Development and Functionalization of Electrolyte-Gated Graphene Field-Effect Transistor for Biomarker Detection
Summary
The present protocol demonstrates the development of electrolyte-gated graphene field-effect transistor (EGGFET) biosensor and its application in biomarker immunoglobulin G (IgG) detection.
Abstract
In the current study, graphene and its derivatives have been investigated and used for many applications, including electronics, sensing, energy storage, and photocatalysis. Synthesis and fabrication of high quality, good uniformity, and low defects graphene are critical for high-performance and highly sensitive devices. Among many synthesis methods, chemical vapor deposition (CVD), considered a leading approach to manufacture graphene, can control the number of graphene layers and yield high-quality graphene. CVD graphene needs to be transferred from the metal substrates on which it is grown onto insulating substrates for practical applications. However, separation and transferring of graphene onto new substrates are challenging for a uniform layer without damaging or affecting graphene's structures and properties. Additionally, electrolyte-gated graphene field-effect transistor (EGGFET) has been demonstrated for its wide applications in various biomolecular detections because of its high sensitivity and standard device configuration. In this article, poly (methyl methacrylate) (PMMA)-assisted graphene transferring approach, fabrication of graphene field-effect transistor (GFET), and biomarker immunoglobulin G (IgG) detection are demonstrated. Raman spectroscopy and atomic force microscopy were applied to characterize the transferred graphene. The method is shown to be a practical approach for transferring clean and residue-free graphene while preserving the underlying graphene lattice onto an insulating substrate for electronics or biosensing applications.
Introduction
Graphene and its derivatives have been investigated and used for many applications, including electronics1,2, sensing3,4,5, energy storage6,7, and photocatalysis1,6,8. Synthesis and fabrication of high quality, good uniformity, and low defects graphene are critical for high-performance and highly sensitive devices. Since the development of Chemical vapor deposition (CVD) in 2009, it has shown colossal promise and set its place as an essential member of the graphene family9,10,11,12,13. It is grown on a metal substrate and, later for practical uses, is transferred onto insulating substrates14. Several transferring methods have been used to transfer CVD graphene recently. The poly (methyl methacrylate) (PMMA) assisted method is the most used among the different techniques. This method is particularly well-suited for industrial usage because of its large-scale capability, lower cost, and high quality of the transferred graphene14,15. The critical aspect of this method is getting rid of the PMMA residue for CVD graphene's applications because the residues can cause declination of the electronic properties of graphene14,15,16, cause an effect on biosensors' sensitivity and performance17,18, and create significant device-to-device variations19.
Nanomaterials-based biosensors have been significantly investigated over the past decades, including silicon nanowire (SiNW), carbon nanotube (CNT), and graphene20. Because of its single-atom-layer structure and distinctive properties, graphene demonstrates superior electronic characteristics, good biocompatibility, and facile functionalization, making it an attractive material for developing biosensors14,21,22,23. Due to field-effect transistors (FET) characteristics such as high sensitivity, standard configuration, and cost-effective mass producibility21,24, FET is more preferred in portable and point-of-care implementations than other electronics-based biosensing devices. The electrolyte-gated graphene field-effect transistor (EGGFET) biosensors are examples of the stated FETs21,24. EGGFET can detect various targeting analytes such as nucleic acids25, proteins24,26, metabolites27, and other biologically relevant analytes28. The technique mentioned here ensures the implementation of CVD graphene in a label-free biosensing nanoelectronics device which offers higher sensitivity and accurate time detection over other biosensing devices29.
In this work, an overall process for developing an EGGFET biosensor and functionalizing it for biomarker detection, including transferring CVD graphene onto an insulating substrate, Raman, and AFM characterizations of the transferred graphene, are demonstrated. Furthermore, fabrication of EGGFET and integration with a polydimethylsiloxane (PDMS) sample delivery well, bioreceptor functionalization, and successful detection of human immunoglobulin G (IgG) from serum by spike-and-recovery experiments are also discussed here.
Protocol
1. Transferring chemical vapor deposition of graphene
- Cut the graphene sheet on a copper substrate in half (2.5 cm x 5 cm) using scissors. Apply heat resistive tape to fix the four corners of the graphene square on a spinner gasket (see Table of Materials).
NOTE: The purchased graphene has a dimension of 5 cm x 5 cm (see Table of Materials). - Spin-coat the sheet of the graphene with a thin layer (100-200 nm) of PMMA 495K A4 spinning at 500 rpm for 10 s and then 2000 rpm for 50 s. Then bake the sample at 150 °C for 5 min.
- Remove the backside of the graphene with oxygen plasma (see Table of Materials) at 30 W, 15 sccm for 5 min.
- Cut the plasma-treated graphene square into smaller dimensions (1 cm x 2 cm) for device fabrication.
- Cut the pre-cleaned substrate (SiO2) into small pieces with an approximate dimension of 2.5 cm x 2 cm.
- Etch the copper off using the graphene commercial etchant (Ferric chloride) (see Table of Materials). Do not dilute the etchant. Float the sample with the copper side down and the PMMA side up on the liquid etchant.
- After copper etching, lift the graphene film slowly using the plasma-treated substrate.
- Air-dry the transferred graphene for 2 h and then bake at 80 °C for 15 min.
- Remove the PMMA following the steps below.
- Warm up the sample with acetone vapor at 70 °C. Keep the sample at ~2 cm above acetone vapor for 4 min with the PMMA side facing down. Then immerse the sample in acetone for 5 min.
- Wash the sample with DI water cautiously and observe the transferred graphene under a microscope. Finally, gently blow-dry the sample with N2.
- Perform Atomic force microscopy (AFM) observation to ensure PMMA residue-free graphene. If PMMA residue is visible in the image, perform the acetone vapor cleaning and immersion once again.
- Perform Raman and AFM characterization to confirm the monolayer of graphene transferring and observe the surface properties (Figure 1A,B).
2. Fabrication of Graphene Field Effect Transistor (GFET)
- Wash the substrate with the transferred graphene using acetone, IPA, and DI water; then bake the substrate on a hot plate at 75 °C for 30 min (Figure 2A).
- Using the E-beam evaporator30 (see Table of Materials), deposit 5 nm nickel and 45 nm gold on the graphene sample (Figure 2B).
- Apply the first photolithography30 process using mask A (Supplementary Figure 1) for the patterning of the electrodes (Figure 2C).
- Spin a positive photoresist (AZ 5214E, see Table of Materials) on the sample (2000 rpm for 45 s) and cure the sample at 120 °C for 1 min.
- Place the sample in the UV flood exposure system and expose it for ~10 s under 200 mJ/cm2.
- Develop the sample with a photoresist developer (AZ300 MIF, see Table of Materials) for ~2 min, and then rinse with DI water.
- Immerse the sample in a gold etchant to etch the gold layer for 10 s; rinse with DI water and remove the remaining photoresist layer by immersing in acetone for 10 min (Figure 2C).
- Using acetone, IPA, and DI water, wash the sample; bake on a hot plate at 75 °C for 30 min. Then apply the second photolithography process using mask B (Supplementary Figure 1) to pattern the graphene channels.
NOTE: Use the same process parameters as the first one (step 2.4-2.6), except the UV exposure system in the mask aligner (Figure 2D). - Immerse the sample in nickel etchant at 60 °C to etch the nickel layer for 10 s; rinse with DI water; blow dry using N2 (Figure 2D).
- Place the sample in the plasma asher and remove the exposed graphene using oxygen plasma (100 W for 90 s with oxygen flow at 49 sccm); after that, remove the photoresist layer by immersing in acetone for 10 min (Figure 2E).
- Wash the sample using acetone, IPA, and DI water; bake on a hot plate at 75 °C for 30 min and apply the third photolithography process using mask C (Supplementary Figure 1) for the patterning of the passivation photoresist layer to protect the underlying graphene on the substrate. Use the same process parameters as the first one (step 2.4-2.6), except the UV exposure system in the mask aligner (Figure 2F).
- After the third photolithography process, immerse the sample in nickel etchant at 60 °C for 10 s to remove the remaining nickel layer; then rinse with DI water and blow dry using N2 (Figure 2G). Finally, bake the sample on a hotplate at 120 °C for 30 min (Figure 2H).
3. Functionalization of GFET for IgG Detection
- Assemble the sample-delivery channel.
- Fabricate the sample-delivery channel in PDMS using soft lithography techniques31.
- Immerse the graphene device in 0.1 M of NaOH solution for 30 s; rinse with DI water and leave a thin water layer on the device surface to assist PDMS well's alignment and bonding. Then activate the PDMS well's surface using oxygen plasma.
- Align the sample delivery channel and the graphene device under a microscope; place the aligned device in a 60 °C oven for 3 h to allow the bonding. The assembled device is shown in Figure 3A.
- Functionalize the GFET.
- Functionalize the graphene surface with IgG aptamer (see Table of Materials). Use pipettes to load and remove each reagent or buffer from the PDMS well. The schematic process is shown in Figure 4.
NOTE: The following steps were operated at room temperature. - After rinsing the graphene surface with DMSO three times, apply 1-pyrene butyric acid N-hydroxysuccinimide ester (PBASE, 10 mM dissolved in DMSO, see Table of Materials) and keep for 2 h.
- After rinsing with DMSO, apply 5'amino-modified IgG aptamer (20 µM in 1x PBS), incubate for 3 h, and rinse with 1x PBS three times.
- Apply bovine serum albumin (BSA, 10% w/v in 1x PBS) on graphene for 1 h and rinse with 1x PBS three times.
- Functionalize the graphene surface with IgG aptamer (see Table of Materials). Use pipettes to load and remove each reagent or buffer from the PDMS well. The schematic process is shown in Figure 4.
4. IgG detection
- Rinse the device with 0.01x PBS three times. Fill the PDMS well with 0.01x PBS (detection buffer) (Figure 3A,B).
- Connect the electrodes with a high-performance parameter analyzer (see Table of Materials). Connect the source electrode to the ground, the drain, and the gate electrodes to Source Measurement Units (SMU 1 and SMU 2) equipped with the parameter analyzer, respectively (Figure 3C).
- Set up the measurement parameters and turn on the sampling process.
- Test the response of the EGGFET to IgG by continuously monitoring the drain current. Dissolve IgG in 0.01x PBS with different concentrations, add the solution into the detection chamber, and monitor the drain current continuously. Save the data.
Representative Results
The representative results show the transferred CVD graphene characterized by Raman and AFM, respectively. The G peak and the 2D peaks of the Raman image give comprehensive information regarding the existence and the quality of the transferred monolayer graphene32 (Figure 1). Standard lithography processes30,31 were applied for fabricating the GFET device, as shown in Figure 2. Figure 3 shows the fabricated GFET with assembled PDMS sample delivery wells and the experimental setup. The PDMS was mixed at a weight ratio of 10:1 and cast into a Petri dish. Then the whole dish with PDMS mixture was baked in an oven at 60 °C for 3 h. The cured PDMS was peeled off the dish and trimmed to a cube (1 cm x 1 cm × 1 cm). The well (6 mm diameter) was then created by punching the PDMS cube with a puncher.
Schematic functionalization processes for IgG detection by EGGFET are shown in Figure 4, and Figure 5 shows the IgG detection under different electrolyte conditions24. PBASE, a widely used functionalization reagent for graphene, can be adsorbed on graphene surface through a π-π interaction24 without damaging graphene's electrical properties (Figure 4A). A 5′amino-modified IgG aptamer is conjugated with PBASE by the amide bond linkages between the reactive N-hydroxysuccinimide (NHS) ester in PBASE and the amine group on the 5′ end of the IgG aptamer (Figure 4B). Bovine serum albumin (BSA) incubation, a standard approach for biosensor detection, was used to block the remaining unconjugated sites after rinsing the device with 1x PBS (Figure 4C). A more detailed discussion can be found in our previously published work24. The Ag/AgCl reference electrode was applied to define the gate potential during the detection. The detection range, the concentration range that a sensor can reliably measure, is determined to be around ~2-50 nM for the EGGFET device. More detailed discussions for chemical and measurement principles involved in IgG detection and EGGFET's sensitivity and detection limit were reported previously24.
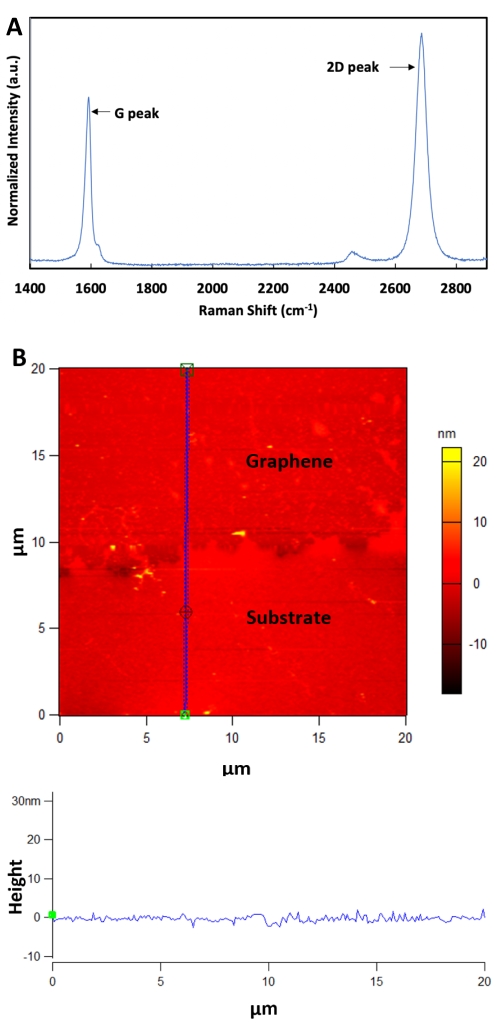
Figure 1: CVD graphene is characterized by Raman and AFM spectroscopy. (A) Representative Raman spectrum of the transferred graphene. The G peak and the 2D peaks are the predominant peaks of pristine graphene. (B) Representative AFM image of the graphene. The corresponding height profiles in the AFM image are shown in the bottom panel along the blue dashed line. Please click here to view a larger version of this figure.
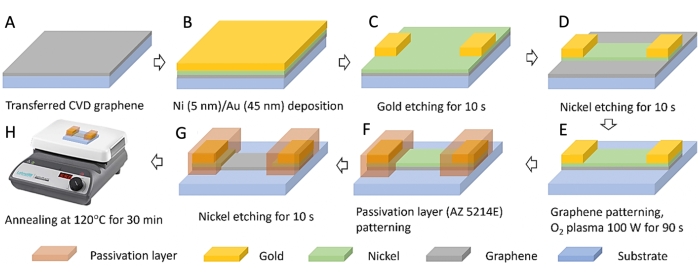
Figure 2: Schematic fabrication of graphene field-effect transistor. (A) Monolayer graphene transferred onto silicon dioxide substrates. (B) Nickel and Gold deposited on transferred graphene. (C) Gold etched after the first photolithography process. (D) Nickel etched after the second photolithography process. (E) Removing unprotected graphene using oxygen plasma. (F) Coating the pattern with photoresist for passivation layering and performing the third photolithography process. (G) Nickel etched after the third photolithography process. (H) Annealing after etching nickel. Please click here to view a larger version of this figure.
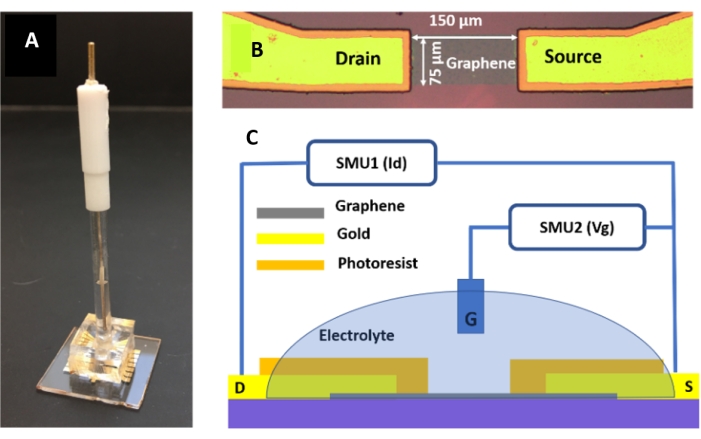
Figure 3: Device and experimental setup for IgG detection. (A) The EGGFET biosensor integrated with a standard Ag/AgCl reference electrode and a PDMS well for containing the sample. (B) The enlarged view of the graphene channel. (C) The schematic diagram of the circuit connection for detecting IgG using the EGGFET biosensor. Please click here to view a larger version of this figure.
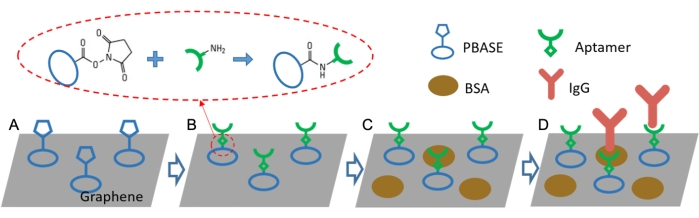
Figure 4: Functionalization of the graphene surface for IgG detection. Reprinted with permission from Reference24. Please click here to view a larger version of this figure.
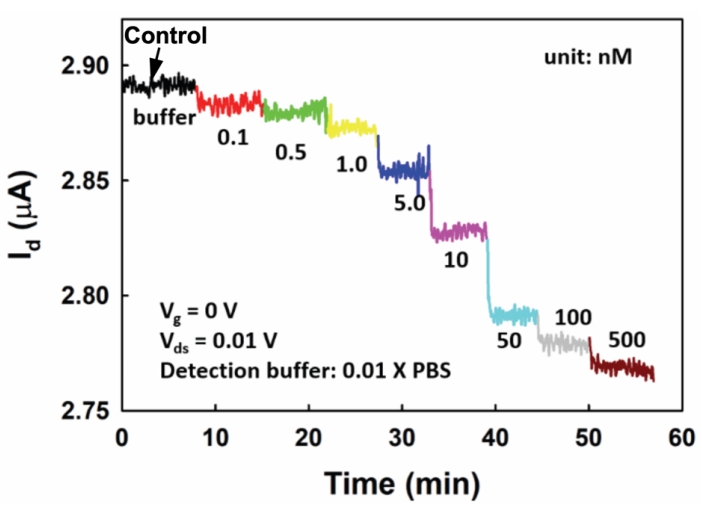
Figure 5: The response of the EGGFET biosensor to biomarker IgG under different diluents. Reprinted with permission from Reference24. Please click here to view a larger version of this figure.
Supplementary Figure 1: Mask designs used for photolithography processes. (A) The mask design used in the first photolithography process. The electrodes are given with dimensions in the enlarged image A1. (B) Mask design used in the second photolithography with dimensions. (C) Mask design used in the third photolithography process. The electrodes are given with dimensions in the enlarged image C1. (D) The final product of all three photolithography processes and the enlarged image D1 shows the electrode configurations. The units for the dimensions are in millimeters (mm). Please click here to download this File.
Discussion
The purchased CVD graphene on copper film needs to be trimmed to the right size for the following fabrication steps. Cutting of the films can cause wrinkling, which needs to be prevented. The parameters provided in the fabrication step can be referred to for plasma etching of graphene, and these numbers could be varied when using different instruments. The etched sample must be closely monitored and inspected to ensure complete graphene etching. Multiple pre-cleaning methods can be applied to clean the substrates, such as sonication in acetone, IPA, and DI water for 5 min, DI water rinsing, and nitrogen gas drying or treatment with O2 plasma (300 W, at ~100 sccm for 5 min). The copper etching rate is about 1.25-1.67 micron/min while using the commercial ferric chloride copper etchant. Close observation is necessary for the etching process. Following the etching, a sufficient rinsing with DI water is needed.
The acetone cleaning technique mentioned in the protocol is the optimum residue cleaning technique. Plasma cleaning has the risk of harming the monolayer graphene. So, the most graphene layer-friendly technique is acetone cleaning. But removing PMMA residue is also of primary importance as it affects the latter processes. Doing Raman spectroscopy and AFM can give the real-time quality of graphene and the PMMA residue. The instruments and the chemicals used in the protocol are critical as these directly influence the quality of the fabricated device. So, the quality of the instruments and the validity of the chemicals need to be checked and updated.
PBASE needs to be kept dry and stored in -20 °C freezer to avoid hydrolysis for bioreceptor functionalization. The stored vial needs to reach room temperature before opening it; otherwise, water could condense inside the vial and hydrolyze the PBASE. To make 10 mM of PBASE, 100 mM of PBASE solution needs to be prepared first by dissolving 38.5 mg of PBASE in 1 mL of DMSO and then diluting it by a factor of 10.
Because the reagents and buffers were added or removed by pipetting directly into the PDMS well, the device demonstrated in the manuscript would not allow for an in-site calibration with negative control. A multichannel array integrated with a properly designed microfluidic device would be necessary for this purpose. Further development of the device, such as combining it with a lateral flow platform, would provide great potential for point-of-care applications33. In addition, the interface between solid and liquid is a topic of great scientific and technological importance34. For example, in the particular case of aqueous media and graphene, it plays a crucial role in many emerging applications of graphene, e.g., analytical chemistry35, energy storage and conversion36, water filtration37, and biosensing38. Unraveling the behavior at the interface has essential scientific and technical significance, especially for an accurate and more in-depth understanding of graphene’s properties and practical applications39,40.
In the present work, an in-detail protocol is provided to demonstrate the development of the EGGFET biosensor and its application in biomarker detection. For practical uses of CVD graphene transferred by the PMMA approach, it is critical to remove PMMA residues completely to get a clean surface. The method effectively removes PMMA residues while preserving the underlying graphene lattice. The functional device shows consistent results for detecting human IgG. Interested researchers could use this protocol as a reference to build devices for specific applications, such as studying interface interactions, biosensing, developing similar devices using other nanomaterials, etc.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The experiments were conducted at West Virginia University. We acknowledge the Shared Research Facilities at West Virginia University for device fabrication and material characterization. This work was supported by the US National Science Foundation under Grant No. NSF1916894.
Materials
| 1-pyreneutyric acid N- hydroxysuccinimide ester | Sigma Aldrich | 457078-1G | functionalization |
| Asylum MFP-3D Atomic Force Microscope | Oxford Instruments | graphene characterization | |
| AZ 300 MIF | MicroChemicals | AZ 300 MIF | photoresist developer |
| AZ 300 MIF | MicroChemicals | AZ 300 MIF | photoresist |
| Bovine Serum Albumin | Sigma Aldrich | 810014 | blocking |
| Branson 1210 Sonicator | SONITEK | sample cleaning | |
| Copper Etchant | Sigma Aldrich | 667528-500ML | removing copper film to release graphene |
| Dimethyl Sulfoxide (DMSO) | VWR | 97063-136 | functionalization |
| Disposable Biopsy Punches, Integra Miltex | VWR | 21909-144 | create well in PDMS |
| Gold etchant | Gold Etch, TFA, Transene | 658148 | enchant |
| Graphene | Graphene supermarket | 2" x 2" sheet | biosensing element of the device |
| IgG aptamer | Base Pair Biotechnologies | customized | bioreceptor |
| Keithley 4200A-SCS Parameter Analyzer | Tektronix | measurement and detection | |
| KMG CR-6 | KMG chemicals | 64216 | Chromium etchant |
| Kurt J. Lesker E-beam Evaporator | Kurt J. Lesker | metal deposition | |
| Laurell Technologies 400 Spinners | Laurell Technologies | WS-400BZ-6NPP/LITE | thin film coating |
| March PX-250 Plasma Asher | March Instruments | sample cleaning | |
| Nickel etchant | Nickel Etchant, TFB, Transene | 600016000 | etchant |
| OAI Flood Exposure | OAI | photolithography | |
| Phosphate Buffered Saline (PBS) | Sigma Aldrich | 806552-500ML | buffer |
| PMMA 495K A4 | MicroChemicals | PMMA 495K A4 | Photoresist for assisting graphene transferring |
| Polydimethylsiloxane (PDMS) | Sigma Aldrich | Sylgard 184 | sample delivery well |
| Renishaw InVia Raman Microscope | Renishaw | graphene characterization | |
| Sodium Hydroxide (NaOH) | Sigma Aldrich | 221465-25G | functionalization |
| Suss Microtech MA6 Mask Aligner | Suss MicroTec | photolithography | |
| Thermo Scientific Cimarec Hotplate | Thermo Scientific | SP131635 | sample and device Baking |
Riferimenti
- Saini, D. Synthesis and functionalization of graphene and application in electrochemical biosensing. Nanotechnology Reviews. 5 (4), 393-416 (2016).
- Emtsev, K. V., Bostwick, A., Horn, K., et al. Towards wafer-size graphene layers by atmospheric pressure graphitization of silicon carbide. Nature Materials. 8 (3), 203-207 (2009).
- Wang, Y., et al. Electrochemical delamination of CVD-grown graphene film: Toward the recyclable use of copper catalyst. ACS Nano. 5 (12), 9927-9933 (2011).
- Carvalho Fernandes, D. C., Lynch, D., Berry, V. 3D-printed graphene/polymer structures for electron-tunneling based devices. Scientific Reports. 10 (1), 1-8 (2020).
- Gao, L., et al. Repeated growth and bubbling transfer of graphene with millimetre-size single-crystal grains using platinum. Nature Communications. 3, 699 (2012).
- Singh, J., Rathi, A., Rawat, M., Gupta, M. Graphene: From synthesis to engineering to biosensor applications. Frontiers of Materials Science. 12 (1), 1-20 (2018).
- Randviir, E. P., Brownson, D. A. C., Banks, C. E. A decade of graphene research: Production, applications and outlook. Materials Today. 17 (9), 426-432 (2014).
- Suvarnaphaet, P., Pechprasarn, S. Graphene-based materials for biosensors: A review. Sensors (Switzerland). 17 (10), 2161 (2017).
- Li, X., Cai, W., An, J., et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science. 324 (5932), 1312-1314 (2009).
- Yu, Q., Lian, J., Siriponglert, S., Li, H., Chen, Y. P., Pei, S. S. Graphene segregated on Ni surfaces and transferred to insulators. Applied Physics Letters. 93 (11), 113103 (2008).
- Xu, S. C., et al. Direct synthesis of graphene on SiO2 substrates by chemical vapor deposition. CrystEngComm. 15 (10), 1840-1844 (2013).
- Zhang, C., et al. Facile synthesis of graphene on dielectric surfaces using a two-temperature reactor CVD system. Nanotechnology. 24 (39), 395603 (2013).
- Zhang, C., et al. Direct formation of graphene-carbon nanotubes hybrid on SiO2 substrate via chemical vapor deposition. Science of Advanced Materials. 6 (2), 399-404 (2014).
- Sun, J., Finklea, H. O., Liu, Y. Characterization and electrolytic cleaning of poly(methyl methacrylate) residues on transferred chemical vapor deposited graphene. Nanotechnology. 28 (12), 125703 (2017).
- Lin, Y. C., Lu, C. C., Yeh, C. H., Jin, C., Suenaga, K., Chiu, P. W. Graphene annealing: How clean can it be. Nano Letters. 12 (1), 414-419 (2012).
- Pirkle, A., et al. The effect of chemical residues on the physical and electrical properties of chemical vapor deposited graphene transferred to SiO2. Applied Physics Letters. 99 (12), 122108 (2011).
- Chen, T. Y., et al. Label-free detection of DNA hybridization using transistors based on CVD grown graphene. Biosensors and Bioelectronics. 41 (1), 103-109 (2013).
- Xu, S., et al. Direct growth of graphene on quartz substrates for label-free detection of adenosine triphosphate. Nanotechnology. 25 (16), 165702 (2014).
- Dan, Y., Lu, Y., Kybert, N. J., Luo, Z., Johnson, A. T. C. Intrinsic response of graphene vapor sensors. Nano Letters. 9 (4), 1472-1475 (2009).
- Zhang, A., Lieber, C. M. -. Nano-Bioelectronics. Chemical Reviews. 116 (1), 215-257 (2015).
- Forsyth, R., Devadoss, A., Guy, O. J. Graphene Field effect transistors for biomedical applications: Current status and future prospects. Diagnostics (Basel). 7 (3), 45 (2017).
- Dankerl, M., et al. Graphene solution-gated field-effect transistor array for sensing applications. Advanced Functional Materials. 20 (18), 3117-3124 (2010).
- He, Q., Wu, S., Yin, Z., Zhang, H. Graphene -based electronic sensors. Chemical Science. 3 (6), 1764-1772 (2012).
- Sun, J., Liu, Y. Matrix effect study and immunoassay detection using electrolyte-gated graphene biosensor. Micromachines. 9 (4), 142 (2018).
- Mohanty, N., Berry, V. Graphene-based single-bacterium resolution biodevice and DNA transistor: Interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Letters. 8 (12), 4469-4476 (2008).
- Ohno, Y., Maehashi, K., Yamashiro, Y., Matsumoto, K. Electrolyte-gated graphene field-effect transistors for detecting pH and protein adsorption. Nano Letters. 9 (9), 3318-3322 (2009).
- Huang, Y., Dong, X., Shi, Y., Li, C. M., Li, L. J., Chen, P. Nanoelectronic biosensors based on CVD grown graphene. Nanoscale. 2 (8), 1485-1488 (2010).
- Jiang, S., et al. Real-time electrical detection of nitric oxide in biological systems with sub-nanomolar sensitivity. Nature Communications. 4 (1), 1-7 (2013).
- Bai, Y., Xu, T., Zhang, X. Graphene-based biosensors for detection of biomarkers. Micromachines. 11 (1), 60 (2020).
- Madou, M. J. . Fundamentals of Microfabrication The Science of Miniaturization. 2nd ed. , (2002).
- Xia, Y., Whitesides, G. M. Soft lithography. Annual Review of Material Sciences. 28 (1), 153-184 (2003).
- Wang, Y. Y., et al. Raman studies of monolayer graphene: The substrate effect. Journal of Physical Chemistry C. 112 (29), 10637-10640 (2008).
- Betancur, V., Sun, J., Wu, N., Liu, Y. Integrated lateral flow device for flow control with blood separation and biosensing. Micromachines. 8 (12), 367 (2017).
- Butt, A. . Physics and Chemistry of Interfaces. 3rd ed. , (2003).
- Sitko, R., Zawisza, B., Malicka, E. Graphene as a new sorbent in analytical chemistry. TrAC Trends in Analytical Chemistry. 51, 33-43 (2013).
- Bai, L., et al. Graphene for energy storage and conversion: Synthesis and Interdisciplinary applications. Electrochemical Energy Reviews. 3 (2), 395-430 (2019).
- Boretti, A., Al-Zubaidy, S., Vaclavikova, M., Al-Abri, M., Castelletto, S., Mikhalovsky, S. Outlook for graphene-based desalination membranes. npj Clean Water. 1 (1), 1-11 (2018).
- Pumera, M. Graphene in biosensing. Materials Today. 14 (7-8), 308-315 (2011).
- Sun, J., Liu, Y. Unique constant phase element behavior of the electrolyte-graphene interface. Nanomaterials. 9 (7), 923 (2019).
- Sun, J., Camilli, L., Caridad, J. M., Santos, J. E., Liu, Y. Spontaneous adsorption of ions on graphene at the electrolyte-graphene interface. Applied Physics Letters. 117 (20), 203102 (2020).

