Measurement of Oxygen Consumption Rate in Acute Striatal Slices from Adult Mice
Summary
Oxygen consumption rate (OCR) is a common proxy for mitochondrial function and can be used to study different disease models. We developed a new method using a Seahorse XF analyzer to directly measure the OCR in acute striatal slices from adult mice that is more physiologically relevant than other methods.
Abstract
Mitochondria play an important role in cellular ATP production, reactive oxygen species regulation, and Ca2+ concentration control. Mitochondrial dysfunction has been implicated in the pathogenesis of multiple neurodegenerative diseases, including Parkinson's disease (PD), Huntington's disease, and Alzheimer's disease. To study the role of mitochondria in models of these diseases, we can measure mitochondrial respiration via oxygen consumption rate (OCR) as a proxy for mitochondrial function. OCR has already been successfully measured in cell cultures, as well as isolated mitochondria. However, these techniques are less physiologically relevant than measuring OCR in acute brain slices. To overcome this limitation, the authors developed a new method using a Seahorse XF analyzer to directly measure the OCR in acute striatal slices from adult mice. The technique is optimized with a focus on the striatum, a brain area involved in PD and Huntington's disease. The analyzer performs a live cell assay using a 24-well plate, which allows the simultaneous kinetic measurement of 24 samples. The method uses circular-punched pieces of striatal brain slices as samples. We demonstrate the effectiveness of this technique by identifying a lower basal OCR in striatal slices of a mouse model of PD. This method will be of broad interest to researchers working in the field of PD and Huntington's disease.
Introduction
Mitochondrial dysfunction has been implicated in several neurological diseases, including Parkinson's disease (PD), Huntington's disease, and Alzheimer's disease1,2,3. PD models such as PINK1 knockout (KO) mice and rats display impaired mitochondrial function4,5,6,7,8,9,10,11. Mitochondria isolated from the striatum (STR) or whole-brain of aged PINK1 KO mouse exhibit defects in complex I7,10,12,13. Directly measuring the oxygen consumption rate (OCR) is one of the most common methods to evaluate mitochondrial function since OCR is coupled with ATP production, the principal function of mitochondria14. Therefore, measuring OCR in disease models or patient-derived samples/tissue can help investigate how mitochondrial dysfunction leads to disease.
Currently, there are several ways to measure mitochondrial OCR, including the Clark electrode and other O2 electrodes, O2 fluorescent dye, and the extracellular flux analyzer15,16,17,18,19. As an advantage, O2 electrode-based methods allow various substrates to be easily added. However, they are insufficient for simultaneously measuring several samples. Compared to traditional O2 electrode-based methods, the extracellular flux analyzer, a commonly used tool for OCR in cell cultures or purified mitochondria, offers improved throughput15,18,20. Nevertheless, all these methods are usually applied to measure OCR in isolated mitochondria or cell cultures6,16,17,19,20,21. The isolation of mitochondria causes inadvertent damage, and extracted mitochondria or cell cultures are less physiologically relevant than intact brain slices22. Even when microelectrodes are used in slices, they are less sensitive and more difficult to operate than in cultured cells23.
To meet these challenges, we developed a method using the XF24 extracellular flux analyzer, which allows for the analysis of multiple metabolic parameters from acute striatal brain slices of mice24. This technique provides continuous direct quantification of mitochondrial respiration via the OCR. In brief, small sections of striatal brain slices are placed into wells of the islet plate, and the analyzer uses oxygen and proton fluorescent-based biosensors to measure the OCR and extracellular acidification rate, respectively17,21,25.
One of the unique features of the analyzer is the four injection wells, which allow the continued measurement of OCR while sequentially injecting up to four compounds or reagents; this enables the measurement of several cellular respiration parameters, such as basal mitochondrial OCR, ATP-linked OCR, and maximal mitochondrial OCR. The compounds injected during the measurements for the protocol shown here were working concentrations of 10 mM pyruvate in the first solution well (port A), 20 µM oligomycin in the second solution well (port B), 10 µM carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) in the third well (port C), and 20 µM antimycin A in the fourth well (port D), based on Fried et al.25. It must be noted that these concentrations were working concentrations, and stock solutions of 10x, 11x, 12x, and 13x were injected into the solution ports A through D, respectively. The purpose of using each solution was as follows: 1) Pyruvate was necessary since, without it, the addition of FCCP would have a decreased OCR response caused by a limitation of available substrates; 2) Oligomycin inhibits ATP synthase and allows the measurement of ATP linked respiration; 3) FCCP uncouples oxidation from phosphorylation and allows the measurement of maximal mitochondrial capacity; 4) Antimycin A inhibits complex III in the electron transport chain and, therefore, allows the measurement of OCR not linked to the mitochondria.
The concentration of oligomycin used was determined based on the following reasons: 1) The recommended dose of oligomycin for most cell types (isolated mitochondria or cell cultures) is 1.5 µM. From experience, usually 3x-10x of the dissociated cells dose is used for the slice experiments since there might be a gradient, and the penetration of solution in the slices takes time. Therefore, the concentration should be in the range of 5 µM to 25 µM. 2) A 20 µM concentration was selected based on Fried et al.25. Higher concentrations were not tried due to the non-specific toxicity of oligomycin. 3) In the report by Underwood et al.26, the authors did a titration experiment for oligomycin and found that doses at 6.25, 12.5, 25, and 50 µg/mL resulted in similar inhibition. The higher concentration of oligomycin (50 µg/mL) did not inhibit more but had a bigger variance. 4) In our observation, the determining factor seems to be the penetrating ability of oligomycin. It is difficult for oligomycin to penetrate the tissue, and that is why it takes at least 7 to 8 cycles to reach the plateau, the maximum response. As long as it reaches the plateau, the inhibition is assumed to be maximal.
A key technical challenge of adapting the extracellular flux analyzer for measuring OCR in striatal slices is to prevent tissue hypoxia. Since the buffer was not oxygenated during the entire duration of measurements (about 4 h), hypoxia was a central issue. This is especially true for thicker tissue samples, where oxygen cannot diffuse throughout the samples. To overcome this problem, slices were sectioned at 150 µm thickness, so that ambient oxygen could penetrate the middle of the brain slices. In addition, 4 mg/mL bovine serum albumin (BSA) was added to the pre-oxygenated artificial cerebrospinal fluid (ACSF) buffer, which facilitated the determination of maximal OCR, as previously suggested23. We examined whether cells were alive. First, Hoechst 33258 (10 µM) and propidium iodide (10 µM) were used to examine whether cells were healthy under these conditions. We then examined whether medium spiny neurons were functionally healthy using patch-clamp recording. We further evaluated whether dopamine (DA) terminals in the striatal slices were functionally healthy by measuring DA release using fast-scan voltammetry. The results showed that striatal slices that were not oxygenated (ACSF/BSA group) were as healthy as the oxygenated control group24.
We then tested different combinations of slice thickness and punch size to determine optimal striatal slice conditions for the flux respiration assay. Dorsal striatal slices with different thicknesses (150 µm and 200 µm) and punch sizes (1.0 mm, 1.5 mm, and 2.0 mm in diameter) were used for OCR analysis using the analyzer. Striatal slices that were 150 µm thick with a punch size of 1.5 mm in diameter had the highest coupling efficiency and OCRs within an optimal range for the analyzer24.
Protocol
All the procedures including animal work were conducted according to national and international guidelines and were approved by the Animal Care and Use Committee of Thomas Jefferson University. Male FVB/NTac mice at the age of 3 to 14 months were used. The following steps were performed in a non-sterile setting, but caution should be taken to keep everything as clean as possible.
NOTE: The method presented here was established and used in the research reported by Zhi et al.24. The experiments described here used a Seahorse XF24 extracellular flux analyzer (see Table of Materials). These methods can be adapted for the XFe24 analyzer, and some results were confirmed using this analyzer.
1. Hydrate cartridge sensors (1 day before the assay)
- Open the extracellular flux assay kit (see Table of Materials) and remove both the sensor cartridge (green) and utility plate (clear; Figure 1A). Place the sensor cartridge aside (sensors up) and do not touch the sensors.
- Add 600 µL of calibrant solution (pH 7.4) to each well of the utility plate. Place the sensor cartridge on top of the utility plate and submerge the sensors in the calibrant solution. Make sure the triangular notch of the utility and sensor cartridge plate are correctly aligned (Figure 1B).
- Seal the extracellular flux assay kit with sealing film to prevent evaporation of the calibrant solution, and then place it in a 37 °C incubator not supplemented with CO2 or oxygen overnight.
2. Prepare the tissue plate (on the day of the assay)
- Open the islet capture microplate and take out the islet plate for tissue sitting.
- Warm an appropriate volume (625 µL per well) of pre-oxygenated modified artificial cerebrospinal fluid (ACSF, composition 140 mM NaCl, 2.5 mM KCl, 2.4 mM CaCl2, 1.3 mM MgSO4, 0.3 mM KH2PO4, 25 mM glucose, 10 mM HEPES, pH 7.2) buffer to 37 °C in a 50 mL tube. Then add BSA to a final concentration of 4 mg/mL to prepare the respiration buffer. In general, 50 mL is enough for one plate.
- Add 625 µL of respiration buffer (pre-oxygenated ACSF buffer with 25 mM glucose and 4 mg/mL BSA) to each well of the islet plate carefully and avoid shaking the plate. Ensure no residual drops are on top of the sensor cartridge and no air bubbles are present in the buffer of each well.
3. Acute striatal slice preparation and excised slice placement
- Decapitate the mouse after cervical dislocation and immediately dissect the brain in 10 mL of ice-cold pre-oxygenated cutting solution (125 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 3.7 mM MgSO4, 0.3 mM KH2PO4, 10 mM glucose, pH 7.4).
- Section coronal striatal slices with a vibratome following the manufacturer's instruction (see Table of Materials) at a thickness of 150 µm in ice-cold, pre-oxygenated cutting solution.
- Recover the slices in 50 mL of oxygenated ACSF (125 mM NaCl, 2.5 mM KCl, 26 mM NaHCO3, 2.4 mM CaCl2, 1.3 mM MgSO4, 0.3 mM KH2PO4, 10 mM glucose, 2 mM HEPES, pH 7.4) and keep in solution for up to 30 min at room temperature (RT).
- After recovery, transfer the slices to a 35 mm x 10 mm Petri dish with 5 mL of respiration buffer.
- Use a stainless-steel biopsy punch (e.g., 1.5 mm diameter) to create a circular piece of tissue in the desired area of the sliced brain. Keep the slice in the buffer while gently pressing down with the punch. Make sure the punch is pushed down hard enough to cut the tissue. Remove the rest of the tissue, lift the punch away, and remove the circular piece of tissue into the buffer.
- Cut the very end of a 1 mL pipette tip to make a hole with a 1.5-2.0 mm diameter and use it to hold and transfer the punched slice to the top of the capture screen. Suction one piece of punched brain tissue and carefully place the tissue onto the mesh side of the capture screen (Figure 2A). The capture screen will be a circular piece of plastic with the mesh attached to one side.
NOTE: The capture screens are included in the microplate pack (see Table of Materials) and are similar to the one used in Schuh et al.23. - Gently use a paper tissue to dry the capture screen briefly. With the moisture removed, the tissue becomes sticky, which allows the slice to attach to the center of the mesh. Do not dry the slice too much as, otherwise, it will be damaged.
- Hold the capture screen slice side down with tweezers and place it into one of the wells of the incubating islet plate (Figure 2B). Be careful not to drop the slice; if so, take the screen out and place a new slice on it.
NOTE: Do not place brain slices into the two background correction wells (A1 and D6) in the islet plate. - Incubate the islet plate at 37 °C in an incubator for at least 30 min to allow temperature and pH equilibration before running the assay.
4. Loading of cartridges with desired compounds
- Dilute the desired compounds in modified ACSF (37 °C) to the final stock concentration of 10x, 11x, 12x, and 13x of the working concentration for ports A to D, respectively, and adjust to pH 7.4. The test will administer the compounds in order from port A to D. For this experiment, the following solutions were used, with their respective working concentration mentioned before them: 10 mM pyruvate (port A), 20 µM oligomycin (port B), 10 µM or other concentrations of FCCP (port C), and 20 µM antimycin A (port D).
- Gently preload 75 µL of the diluted compounds into the appropriate injection ports of the sensor cartridge. Place the tips halfway into the injection ports at a 45° angle with the tip against the wall of the injection port. Tips should not be inserted completely to the bottom of the injection ports as this may cause compound leakage through the port.
- Withdraw the tips from the ports carefully to avoid creating air bubbles. Do not tap any portion of the cartridge to avoid alleviating air bubbles.
- Visually inspect the injection ports for even loading. Ensure all liquid is in the port and no residual drops are present on top of the cartridge.
- Place the sensor cartridge onto the utility plate and put it into an incubator (non-CO2) for 30 min to allow it to heat up to 37 °C again. Handle carefully by only holding onto the utility plate. Move as little as possible.
5. Calibration and performing the assay
- Load the assay template in the software. Press the green START button.
- Make sure to load the correct protocol. The protocol contains a combination of 3 min mix, 3 min wait, and 2 min measure sequences (these three steps form one measurement). Change the distance of the probe head from 26,600 to 27,800 in the hardware settings under instrument settings25. There is no need to change the probe head for the XFe24 analyzer. Press START.
- Load the sensor cartridge on the utility plate into the instrument tray. The notch goes in the front, left corner. Ensure that the plate sits correctly and is flat. Load the drug-filled sensor cartridge into the analyzer for calibration.
- Follow the instructions on the screen in order to calibrate and equilibrate the sensors. This takes around 30 min.
- Once the calibration step is done, remove the calibration plate and replace it with the islet plate (containing the mesh and tissue slices).
- Measure the oxygen consumption in each well of the plate using the assay protocols. The protocol contains a combination of 3 min mix, 3 min wait, and 2 min measure sequences (these three steps form one measurement), at which point the OCR is calculated.
- For the entire experiment, include 4x OCR measurements to create a baseline, followed by the injection of port A (pyruvate) with 4x measurements, then the injection of port B (oligomycin) with 8x measurements, then the injection of port C (FCCP) with 5x measurements, and, finally, the injection of port D (antimycin A) with 6x measurements.
NOTE: This number of measurements after each compound injection is determined depending on when the measurement reaches the plateau. - Analyze the OCR measurement data and the coupling efficiency data.
Representative Results
The first step of this study was to optimize the slice thickness and punch size used to excise a section of the striatum from the slice (Figure 3A). A slice at 150 µm thickness and a 1.5 mm punch size gave the best results determined by the coupling efficiency (Figure 3B–C). As shown in Figure 3B, OCR is relatively stable for 5 h with less than 10% run down. In addition, functional measurements were used, as well as patch-clamp recording of cortical neurons and medium spiny neurons in the striatum, and fast-scan cyclic voltammetry (FSCV) measurement of DA release, to demonstrate that the neurons and terminals were fully functional in this preparation, as shown in Zhi et al.24. Included with this, we then tested different concentrations of FCCP to discover which would give the best response; 10 µM FCCP gave the best readout (Figure 3D1,D2) determined by the spare respiratory capacity:
Spare respiratory capacity = maximal respiration – basal respiration
These slice conditions were used to measure the differences in the OCR of striatal slices from PINK1 KO mice and wild-type (WT) littermates. For the results of PINK1 KO and WT mice, 3-4 mice were used and 4-6 replicates per mouse were used and averaged to obtain one data point.Since PINK1 is a key regulator of mitochondrial function, mitochondrial dysfunction was expected to be found in the KO mice, measured by decreased mitochondrial OCR. Indeed, an age-dependent decrease in OCR was evident in the KO mice compared to their WT controls (Figure 4A,D). Basal OCR was similar in both the KO and WT groups for young mice (3-4 months); however, basal OCR decreased for the KO mice in the old group (10-14 months; Figure 4B,E). Coupling efficiency, determined as below,
Coupling efficiency = [ATP production rate] / [basal respiration rate] × 100
however, decreased in the KO mice for both the young and old groups (Figure 4C,F). This data demonstrates that the sections of striatal tissue from PINK1 KO mice had mitochondrial dysfunction. This dysfunction in the KO mice also started from a young age, indicated by the decreased coupling efficiency, although the basal OCR was only decreased in the old group. This age-dependent decrease was likely a result of accumulating mitochondrial defects caused by the knockout of PINK1.
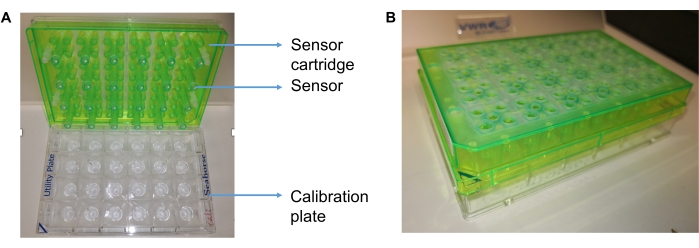
Figure 1: Images showing the extracellular flux analyzer hydrate cartridge sensors and plate. (A) The sensor cartridge sitting on top of a calibration plate (utility plate). (B) Side view of the utility plate and sensor cartridge plate. The triangular notch of the utility and sensor cartridge plate should be correctly aligned. Please click here to view a larger version of this figure.
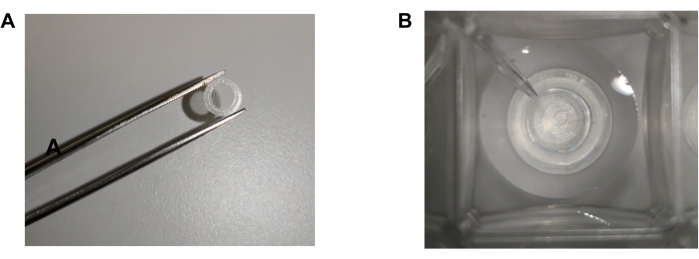
Figure 2: Punched slice attachment and placement. (A) Punched slice is attached to the mesh insert of the capture screen, and then (B) placed into one of the wells of the incubating islet plate carefully to ensure the slice does not detach or move during the measurement. Please click here to view a larger version of this figure.
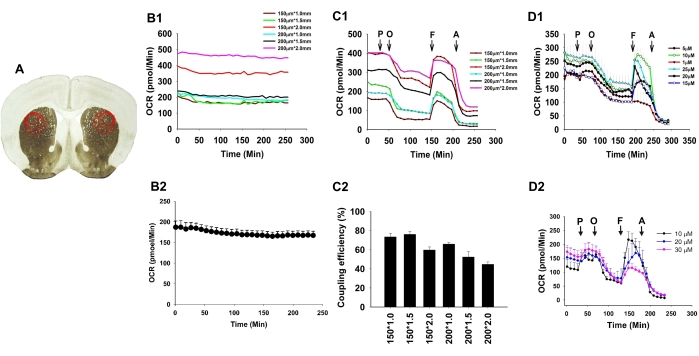
Figure 3: Optimal striatal slice condition for flux respiration assay. (A) Diagram of tissue punch sizes (1.0 mm, 1.5 mm, and 2.0 mm in diameter) for the striatum (STR) with red circles representing the areas that were obtained for the analysis. (B1) O2 consumption rates (OCRs) for different thicknesses and punch sizes of slices in the control group showed stable basal respiration over the whole measurement (4 h), and OCR was proportional to the volume of the slice. OCRs were measured in 150 µm and 200 µm thickness slices punched by 1.0 mm, 1.5 mm, and 2.0 mm punch. The combinations used are mentioned in the legends as thickness * punch size (e.g., 150 µm * 1 mm). (B2) Averaged OCRs measured in 150 µm thickness slices punched by 1.5 mm punch; n = 7. (C1) Representative OCR responses of slices at different thicknesses and diameters with 10 mM pyruvate (P), 20 µM oligomycin (O), 10 µM FCCP (F), and 20 µM antimycin A (A) injected sequentially. The arrows indicate the time point of injection of these compounds. (C2) The mitochondrial coupling efficiency was compared among different groups. Slices of 150 µm * 1.5 mm had the high coupling efficiency but the smallest variance; n = 4. (D1) Titration experiment for FCCP was performed, and 10 µM FCCP gave the biggest spare capacity; n = 4 for each group. (D2) Titration experiments for FCCP were performed with an analyzer, and 10 µM FCCP gave the biggest spare capacity; n = 4 for each group. The values given in the figures are mean ± standard error of the mean (SEM). This figure has been modified from Zhi et al.24. Please click here to view a larger version of this figure.
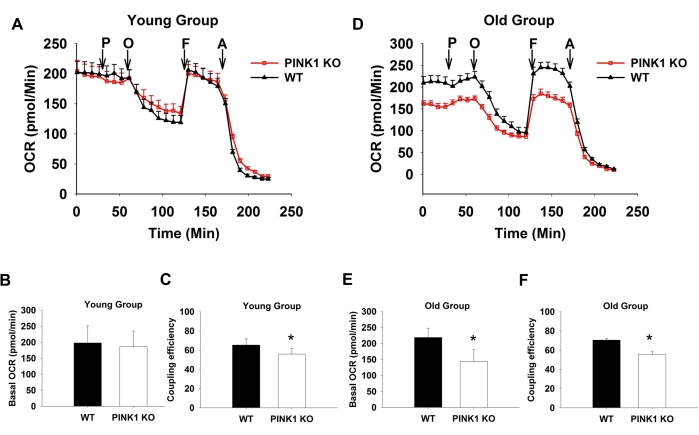
Figure 4: PINK1 KO slices from the old group showing significantly lower OCR. OCRs of acute STR slices (150 µm * 1.5 mm) from mice in the young (A, B, and C) and the old groups (D, E, and F), exposed to successive additions of respiratory modulators (showed using arrows). The OCR of the young group was not significantly different between different genotypes (B), while the coupling efficiency (determined as a percentage value using the formula mentioned above %) was decreased in PINK1 KO slices (C). In the old group, the PINK1 KOslices showed significantly decreased basal respiration level (E) and coupling efficiency (F). The following solution, 10 mM pyruvate (P), 20 µM oligomycin (O), 10 µM FCCP (F), and 20 µM antimycin A (A), was injected sequentially. n = 4 for each genotype and age. The values given in the figures are mean ± SEM. The difference was considered significant when p < 0.05 (*). This figure has been modified from Zhi et al.24. Please click here to view a larger version of this figure.
Discussion
The method we developed allowed an XF analyzer to be used for measuring OCR in striatal slices from adult mice over a time span of 4 h. This method provides a new way to measure cellular bioenergetics in punches excised from anatomically defined brain structures. Since the tissue samples being analyzed are rather small, the metabolic parameters of specific brain areas involved in a disease can be investigated. In addition, using acute slices more closely mimics the physiological cellular environment, which cannot be achieved with isolated mitochondria or cultured cells and organotypic slices. Further, measuring OCR in multiple brain regions from a single animal or across multiple animals in one 24-well plate vastly increases the statistical power of studies that implement this new technique.
This is the first protocol of OCR measurement in acute striatal slices from adult mice. The focus was on the STR as it is one of the brain areas mainly involved in PD and Huntington's disease. The basal OCR and coupling efficiency in our study are comparable to the other studies measuring OCR in acute brain slices using the XF analyzer25,26,27. However, the spare respiratory capacity of striatal slices in our study seems smaller compared to the other reports that measured other brain areas. Still unknown is whether this difference reflects true differences in spare respiratory capacity between brain areas or slight differences in slice preparation, excised slice placement, or mouse strain. These differences warrant further investigation.
There are several critical steps in this protocol, including preparing acute brain slices, transferring the punched slice to the top of the capture screen, and placing the slice into the well. The slices should be kept attached to the capture screen during the measurement. Otherwise, if the slice detaches from the screen and sits in the well of the plate, it will be far away from the oxygen sensor, resulting in a much lower readout of the OCR and responses to the drugs. We recommend at least four replicates (four excised brain slices in the same area) for each condition and excluding the results of the detached slices. OCR should be proportional to tissue content. This study did not measure tissue amount since the brain was sliced at the same thickness, and the slice was punched using the same puncher. Thus, the tissue amount was constant, and there was no need to determine the tissue content. It is critical to use a new puncher for every plate (24 slices) to ensure the sharpness and, thus, the same amount of tissue. In addition, sectioning, preparing tissue punches, and attaching the slices per mouse takes around 30 min and takes around 2 h in total for two pairs of mice. The stability of acute brain slices is only good for 7-8 h after sectioning even at optimal conditions (assessed using sensitive functional assays-patch-clamp recording to evaluate the healthy state of the neurons) and, thus, using a 96 well setup might not be practical.
To illustrate the utility of the technique, we measured the OCR from striatal slices from PINK1 KO mice and their WT littermates. A significant decrease was found in the basal respiration of aged PINK1 KO mice compared to age-matched WT controls, consistent with age-dependent DA release deficits24. This observation also aligns with previously published studies, confirming the validity of this protocol. There are also limitations of the methods. For example, the OCR was measured from the acute striatal brain slice, which is likely brain activity-independent and mostly offers inactive medium spiny neurons and dopaminergic terminals, as well as astrocytes. In addition, the method does not have a cell type-specific resolution.
In summary, this novel method measures OCR in acute brain slices from adult mice and demonstrates how PINK1, a PD-related gene, affects mitochondrial function in mice. This method is easy to implement and widely applicable to researchers working in the field of PD and Huntington's disease.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Wangchen Tsering and Pamela Walter for their critical reading and editing of this manuscript. This work was supported by the National Institute of Neurological Disorders and Stroke (NINDS) (NS054773 to C.J. L. and NS098393 to H.Z.) and the Department of Neuroscience at Thomas Jefferson University (Startup Funds to H.Z.).
Materials
| Accumet AB150 pH benchtop meter | Thermo Fisher Scientific | 13-636-AB150 | To measure pH |
| Antimycin A from streptomyces sp. | SIGMA | A8674 | To inhibit complex III of the mitochondria |
| Bovine Serum Albumin (BSA) | SIGMA | A6003 | To make modified artificial cerebrospinal fluid (BSA-ACSF) |
| Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP) | SIGMA | C2920 | To uncouple mitochondrial respiration |
| D-Glucose | SIGMA | G8270 | To make artificial cerebrospinal fluid (ACSF) |
| DMSO | SIGMA | D8418 | To dissovle compounds |
| HEPES | SIGMA | H3375 | To make artificial cerebrospinal fluid (ACSF) |
| Humidified non-CO2 incubator | Fisher Scientific | 11-683-230D | To hydrate plates at 37 °C |
| Oligomycin from Streptomyces diastatochromogenes | SIGMA | O4876 | To inhibit mitochondrial ATP synthase |
| Parafilm | SIGMA-ALDRICH | sealing film | |
| Rotenone | Tocris | 3616 | To inhibit complex I of the mitochondria |
| Seahorse XF Calibrant Solution 500 mL | Seahorse Bioscience | 103681-100 | Solution for seahorse calibration |
| Seahorse XF Extracellular Flux Analyzer | Seahorse Bioscience | Equipment used to analyze oxygen consumption rate, old generation | |
| Seahorse XFe24 Extracellular Flux Analyzer | Seahorse Bioscience | Equipment used to analyze oxygen consumption rate, new generation | |
| Seahorse XF24 FluxPaks | Seahorse Bioscience | 101174-100 | Package of flux analyzer sensor cartridges, tissue culture plates, capture screens, calibrant solution and calibration plates; assay kit. |
| Sodium pyruvate | SIGMA | P2256 | To prevent any substrate-limiting constraints of substrate supply |
| Stainless steel biopsy punches | Miltex | Device used to punch slices | |
| Sterile cell culture dish, 35 x 10 mm | Eppendrof | 0030700102 | Used for slice punch |
| Vibratome | Leica | VT1200 | To slice brain tissue |
| Water bath | Thermo Scientific Precision | 282-115 | To heat buffer and solutions |
Riferimenti
- Hauser, D. N., Hastings, T. G. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease and monogenic parkinsonism. Neurobiology of Disease. 51, 35-42 (2013).
- Swerdlow, R. H. Mitochondria and mitochondrial cascades in Alzheimer’s disease. Journal of Alzheimer’s Disease: JAD. 62 (3), 1403-1416 (2018).
- Lou, S., et al. Oxygen consumption deficit in Huntington disease mouse brain under metabolic stress. Human Molecular Genetics. 25 (13), 2813-2826 (2016).
- Amo, T., et al. Mitochondrial membrane potential decrease caused by loss of PINK1 is not due to proton leak, but to respiratory chain defects. Neurobiology of Disease. 41 (1), 111-118 (2011).
- Clark, I. E., et al. Drosophila pink1 is required for mitochondrial function and interacts genetically with parkin. Nature. 441 (7097), 1162-1166 (2006).
- Cooper, O., et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Science Translational Medicine. 4 (141), (2012).
- Gautier, C. A., Kitada, T., Shen, J. Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 105 (32), 11364-11369 (2008).
- Gispert, S., et al. Parkinson phenotype in aged PINK1-deficient mice is accompanied by progressive mitochondrial dysfunction in absence of neurodegeneration. PLoS One. 4 (6), 5777 (2009).
- Heeman, B., et al. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. Journal of Cell Science. 124, 1115-1125 (2011).
- Liu, W., et al. Pink1 regulates the oxidative phosphorylation machinery via mitochondrial fission. Proceedings of the National Academy of Sciences of the United States of America. 108 (31), 12920-12924 (2011).
- Villeneuve, L. M., Purnell, P. R., Boska, M. D., Fox, H. S. Early expression of Parkinson’s disease-related mitochondrial abnormalities in PINK1 knockout rats. Molecular Neurobiology. 53 (1), 171-186 (2016).
- Morais, V. A., et al. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 344 (6180), 203-207 (2014).
- Morais, V. A., et al. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Molecular Medicine. 1 (2), 99-111 (2009).
- Mookerjee, S. A., Gerencser, A. A., Nicholls, D. G., Brand, M. D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. The Journal of Biological Chemistry. 292 (17), 7189-7207 (2017).
- Ferrick, D. A., Neilson, A., Beeson, C. Advances in measuring cellular bioenergetics using extracellular flux. Drug Discovery Today. 13 (5-6), 268-274 (2008).
- Jekabsons, M. B., Nicholls, D. G. In situ respiration and bioenergetic status of mitochondria in primary cerebellar granule neuronal cultures exposed continuously to glutamate. The Journal of Biological Chemistry. 279 (31), 32989-33000 (2004).
- Zhang, J., et al. Measuring energy metabolism in cultured cells, including human pluripotent stem cells and differentiated cells. Nature Protocols. 7 (6), 1068-1085 (2012).
- Gerencser, A. A., et al. Quantitative microplate-based respirometry with correction for oxygen diffusion. Analytical Chemistry. 81 (16), 6868-6878 (2009).
- Land, S. C., Porterfield, D. M., Sanger, R. H., Smith, P. J. The self-referencing oxygen-selective microelectrode: detection of transmembrane oxygen flux from single cells. TheJournal of Experimental Biology. 202, 211-218 (1999).
- Sure, V. N., et al. A novel high-throughput assay for respiration in isolated brain microvessels reveals impaired mitochondrial function in the aged mice. Geroscience. 40 (4), 365-375 (2018).
- Sperling, J. A., et al. Measuring respiration in isolated murine brain mitochondria: implications for mechanistic stroke studies. Neuromolecular Medicine. 21 (4), 493-504 (2019).
- Picard, M., et al. Mitochondrial structure and function are disrupted by standard isolation methods. PLoS One. 6 (3), 18317 (2011).
- Schuh, R. A., et al. Adaptation of microplate-based respirometry for hippocampal slices and analysis of respiratory capacity. Journal of Neuroscience Research. 89 (12), 1979-1988 (2011).
- Zhi, L., et al. Loss of PINK1 causes age-dependent decrease of dopamine release and mitochondrial dysfunction. Neurobiology of Aging. 75, 1-10 (2019).
- Fried, N. T., Moffat, C., Seifert, E. L., Oshinsky, M. L. Functional mitochondrial analysis in acute brain sections from adult rats reveals mitochondrial dysfunction in a rat model of migraine. American Journal of Physiology. Cell Physiology. 307 (11), 1017-1030 (2014).
- Qi, G., Mi, Y., Yin, F. Characterizing brain metabolic function ex vivo with acute mouse slice punches. STAR Protocols. 2 (2), 100559 (2021).
- Underwood, E., Redell, J. B., Zhao, J., Moore, A. N., Dash, P. K. A method for assessing tissue respiration in anatomically defined brain regions. Scientific Reports. 10 (1), 13179 (2020).

