Frugal Imaging Technique of Capillary Flow Through Three-Dimensional Polymeric Printing Powders
Summary
The proposed technique will provide a novel, efficient, frugal, and non-invasive approach for imaging fluidic flow through a packed powder bed, yielding high spatial and temporal resolution.
Abstract
The development of novel imaging techniques of molecular and colloidal transport, including nanoparticles, is an area of active investigation in microfluidic and millifluidic studies. With the advent of three-dimensional (3D) printing, a new domain of materials has emerged, thereby increasing the demand for novel polymers. Specifically, polymeric powders, with average particle sizes on the order of a micron, are experiencing a growing interest from academic and industrial communities. Controlling material tunability at the mesoscopic to microscopic length scales creates opportunities to develop innovative materials, such as gradient materials. Recently, a need for micron-sized polymeric powders has been growing, as clear applications for the material are developing. Three-dimensional printing provides a high-throughput process with a direct link to new applications, driving investigations into the physio-chemical and transport interactions on a mesoscale. The protocol that is discussed in this article provides a non-invasive technique to image fluid flow in packed powder beds, providing high temporal and spatial resolution while leveraging mobile technology that is readily available from mobile devices, such as smartphones. By utilizing a common mobile device, the imaging costs that would normally be associated with an optical microscope are eliminated, resulting in a frugal-science approach. The proposed protocol has successfully characterized a variety of combinations of fluids and powders, creating a diagnostic platform for quickly imaging and identifying an optimal combination of fluid and powder.
Introduction
Inkjet-based binder jetting into powder media represents an important technology in additive manufacturing (3D printing). The binder jetting process begins with the deposition of functional fluids into powder media using a scanning inkjet printing process. Specifically, an inkjet print head translates over the powder surface, depositing the liquid binding agent onto a powder surface, and thereby forming a solid part in a layer-by-layer fashion1. Inkjet-based binder jetting technologies generally include sand, metal powders, and polymeric powders. However, to expand the materials' space in binder jetting, a fundamental approach to investigating fluid-powder and powder-powder interactions, tribology, powder packing density, and particle aggregation is required. Specifically, for fluid-powder interactions, a critical need exists for the ability to image fluid flow through powder beds in real-time. This promises to be a powerful tool for researchers to include as a characterization technique and potentially as a screening method for different combinations of fluids and powders2,3,4, as well as more complex systems, such as concrete 3D-printing systems that utilize particle-bed methods.
The development of novel imaging techniques of molecular and colloidal transport, including nanoparticles, is an active area of investigation in microfluidic and millifluidic studies. Probing intermolecular interactions by imaging techniques can be challenging, as little work has been done to probe these types of interactions under the conditions of unsaturated and unsteady fluid flow. Many of the studies that are reported in the literature have focused on a saturated, pre-wetted, porous media, such as glass bead5,6,7,8,9,10,11,12 and soils13,14,15,16,17,18. This technique provides a non-invasive approach, resulting in high temporal and spatial resolution2,3,4,19. Furthermore, the developed technique provides a novel method for characterizing and quantifying nano-scale and micron-scale particle transport in a variety of porous media, focusing on polymeric powders.
The proposed technique utilizes a mobile device to record unsaturated, unsteady fluidic transport through porous polymeric media with particle dimensions that are representative of the powders used in 3D printing systems that utilize fluidic powder-bed fusion technologies. This technique is advantageous as the flow cells are cost-effective, reusable, small, and easily handled, illustrating the dominant aspects of frugal science. The ability to implement these simple experiments into a field study is very straightforward, eliminating the complications, cost, and time that are required in optical microscopy. Given the ease of creating the setup, the access to quick results, and the minimal number of sample requirements, this technique is an optimal platform for diagnostic screening.
Protocol
1. Preparing the microfluidic flow cell
NOTE: For this protocol, a commercial microfluidic flow cell will be utilized. By using a commercial product that is designed for light penetration from an optical microscope, any challenges regarding brightfield illumination of the media will be minimized.
- Start preparing the microfluidic flow cell by covering the outlet with parafilm to seal one end of the channel so that the empty flow cell may be packed with polymeric powder. Before starting the experiment, confirm that the microfluidic channel is clean and dry.
- Tape the metric paper ruler directly beneath the flow channel.
- Weigh the microfluidic flow cell with the parafilm and the ruler attached. The mass of the flow cell is the unpacked flow cell mass (mu).
2. Packing the powder into the channel
- When packing the powder, use a plastic pipet to transfer the powder. Note that particles may adhere to the outside of the pipet tip, which is a result of tribocharging.
- While introducing the powder into the channel, tap the flow cell at least five times to compact the powder. Continue packing until the powder reaches the beginning of the opening of the flow channel.
NOTE: Tapping compacts the powder within the channel with the goal of providing a reproducible diagnostic tool. For certain applications, this effort can be a higher, lower, or equivalent level of powder compaction than the compaction that is observed in the application of interest. If there are issues with the reproducibility of tapping or with packing powder within the application, consider performing ASTM D7481-1820. - Remove the powder present on the outer surface of the flow cell with a wipe soaked in alcohol.
NOTE: Some types of particles can be hydrophobic, so water may not remove the particles well.
- While introducing the powder into the channel, tap the flow cell at least five times to compact the powder. Continue packing until the powder reaches the beginning of the opening of the flow channel.
- Once the powders are packed, visually inspect the flow cell for loosely packed powder. If the powder within the flow cell appears loosely packed (Figure 1), tap the flow cell five more times. If the powder packing appears consistent and compact, weigh the flow cell to measure the mass of the polymeric powder (mp – mu; see Equation 1).
- Calculate the bulk packing density (ρ) using the difference between the unpacked (mu) and packed flow cell mass (mp) and dividing it by the volume of the flow cell. The volume of the flow cell is then known [length (l): 50 mm, width (w): 5 mm, channel depth (h): 0.8 mm].
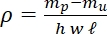 Eq 1
Eq 1 - Confirm that the packing density is in the typical range of 0.45 g/mL to 0.55 g/mL for polymeric powders2,3,4,21. Leave the flow cells in the fume hood until steps 3 and 4 are completed.
CAUTION: Particles with a diameter of less than 10 µm can penetrate into the lungs and potentially enter the bloodstream, which can cause health problems related to the pulmonary and cardiovascular systems. The polymeric powders that were used in this experiment have a particle diameter of approximately 50 μm. Therefore, inhalation of the particles has less potential to cause health problems, but smaller particles are present even in narrow particle size distributions. For the safest environment, preparation of the flow cells should be done in a fume hood.
- Calculate the bulk packing density (ρ) using the difference between the unpacked (mu) and packed flow cell mass (mp) and dividing it by the volume of the flow cell. The volume of the flow cell is then known [length (l): 50 mm, width (w): 5 mm, channel depth (h): 0.8 mm].
3. Preparing the solvent
- Prepare a 75 wt% solution of ethanol in water. Note that the solvent will be referred to as the fluid in the rest of this manuscript.
CAUTION: Make sure that the beaker used to prepare the solution is free from any surfactants, as surfactants will affect the results.
4. Preparing the white light table
- To prevent flooding the detector (camera) with too much light, cover the light table with an opaque material, such as a 3D printed cover in black polylactic acid (PLA) filament (Supplementary Figure 1). Ensure that the material has an opening that is the size of the microchannel (5 mm x 55 mm) to allow light to illuminate the powder.
NOTE: Too much light means the screen or monitor of the camera will appear white and the microchannel will not be visible. Therefore, the detector will be unable to focus the lens on the microchannel. - To ensure that the camera on the mobile device can capture the contrast between the wet and dry powder, use the light table at a low to medium light intensity.
NOTE: High light intensity is at 100%. The other two settings are relative to the high light intensity; the setting for low light intensity is at ~30%, and medium light intensity is at ~65%. - Align the camera on the mobile device directly above the light table. Confirm that the camera is perpendicular to the top of the light table (Figure 2).
- Orient the camera on the mobile device so that the long axis of the mobile device aligns with the longest axis of the flow cell.
5. Starting the experiment
- Place the flow cell on the light table and focus the camera on the mobile device on the flow channel.
NOTE: For optimal results, a darker (reduced overhead lighting) recording space will typically provide better image resolution. If a dark space is not available, minimizing changes in overhead lighting (lights being turned on, turned off, or being dimmed) during recording should improve graphic signals and minimize undesired noise in the experiment. - After focusing the camera on the mobile device, select the record button. Add 125 μL of fluid to the open inlet of the microchannel using a pipet.
- Record the flow for 2 min or until all the powder is wetted visibly.
6. Analyzing the data
- Transfer the video file from the mobile device to the computer for easy access. Note that videos over 2 min may not load in the software at this time, as the file size can be excessively large.
- Download Tracker, a free software from the Physlets website22. This software can track position, velocity, and acceleration in the following video files: .mov, .avi, .mp4, .flv, .wmv, etc. For the following steps, please refer to Supplemental File.
NOTE: For Mac users, install the latest version of the software for the software to function properly. Additionally, Mac users may require a video engine (Xuggle), animated GIF files (.gif), or image sequences that consist of one or more digital images (.jpg, .png, or pasted from the clipboard). - Once the software is installed, open the Tracker software. From the File menu, select Open File to load the transferred video file from step 6.1 on the desktop of the computer.
- Click the Clip Settings icon, which looks like the film strip, to define the Starting Frame and the Step Size.
NOTE: Placing the mouse over an icon will identify the icon.- Define the Starting Frame. The Starting Frame is defined as the frame in which the first contrast (the contrast between the wet and dry powder) is observed.
- Set the Step Size. Step Size refers to the frame step size, which the software would analyze. From prior experiments, the optimum Step Size is 10.
- Click on the Calibration Tool, the icon with the blue ruler, to the right of the Clip Settings button. From Nuovo, select Calibration Stick.
- To zoom in on the ruler in the video, right-click on the area to magnify and Select Zoom in from the list. Once appropriately magnified, define the beginning and end of 1 mm on the ruler taped to the microchannel, and Type 1 mm to define the distance.
- Click on the Coordinate Axis Tool, which is the purple icon, to the right of the Calibration Tool. Set the Origin for the x- and y-axis, using the starting frame while doing this step.
- To define the initial point of analysis, create a Point Mass. Click on Create, then select Point Mass. Use Shift + Control to change the size of the rectangle. The initial point is where the inlet and the channel connect.
NOTE: The rectangle indicates the domain, defined by the user, that the software will scan to find the contrasting wet and dry powder. The boundary allows the user to define the region where the initial point will be observed.- Click on Search Next a couple of times to verify that the software is analyzing the correct area. If the software is functioning properly, click on Search and wait for the software to finish analyzing the video. If the software cannot automatically find a matching image intensity from the previous frame to the current frame, the software will stop and wait for the user to redefine the search area.
NOTE: For reproducibility and the ability to compare different experimental results, choose the fastest or the slowest point of the fluidic flow front (region of contrast between the wetted and dry powder) for every sample. - If an analysis error is observed on the live plotted data on the right-hand side of the Tracker screen, click on the Data Point once on the step prior to the erred data point. On the main screen, modify the red rectangular search area location to search the region of interest and repeat step 6.8.1.
NOTE: If an error exists, right-click on the inaccurate data point and deselect the point for further analysis.
- Click on Search Next a couple of times to verify that the software is analyzing the correct area. If the software is functioning properly, click on Search and wait for the software to finish analyzing the video. If the software cannot automatically find a matching image intensity from the previous frame to the current frame, the software will stop and wait for the user to redefine the search area.
- Once the analysis is complete, copy and paste the results into a spreadsheet. The results saved in the spreadsheet comprise the distance and time data.
- Plot the copied data in the spreadsheet as the distance of fluid transport through the powder bed as a function of time.
Representative Results
In the section on analyzing data, the data for the time-lapsed images in Figure 3 illustrate the 75 wt% ethanol solution infiltrating the polycarbonate (PC) powder. Fluorescein was added to the solution to enhance the image quality for this publication. In the time-lapse images, the time-resolved process begins when the fluid is added to the inlet. Time, t, starts as soon as the fluid begins to penetrate the channel. The series of images demonstrates the progression of the fluid and fluorescein. In PC, the fluid and fluorescein are transported at the same flow rate. The open red circles on the plot in Figure 4 represent the exact time and distance of the compiled information found in Table 1. Infiltration of the fluid into the powder bed combined with the incremental time steps (red circles) are visually represented in Figure 3.
In the interval from 1 s to 2 s, the distance traveled by the fluid has doubled. During the interval from 2 s to 5 s, the distance that the fluid has traveled also doubles. From 5 s to 10 s, the fluid is still moving quickly. However, after 15 s, the flow rate is slowing to a rate of approximately 2 mm every 5 s. For a single powder and fluid combination, five tests are performed in a single group. The number of total tests may vary for each group. For example, if one of the five experiments fails, then a new packed microchannel will be analyzed in lieu of the failed test. Failure is defined as a fluid that does not penetrate the powder bed or only partially penetrates the powder bed because of the bubbles forming in the channel resulting from inconsistent powder packing. To observe the standard deviation between a set of tests in a group refer to Donovan21, specifically Figure 19 and Figure 21.
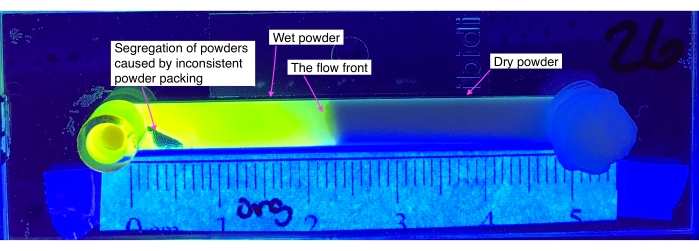
Figure 1: Polymeric powder loosely packed into a microfluidic flow cell that could result in a failed experiment if not addressed. Please click here to view a larger version of this figure.
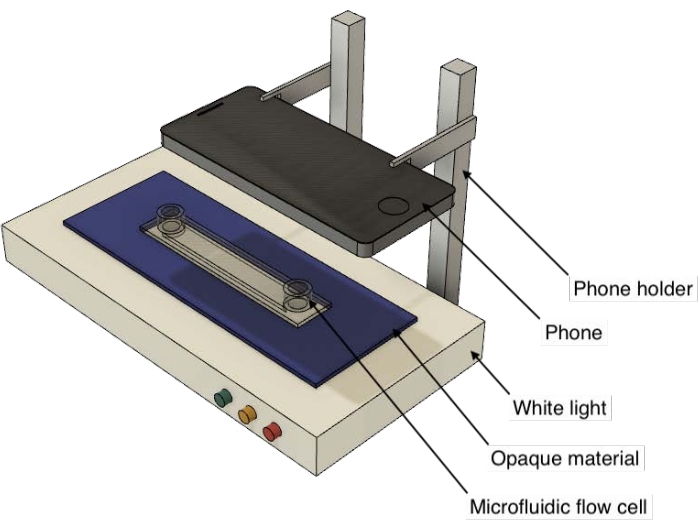
Figure 2: A cartoon representation of the experimental setup. This image is not drawn to scale. Please click here to view a larger version of this figure.

Figure 3: A representative time-lapse series of images from a single experiment. Images from left to right exemplify the flow of solvent (enhanced with fluorescent dye for visualization) through the packed porous bed. Note that the leading front is not uniform, so an average distance of the propagating front is typically used. Please click here to view a larger version of this figure.
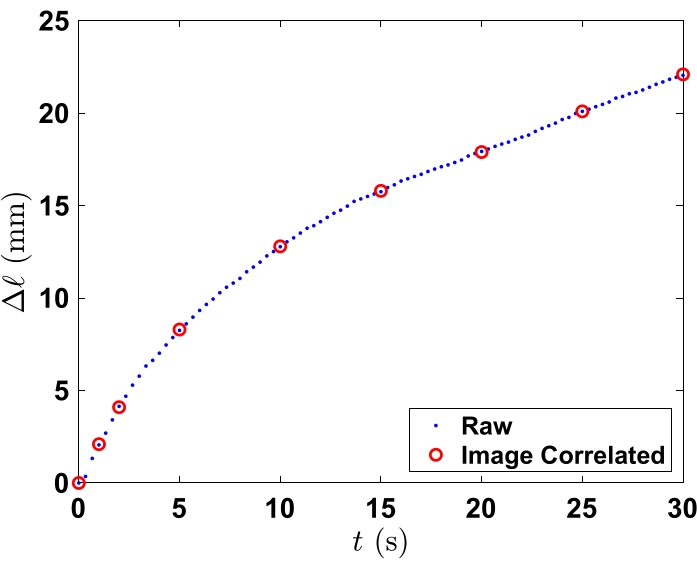
Figure 4: Quantitative representation of the average distance of propagation (Δl) versus time (t) as the fluid penetrates the packed powder bed. Red circles represent data points for each time increment that is seen in Figure 3. Please click here to view a larger version of this figure.
| Time (s) | Distance (mm) |
| 0 | 0 |
| 1 | 2.1 |
| 2 | 4.1 |
| 5 | 8.3 |
| 10 | 12.8 |
| 15 | 15.8 |
| 20 | 17.9 |
| 25 | 20.1 |
| 30 | 22.1 |
Table 1: Distance and time values for the red dots that are shown in Figure 4.
Supplementary Figure 1: CAD drawing of the opaque 3D printer cover in black polylactic acid (PLA) filament. Please click here to download this File.
Supplemental File: Screenshots of the steps involved in data analysis using the tracking software. Please click here to download this File.
Discussion
The protocol that is provided is highly dependent on the material characteristics of the particles that are chosen. Material properties impacting flow include particle size distribution2,3,4,5,11,21, particle surface roughness11, chemical properties at the particle surface2,3,4,5,11,16,21,23,24,25, molecular dipole moments, particle shape11, and particle-particle interactions2,3,4,5,11,16,23,24,25,26,27. These properties directly affect the packing density of the powder in the microfluidic channel and, consequently, the capillary flow behavior of the fluid as it wets the particles2,3,4,5,7,8,14,15.
The powder packing density plays a very important role in this technique. If the powder is not packed densely enough, air bubble formation or the segregation of powder during imaging may occur, preventing a reproducible sample. Therefore, tapping the microfluidic channel (step 2.1.1) while packing powder is a very crucial step. Figure 1 represents a microfluidic flow cell with inconsistent packed powder after the fluid infiltrated the entire channel. The segregation of the powders can be seen toward the input of the channel. Once the cell is packed, before running the experiment, checking the powder packing density on the lightbox is a helpful way to avoid these types of failed experiments. The powders that are presented in this protocol have been analyzed utilizing a standardized tap density test, specifically ASTM D7481-18, to report bulk packing density as a function of taps20. ASTM D7481-18 does not need to be conducted for the proposed protocol to be completed, but the ASTM will provide supplemental information on the powder.
Particle size distribution, a measurable characteristic, directly impacts the bulk packing density23,24,25. In a packing system, larger particles will create a large void space, providing a position for the small particles to settle. Measuring the ratio of large to small particles provides insight into the volume of void space for the fluid to penetrate the powder. When packing the microfluidic flow cell for an experiment, all the small particles will fill the void space that is made by larger particles. Minimizing the available void spaces will impact the fluid transport, as well as provide more sites for molecular and particle retention to occur. For further technique improvement, the similar-sized particles (e.g., those particles from 60 μm to 65 μm) need to be further investigated to determine if this technique has the sensitivity to differentiate between particles with an average particle size of only a couple microns difference.
Bulk density is not an intrinsic property of the powder, as it is very dependent on how the material is handled26. Whether the powder was made in-house or transported by plane, train, or car may greatly impact the value for the bulk packing density, impacting the particle size distribution. Whether the powder samples are selected from the top versus the bottom of a container can also impact the results. Imagine opening a box of cereal; the material at the top is comprised of all the large pieces, and the material at the bottom of the box is comprised of all the smaller pieces. In the same way, a powder that has experienced stress (vibrations) from travel will have a particle size gradient throughout the container.
For polymeric powders, verifying that the internal surfaces of the flow cells have received a hydrophobic treatment is integral. If the walls of the microfluidic flow cell have not been treated, then wall effects often occur when imaging the fluid transport. Wall effects are observed when the fluid travels along the wall much faster and farther than the bulk fluid flow through the powder of interest. If the wall is not hydrophobic, it allows for a path of least resistance to form, and fluid will flow along that path (the wall) and not through the powder. Therefore, utilizing hydrophobic cells allows for a more representative study of the flow of aqueous systems through a porous media, whereas hydrophilic cells should be utilized for organic systems.
For some polymeric powders, a tribocharging effect26,27 that occurs between the powder particles and the tip of the plastic pipet can be present. As a result, the powder may adhere to the outside of the pipet tip when loading the pipet with powder particles. The powder adhesion has not caused any issue with transferring the powder or the particle packing. However, if the particle adhesion does become an issue, a couple of modifications that may reduce the occurrence of particles adhering to the pipet can be attempted. One option is to dampen the outer tip of the pipet with water and blot the tip dry to disrupt the static electricity. Another option is to use a glass pipet instead of plastic. A third option is to transfer the powder particles in a more humid environment.
The technique is a frugal method for a one-dimensional (1D) measurement of the fluid intrusion length within a 3D particle bed. Therefore, the technique will only be able to account for the preferential flow path in the direction of interest.
The current protocol discusses fluidic transport through a porous media, utilizing a frugal setup and eliminating the complications and expenses of an optical microscope. Additionally, with a UV-transilluminating table to excite the fluorescing and photoluminescent species, the technique could also be used to image molecular and nanoparticle fate and transport. For this setup, the solvent protocol would need to be modified for the molecular and nanoparticle systems.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| µ-Slide I Luer | ibidi | 80191 | Microfluidic flow cell |
| Beaker | Southern Labware | BG1000-800 | Glassware |
| CALIBRE 301-58 LT Natural Polycarbonate Resin | TRINSEO LLC | CALIBRETM 301-58 LT | Natural polycarbonate resin |
| Ethanol | Sigma Aldrich | 1.00983 | Solvent |
| Fume Hood | Kewaunee | Supreme Air LV Fume Hoods | Used with 92 FPM at 18" opening |
| iPhone 7 plus | Apple | Camera | |
| Opaque 3D printed material | The CAD drawing is provided in the supplemental file | ||
| ORGASOL 2002 ES 6 NAT 3 | ARKEMA | A12135 | Polyamide powder |
| Pipet | VWR | 10754-268 | Disposable Transfer Pipet |
| Pipette | Globe Scientific Inc. | 3301-200 | Pipette that can hold 125 µL of fluid |
| Polystyrene | Advanced Laser Materials, LLC. | PS200 | Polystyrene for sintering |
| Tracker | Video analysis and modeling tool | ||
| VariQuest 100 White Light Model 3-3700 | FOTODYNE | 3-3700 | White light |
| Water | Distilled water |
Riferimenti
- Redwood, B., Schoffer, F., Garret, B. . The 3D Printing Handbook. , (2018).
- . Three dimensional printing, Patent ID: 20210087418 Available from: https://uspto.report/patent/app/20210087418 (2021)
- . Three dimensional printing, Patent ID: 20210095152 Available from: https://uspto.report/patent/app/2021009515.2 (2021)
- Three dimensional printing, Patent ID: 20210107216. Available from: https://uspto.report/patent/app/20210107216#C00011 (2021)
- Petosa, A. R., Brennan, S. J., Rajput, F., Tufenkji, N. Transport of two metal oxide nanoparticles in saturated granular porous media: Role of water chemistry and particle coating. Water Research. 46 (4), 1273-1285 (2012).
- Giordano, S. Effective medium theory for dispersions of dielectric ellipsoids. Journal of Electrostatics. 58 (1-2), 59-76 (2003).
- Toloni, I., Lehmann, F., Ackerer, P. Modeling the effects of water velocity on TiO2 nanoparticles transport in saturated porous media. Journal of Contaminant Hydrology. 171, 42-48 (2014).
- Dang-Vu, T., Hupka, J. Characterization of porous materials by capillary rise method. Physicochemical Problems of Mineral Processing. 39, 47-65 (2005).
- Huang, W. E., Smith, C. C., Lerner, D. N., Thornton, S. F., Oram, A. Physical modelling of solute transport in porous media: evaluation of an imaging technique using UV excited fluorescent dye. Water Research. 36 (7), 1843-1853 (2002).
- Zhao, J., Li, H., Cheng, G., Cai, Y. On predicting the effective elastic properties of polymer nanocomposites by novel numerical implementation of asymptotic homogenization method. Composite Structures. 135, 297-305 (2016).
- Seymour, M. B., Chen, G., Su, C., Li, Y. Transport and retention of colloids in porous media: Does shape really matter. Environmental Science and Technology. 47 (15), 8391-8398 (2013).
- Ochiai, N., Kraft, E. L., Selker, J. S. Methods for colloid transport visualization in pore networks. Water Resources Research. 42 (12), (2006).
- Rottman, J., Sierra-Alvarez, R., Shadman, F. Real-time monitoring of nanoparticle retention in porous media. Environmental Chemistry Letters. 11 (1), 71-76 (2013).
- Xing, Y., Chen, X., Chen, X., Zhuang, J. Colloid-mediated transport of pharmaceutical and personal care products through porous media. Scientific Reports. 6 (1), 1-10 (2016).
- Dathe, A., et al. Functional models for colloid retention in porous media at the triple line. Environmental Science and Pollution Research. 21 (15), 9067-9080 (2014).
- Zhang, T., et al. Investigation of nanoparticle adsorption during transport in porous media. SPE Journal. 20 (4), 667-677 (2015).
- Zhang, Q., Karadimitriou, N. K., Hassanizadeh, S. M., Kleingeld, P. J., Imhof, A. Study of colloids transport during two-phase flow using a novel polydimethylsiloxane micro-model. Journal of Colloid and Interface Science. 401, 141-147 (2013).
- Health and environmental effects of particulate matter (PM). EPA Available from: https://www.epa.gov/pm-pollution/health-and-environmental-effects-particulate-matter-pm (2021)
- Bridge, J. W., Banwart, S. A., Heathwaite, A. L. Noninvasive quantitative measurement of colloid transport in mesoscale porous media using time lapse fluorescence imaging. Environmental Science & Technology. 40 (19), 5930-5936 (2006).
- ASTMInternational. Standard test methods for determining loose and tapped bulk densities of powders using a graduated cylinder. ASTMInternational. , (2018).
- Donovan, K. J. . Microfluidic investigations of capillary flow and surface phenomena in porous polymeric media for 3D printing. , (2019).
- . 34;Try Tracker Online." Tracker Video Analysis and Modeling Tool for Physics Education Available from: https://physlets.org/tracker/ (2022)
- Janssen, P. H. M., Depaifve, S., Neveu, A., Francqui, F., Dickhoff, B. H. J. Impact of powder properties on the rheological behavior of excipients. Pharmaceutics. 13 (8), 1198 (2021).
- Boschini, F., Delaval, V., Traina, K., Vandewalle, N., Lumay, G. Linking flowability and granulometry of lactose powders. International Journal of Pharmaceutics. 494 (1), 312-320 (2015).
- Yablokova, G., et al. Rheological behavior of β-Ti and NiTi powders produced by atomization for SLM production of open porous orthopedic implants. Powder Technology. 283, 199-209 (2015).
- Lumay, G., Fiscina, J., Ludewig, F., Vandewalle, N. Influence of cohesive forces on the macroscopic properties of granular assemblies. AIP Conference Proceedings. 1542, 995 (2013).
- Lumay, G., et al. Effect of relative air humidity on the flowability of lactose powders. Journal of Drug Delivery Science and Technology. 35, 207-212 (2016).

