morning between 8 am to 12 pm as zebrafish were more active during this period40. A longer acclimatization period is necessary if zebrafish show any abnormal behavior with clear signs of stress and anxiety (freezing and erratic behavior)41. However, to avoid inconsistency in the swimming behavior of the earlier and the last batch of recordings, the acclimatization should not exceed 10 min30. For the open field test, an experimental tank of any size, color, shape, and texture could be used for recordings that range from a minimum of 5 to 30 min42,43. Zebrafish behavior is greatly influenced by the temperature of its surroundings. Small fluctuations of more than 4 °C could greatly impact the swimming speed44. Hence, the temperature of water in the experimental tank should be strictly maintained under a controlled temperature of 28 ± 1.0 °C using a commercial heater, and the water level was kept around 12 cm deep throughout the experiment. The walls of the tank were covered with white paper to create contrast between the test subject and experimental arena as well as to reduce external stimuli that may cause an unprompted reaction from test subjects45. The fish from each group were tested individually in accordance with the current standard practice for zebrafish neurobehavioural research23,37,46. Given the zebrafish propensity to social interactions, there is concern that isolation during the testing period might impact their behavior47. However, the current experimental setup was limited to a maximum of 10 min per trial and this short period of isolation was found to not have any effect on the locomotor activity of adult zebrafish48. In order to provide accurate data collection for the behavioral study, the assessment was performed by randomly selecting the zebrafish from different experimental groups (i.e., alternating two zebrafish from either the sham and 6-OHDA lesioned group until n = 6) during the open tank test49. The recorded videos were analyzed using a video tracking system that is commonly used for rodents' behavioral tracking. As zebrafish is an emerging animal model, the behavioral tests conducted using zebrafish are usually adapted from the established scientific literature on rodents50. Here, we demonstrated the ability of the video tracking software to automatically track zebrafish in the experimental arena and effectively compute the desired parameters. The video tracking software stood out from the other available software due to the variety of video files supported by the software, regular update package releases, and support provided for different operating systems51.
One of the limitations of existing animal-based PD models is the lack of mechanistic similarities that mimic the motor impairment as observed after dopaminergic neuronal loss in the substantia nigra pars compacta of the human brain52. The emergence of the adult zebrafish-based PD model may, however, address this particular limitation. As observed in the present study, the reduced swimming speed corresponded to our previous cellular findings whereby more than 85% dopaminergic neuronal loss in the ventral diencephalon of 6-OHDA-induced adult zebrafish model three days postlesion22. It appears that specific ablation of dopaminergic neurons in this area of interest is required to disrupt the descending motor signaling from the brain, causing slowness in movement53. L. J. Caldwell, et al.23 who performed ICV injection of 6-OHDA at the optic tectum (point of entry), for instance, observed only changes in zebrafish shoaling and mating behavior. Ablation of DpN in ventral Dn is crucial as the population of DpN in the Dn of adult zebrafish acts as the only dopamine source for zebrafish motor neurons. This is analogous to the human substantia nigra54. The present study also observed faster-swimming speed and longer distance traveled by lesioned zebrafish in later postlesion time points, indicating continued and ultimately full restoration of dopamine signaling that governs zebrafish swimming behavior. These findings thus validated the capability of newly regenerated dopaminergic neurons in regaining their functional activities in lesioned adult zebrafish.
The current application route of 6-OHDA involved a slightly invasive injection paradigm that required insertion of microcapillary deep into the brain, toward the lesion area of ventral Dn. This method is slightly laborious in comparison to peripheral injection and needs to be performed within 3 min per fish to reduce the risk of mortality following injection. As such, prior practice of ICV injection is required to ensure that the method can be performed within the critical duration at the targeted area (Dn). In order to achieve a valid locomotor assessment of adult zebrafish, the open tank test is limited to only 4 h of assessment period per day. Hence, prior planning is required in an experimental framework that involves a large number of animals whereby extra time should be allocated to ensure that the setup met the minimum requirements for each recording (e.g., temperature and water depth). This planning is especially crucial in time-based experiments such as that of the current study, as each recording needs to be performed on the intended timepoint. The present experimental setup was limited to the study of two swimming parameters that specifically assessed the zebrafish motor function. Other behavioral parameters such as shoaling and anxiety-like behavior, however, required other experimental setups and different analytical methods. In summary, this is a reproducible and useful method to study the DpN neuroregeneration process in 6-OHDA-induced adult zebrafish which may yield important insights into cell replacement treatment strategies against PD.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Ministry of Higher Education Malaysia under the Fundamental Research Grant Scheme [600-IRMI/FRGS 5/3 (033/2019)].
Materials
| Materials | |||
| 6-Hydroxydopamine (6-OHDA) | Sigma-Aldrich, Missouri, USA | 162957 | |
| Ascorbic acid | Thermo Fisher Scientific, California, USA | FKC#A/8882/53 | |
| Disposable pasteur pipette, 3 mL | Thermo Fisher Scientific, California, USA | FB55348 | |
| Microcentrifuge tube, 0.2 mL | Eppendorf, Hamburg, Germany | 30124332 | |
| Nice conical flask, 100 mL | Evergreen Engineering & Resources, Semenyih, Malaysia | SumYau0200 | |
| Phosphate buffered saline (PBS) | Sigma-Aldrich, Missouri, USA | P4417 | |
| Sodium bicarbonate | Sigma-Aldrich, Missouri, USA | S5761 | |
| Sodium chloride | Merck, Darmstadt, Germany | 106404 | |
| Stereomicroscope | Nikon, Tokyo, Japan | SMZ745 | |
| Tricaine methanesulfonate (MS-222) | Sigma-Aldrich, Missouri, USA | E10521 | |
| Equipment | |||
| ANY-maze software | Stoelting Co., Illinois, USA | – | version 7.0; video tracking software |
| Cubis II Micro Lab Balance | Sartorius, Göttingen, Germany | SE 2 | |
| FemtoJet IV microinjector | Eppendorf, Hamburg, Germany | 5192000035 | |
| Femtotip II, sterile injection capillary | Eppendorf, Hamburg, Germany | 5242957000 | |
| InjectMan 4 micromanipulator | Eppendorf, Hamburg, Germany | 5192000027 | |
| LED Portable Lamp | MR. DIY, Selangor, Malaysia | 9023251 | 20 mAh |
| PELCO Pro Superalloy, offset, fine tips | Ted Pella, California, USA | 5367-12NM | |
| Shanda aquarium heater | Yek Fong Aquarium, Selangor, Malaysia | SDH-228 | |
| Thermometer | Sera Precision, Heinsberg, Germany | 52525 | |
| Video camera | Nikon, Tokyo, Japan | D3100 |
Riferimenti
- Dorsey, E. R., et al. regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology. 17 (11), 939-953 (2018).
- Maserejian, N., Vinikoor-Imler, L., Dilley, L. Estimation of the 2020 global population of Parkinson’s Disease (PD). Movement Disorder Council. 35 (1), 198 (2020).
- Hirsch, L., Jette, N., Frolkis, A., Steeves, T., Pringsheim, T. The Incidence of Parkinson’s Disease: A Systematic Review and Meta-Analysis. Neuroepidemiology. 46 (4), 292-300 (2016).
- Przedborski, S. The two-century journey of Parkinson disease research. Nature Review Neuroscience. 18 (4), 251-259 (2017).
- Cookson, M. R. . Disease-Modifying Targets in Neurodegenerative Disorders. , 157-174 (2017).
- Jamebozorgi, K., et al. Cellular and molecular aspects of Parkinson treatment: Future therapeutic perspectives. Molecular Neurobiology. 56 (7), 1-13 (2018).
- Parmar, M., Grealish, S., Henchcliffe, C. The future of stem cell therapies for Parkinson disease. Nature Review Neuroscience. 21 (1), 1-13 (2020).
- Foltynie, T. Can Parkinson’s disease be cured by stimulating neurogenesis. Journal of Clinical Investigation. 125 (3), 978-980 (2015).
- Winner, B., Winkler, J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harbour Perspect Biology. 7 (4), 021287 (2015).
- Huang, C., et al. Nerve guidance conduits from aligned nanofibers: improvement of nerve regeneration through longitudinal nanogrooves on a fiber surface. ACS Applied Materials & Interfaces. 7 (13), 7189-7196 (2015).
- Alunni, A., Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development. 143 (5), 741-753 (2016).
- Dietz, V., Schwab, M. E. From the rodent spinal cord injury model to human application: promises and challenges. Journal of Neurotrauma. 34 (9), 1826-1830 (2017).
- La Rosa, C., Bonfanti, L. Brain plasticity in mammals: An example for the role of comparative medicine in the neurosciences. Frontiers in Veterinary Science. 5 (274), 1-8 (2018).
- Ferretti, P., Prasongchean, W. . Neural Stem Cells in Development, Adulthood and Disease. , 1-21 (2015).
- Vijayanathan, Y., et al. Adult endogenous dopaminergic neuroregeneration against Parkinson’s Disease: Ideal animal models. Neurotoxicity Research. 39 (2), 504-532 (2021).
- Vaz, R. L., Outeiro, T. F., Ferreira, J. J. Zebrafish as an animal model for drug discovery in Parkinson’s disease and other movement disorders: a systematic review. Frontier Neuroscience. 9, 347 (2018).
- Nie, S., et al. Small molecule TrkB agonist deoxygedunin protects nigrostriatal dopaminergic neurons from 6-OHDA and MPTP induced neurotoxicity in rodents. Neuropharmacology. 99, 448-458 (2015).
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Research. 318 (1), 215-224 (2004).
- Betarbet, R., Sherer, T. B., Greenamyre, J. T. Animal models of Parkinson’s disease. Bioessays. 24 (4), 308-318 (2002).
- Anichtchik, O. V., Kaslin, J., Peitsaro, N., Scheinin, M., Panula, P. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Journal of Neurochemistry. 88 (2), 443-453 (2004).
- Fiametti, L. O., Correa, C. N., Castro, L. M. d. Peptide profile of zebrafish brain in a 6-OHDA-induced Parkinson model. Zebrafish. 18 (1), 55-65 (2021).
- Vijayanathan, Y., et al. 6-OHDA-lesioned adult zebrafish as a useful Parkinson’s disease model for dopaminergic neuroregeneration. Neurotoxicity Research. 32 (3), 496-508 (2017).
- Caldwell, L. J., et al. Regeneration of dopaminergic neurons in adult zebrafish depends on immune system activation and differs for distinct populations. Journal of Neuroscience. 39 (24), 4694-4713 (2019).
- Zupanc, G. K., Hinsch, K., Gage, F. H. Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. Journal of Comparative Neurology. 488 (3), 290-319 (2005).
- Lawrence, C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture Research. 269 (1-4), 1-20 (2007).
- Reed, B., Jennings, M. Guidance on the Housing and Care of Zebrafish Danio rerio. Royal Society for the Prevention of Cruelty to Animals (RSPCA). , 7-53 (2011).
- Avdesh, A., et al. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. Journal of Visualized Experiments: JoVE. (69), e4196 (2012).
- Altenhofen, S., et al. Tebuconazole alters morphological, behavioral and neurochemical parameters in larvae and adult zebrafish (Danio rerio). Chemosphere. 180, 483-490 (2017).
- Bridi, D., Altenhofen, S., Gonzalez, J. B., Reolon, G. K., Bonan, C. D. Glyphosate and Roundup alter morphology and behavior in zebrafish. Toxicology. 392, 32-39 (2017).
- Wright, D., Krause, J. Repeated measures of shoaling tendency in zebrafish (Danio rerio) and other small teleost fishes. Nature Protocols. 1 (4), 1828-1831 (2006).
- Pienaar, I. S., Götz, J., Feany, M. B. Parkinson’s disease: insights from non-traditional model organisms. Progress in Neurobiology. 92 (4), 558-571 (2010).
- Becker, T., Becker, C. G. Axonal regeneration in zebrafish. Current Opinion in Neurobiology. 27, 186-191 (2014).
- Collymore, C., Tolwani, A., Lieggi, C., Rasmussen, S. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science. 53 (2), 198-203 (2014).
- Katz, E. M., et al. The stability and efficacy of tricaine methanesulfonate (MS222) solution after long-term storage. Journal of the American Association for Laboratory Animal Science. 59 (4), 393-400 (2020).
- Thiele, S. L., Warre, R., Nash, J. E. Development of a unilaterally-lesioned 6-OHDA mouse model of Parkinson’s disease. Journal of Visualized Experiments: JoVE. (60), e3234 (2012).
- Neiffer, D. L., Stamper, M. A. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. Institute for Laboratory Animal Research. 50 (4), 343-360 (2009).
- Barbosa Júnior, A., et al. . Zebrafish Protocols for Neurobehavioral Research. 66, 323-330 (2012).
- Cocchiaro, J. L., Rawls, J. F. Microgavage of zebrafish larvae. Journal of Visualized Experiments: JoVE. (72), e4434 (2013).
- Stewart, A., et al. Modeling anxiety using adult zebrafish: a conceptual review. Neuropharmacology. 62 (1), 135-143 (2012).
- Sykes, D. J., Suriyampola, P. S., Martins, E. P. Recent experience impacts social behavior in a novel context by adult zebrafish (Danio rerio). PLOS ONE. 13 (10), 0204994 (2018).
- Collymore, C., Tolwani, R. J., Rasmussen, S. The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science. 54 (3), 280-285 (2015).
- Grossman, L., et al. Characterization of behavioral and endocrine effects of LSD on zebrafish. Behavioural Brain Research. 214 (2), 277-284 (2010).
- Stewart, A., et al. Homebase behavior of zebrafish in novelty-based paradigms. Behavioural Processes. 85 (2), 198-203 (2010).
- Abozaid, A., Tsang, B., Gerlai, R. The effects of small but abrupt change in temperature on the behavior of larval zebrafish. Physiology and Behavior. 227, 113169 (2020).
- Sekhar, M., Singh, R., Bhat, A., Jain, M. Feeding in murky waters: acclimatization and landmarks improve foraging efficiency of zebrafish (Danio rerio) in turbid waters. Biology Letters. 15 (7), 1-5 (2019).
- Valcarce, D. G., Martínez-Vázquez, J. M., Riesco, M. F., Robles, V. Probiotics reduce anxiety-related behavior in zebrafish. Heliyon. 6 (5), 03973 (2020).
- Tunbak, H., Vazquez-Prada, M., Ryan, T. M., Kampff, A. R., Dreosti, E. Whole-brain mapping of socially isolated zebrafish reveals that lonely fish are not loners. eLife. 9, 55863 (2020).
- Shams, S., Seguin, D., Facciol, A., Chatterjee, D., Gerlai, R. Effect of social isolation on anxiety-related behaviors, cortisol, and monoamines in adult zebrafish. Behavioral Neuroscience. 131 (6), 492-504 (2017).
- Burghardt, G. M., et al. Perspectives – Minimizing observer bias in behavioral studies: A review and recommendations. Ethology. 118 (6), 511-517 (2012).
- Kalueff, A. V., et al. Towards a comprehensive catalog of zebrafish behavior 1.0 and beyond. Zebrafish. 10 (1), 70-86 (2013).
- Franco-Restrepo, J. E., Forero, D. A., Vargas, R. A. A review of freely available, open-source software for the automated analysis of the behavior of adult zebrafish. Zebrafish. 16 (3), 223-232 (2019).
- Beal, M. F. Parkinson’s disease: a model dilemma. Nature. 466 (7310), 8-10 (2010).
- Jha, U., Thirumalai, V. Neuromodulatory selection of motor neuron recruitment patterns in a visuomotor behavior increases speed. Current Biology. 30 (5), 788-801 (2020).
- Reimer, M. M., et al. Dopamine from the brain promotes spinal motor neuron generation during development and adult regeneration. Developmental Cell. 25 (5), 478-491 (2013).

