高分辨率中子光谱研究蛋白质和水合水的皮秒纳秒动力学
Summary
中子背散射光谱法提供了对蛋白质及其水合水合的ps-ns动力学的无损和无标记访问。该工作流程介绍了两项关于淀粉样蛋白的研究:关于溶菌酶在聚集过程中的时间分辨动力学和纤维形成时tau的水合水动力学。
Abstract
中子散射提供了以非破坏性方式探测样品内各种能量的动力学的可能性,并且不需要标记氘以外的其他标记。特别是中子背散射光谱同时记录多个散射角的散射信号,非常适合在ps-ns时间尺度上研究生物系统的动力学。通过使用D2O和可能的氘代缓冲液组分,该方法可以监测液态蛋白质的质心扩散以及骨架和侧链运动(内部动力学)。
此外,可以通过使用用H2O水合的全氘代蛋白质粉末来研究水合水动力学。本文介绍了劳埃-朗格文研究所 (ILL) 的仪器 IN16B 上采用的工作流程,用于研究蛋白质和水合水动力学。解释了使用蒸汽交换的溶液样品和水合蛋白粉样品的制备。蛋白质和水合水动力学的数据分析程序针对可以在中子背散射光谱仪上获得的不同类型的数据集(准弹性光谱或固定窗口扫描)进行了描述。
该方法通过两项涉及淀粉样蛋白的研究来说明。溶菌酶聚集成μm大小的球形聚集体 – 表示颗粒 – 显示在IN16B探测的空间和时间范围内的一步过程中发生,而内部动力学保持不变。此外,还研究了tau水合水合水在全氘化蛋白水合粉末上的动力学。结果表明,水的平移运动在淀粉样纤维形成时被激活。最后,讨论了协议中的关键步骤,即中子散射相对于其他实验生物物理方法的动力学研究如何定位。
Introduction
中子是一种无电荷的大质量粒子,多年来已成功用于探测从基础物理学到生物学1等各个领域的样品。对于生物应用,小角中子散射,非弹性中子散射以及中子晶体学和反射计被广泛使用2,3,4。非弹性中子散射提供了动力学的集合平均测量,而不需要特定的标记本身,并且信号质量不依赖于大小或蛋白质5。对于模拟细胞内培养基的所研究蛋白质,例如氘代细菌裂解物甚至体内3,6,7,可以使用高度复杂的环境进行测量。可以使用不同的实验装置来研究动力学,即i)飞行时间访问sub-ps-ps动力学,ii)反向散射-访问ps-ns动力学,以及iii)自旋回波访问从ns到数百ns的动力学。中子反向散射利用布拉格定律 2d sinθ = nλ,其中 d 是晶体中平面之间的距离,θ 是散射角,n 是散射阶数,λ 是波长。使用晶体向检测器进行反向散射可以实现高分辨率的能量,通常为~0.8μeV。为了测量能量交换,可以使用携带反向散射晶体的多普勒驱动器来定义和调整入射中子波长8,9,10(图1),或者可以使用飞行时间设置,但代价是能量分辨率降低11。
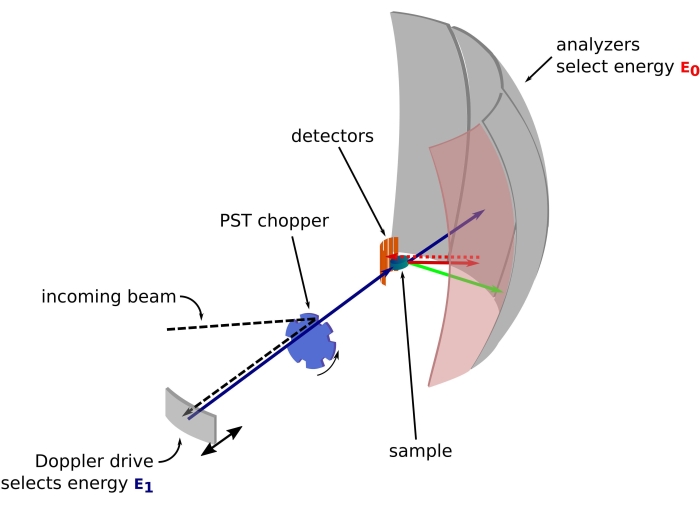
图 1:带多普勒驱动器的中子背散射光谱仪的草图。入射光束撞击相空间变换(PST)斩波器42,这增加了样品位置的通量。然后通过多普勒驱动器将其反向散射到样品,多普勒驱动器选择能量E1(青色箭头)。然后中子被样品散射(箭头的颜色表示不同的能量),由Si 111晶体制成的分析仪只会反向散射具有特定能量E0的中子(此处为红色箭头)。因此,动量传递q是从探测器阵列上中子的检测位置获得的,能量转移是从差值E1– E0获得的。PST产生的中子脉冲的预期飞行时间用于丢弃直接散射到探测器管的中子的信号。缩写:PST = 相空间变换。请点击此处查看此图的大图。
对于背散射光谱,富含氢质子的样品(如蛋白质)信号的主要贡献来自非相干散射,其散射强度 Sinc(q, ω) 由方程 (1)12 表示
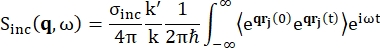 (1)
(1)
其中σinc是所考虑元素的非相干截面,k’是散射波矢量的范数,k是入射波矢量的范数,q(= k – k’)是动量传递,r j(t)是原子j在时间t的位置矢量,ω是对应于入射中子和系统之间能量转移的频率。尖括号表示融合平均值。因此,非相干散射探测原子位置随时间的集合平均单粒子自相关性,并给出系统中所有原子和不同时间起源的自动力学平均值(集合平均值)。散射函数是中间散射函数 I(q, t) 在时间上的傅里叶变换,可以看作是方程 (2) 所示的范霍夫相关函数在空间中的傅里叶变换:
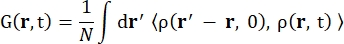 (二)
(二)
其中 ρ(r,t) 是在位置 r 和时间t 13 处找到原子的概率密度。
对于菲克扩散过程,自扩散函数在散射函数中进行双傅里叶变换后产生(参见方程(3)),散射函数由γ = Dq2给出的线宽洛伦兹组成。
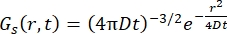 (三)
(三)
开发了更复杂的模型并发现有用,例如Singwi和Sjölander用于ps-ns内部蛋白质动力学14的跳跃扩散模型或Sears用于水合水15,16,17的旋转模型。
在法国格勒诺布尔ILL的中子背散射(NBS)仪器IN16B8,9上(补充图S1),蛋白质常用的设置由Si 111晶体组成,用于分析仪的Si 111晶体,带有用于调整入射波长的多普勒驱动器(补充图S2A),从而可以访问动量转移范围~0.2 Å-1 < q < ~2 Å-1和-30μeV的能量转移范围< 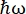 < 30 μeV – 对应于从几ps到几ns的时间尺度和几Å的距离。此外,IN16B还提供了执行弹性和非弹性固定窗口扫描(E/IFWS)10的可能性,其中包括固定能量传输的数据采集。由于在处理中子时通量受到限制,E/IFWS允许在一次能量转移中最大化通量,从而减少获得令人满意的信噪比所需的采集时间。最近的选择是反向散射和飞行时间光谱仪(BATS)模式11,它允许测量广泛的能量传输(例如,-150μeV<
< 30 μeV – 对应于从几ps到几ns的时间尺度和几Å的距离。此外,IN16B还提供了执行弹性和非弹性固定窗口扫描(E/IFWS)10的可能性,其中包括固定能量传输的数据采集。由于在处理中子时通量受到限制,E/IFWS允许在一次能量转移中最大化通量,从而减少获得令人满意的信噪比所需的采集时间。最近的选择是反向散射和飞行时间光谱仪(BATS)模式11,它允许测量广泛的能量传输(例如,-150μeV< 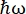 <150μeV),具有比多普勒驱动器更高的通量,但代价是能量分辨率较低(补充图S2B)。
<150μeV),具有比多普勒驱动器更高的通量,但代价是能量分辨率较低(补充图S2B)。
中子散射的一个重要特性是,inc σ非相干截面的氢值比氘高40倍,对于生物样品中常见的其他元素可以忽略不计。因此,可以使用氘代缓冲液研究液体环境中蛋白质的动力学,并且粉末状态允许研究用D 2 O水合的氢化蛋白粉的蛋白质内部动力学,或研究用H2O水合的全氘化蛋白粉的水合水。在液态下,中子背散射通常允许同时访问蛋白质的质心自扩散(Fickian型扩散)及其内部动力学。后者是骨干和侧链运动,通常由所谓的跳跃扩散模型或其他模型描述 3,18.在氢化蛋白粉中,不存在蛋白质扩散,只需要对内部动力学进行建模。对于水合水,水分子的平移和旋转运动的贡献对动量传递q具有不同的依赖性,这使得它们在数据分析过程中得以区分17。
本文通过研究蛋白质来说明中子背散射方法,这些蛋白质被发现能够展开,聚集成由β链堆栈组成的规范形式 – 所谓的交叉β模式19,20 – 并形成细长的纤维。这就是所谓的淀粉样蛋白聚集,由于其在阿尔茨海默氏症或帕金森病等神经退行性疾病中的核心作用而被广泛研究21,22。淀粉样蛋白的研究还受到它们可以发挥的功能作用23,24或其在开发新型生物材料方面的高潜力的动机25。淀粉样蛋白聚集的物理化学决定因素尚不清楚,尽管在过去几年中取得了巨大进展,但没有淀粉样蛋白聚集的一般理论可用21,26。
淀粉样蛋白聚集意味着蛋白质结构和稳定性随时间的变化,其研究自然意味着动力学,与蛋白质构象稳定性、蛋白质功能和蛋白质能量景观27 有关。动力学通过对最快运动28的熵贡献与特定状态的稳定性直接相关,蛋白质功能可以通过从光敏蛋白29 的sub-ps到域运动的ms的各种时间尺度上的运动来维持,这可以通过皮秒-纳秒动力学30来促进。
将介绍两个使用中子背散射光谱研究淀粉样蛋白的例子,一个在液态研究蛋白质动力学,另一个在水合粉末状态下研究水合水动力学。第一个示例涉及实时将溶菌酶聚集成μm大小的球体(称为颗粒)5,第二个示例比较人类蛋白质tau31的天然和聚集状态下的水动力学。
溶菌酶是一种参与免疫防御的酶,由129个氨基酸残基组成。溶菌酶可以在pD为10.5和温度为90°C的氘代缓冲液中形成颗粒。 通过中子散射,我们发现溶菌酶质心扩散系数的时间演变遵循硫黄素T荧光(用于监测淀粉样蛋白交叉β模式形成的荧光探针32)的单指数动力学,表明形成颗粒上层结构和交叉β模式以相同的速率在单个步骤中发生。此外,内部动力学在整个聚集过程中保持不变,这可以通过在NBS仪器上无法观察到的快速构象变化来解释,或者可以通过聚集时蛋白质内能没有显着变化来解释。
人类蛋白tau是一种固有的无序蛋白(IDP),由441个氨基酸组成,用于所谓的2N4R亚型,其主要参与阿尔茨海默病33。在全氘化蛋白tau粉末上使用中子反向散射,我们发现在纤维状态下水合水动力学增加,更多的水分子群经历平移运动。结果表明,水合水熵的增加可能驱动tau的淀粉样蛋白颤动。
Protocol
Representative Results
Discussion
中子光谱是唯一允许探测蛋白质样品的集合平均ps-ns动力学的方法,无论使用氘代时蛋白质的大小或溶液的复杂性如何6。具体来说,通过探测溶液中蛋白质组装体的自扩散,可以明确地确定这种组装体的流体动力学尺寸。尽管如此,该方法通常受到低中子通量的限制,这意味着采集时间长,并且需要大量样品(通常为100 mg蛋白质)才能在分配的光束时间内获得良好的信噪比。
…Divulgazioni
The authors have nothing to disclose.
Acknowledgements
作者感谢德国加兴Heinz Maier-Leibnitz Zentrum的Jülich中子科学中心的Michaela Zamponi在仪器SPHERES上进行的部分中子散射实验。这项工作得益于欧洲联盟根据HPRI-2001-50065和RII3-CT-2003-505925合同资助的氘实验室(DLAB)财团的活动,以及英国工程和物理科学研究理事会(EPSRC)在劳厄·朗格文研究所EMBL实验室内根据赠款GR/R99393/01和EP/C015452/1资助的活动。欧盟委员会在第7个框架计划下通过关键行动提供支持:加强欧洲研究领域,研究基础设施得到认可[合同226507(NMI3)]。Kevin Pounot和Christian Beck感谢联邦教育和研究部(BMBF,批准号05K19VTB)资助他们的博士后奖学金。
Materials
| Aluminum sample holder | Not commercially available. Either the local contact on the instrument can provide them or they can be manufactured based on a technical drawing that can be provided by the local contact. | ||
| Deuterium chloride, 35 wt. % in D2O, ≥99 atom % D | Sigma-Aldrich | 543047 |
|
| Deuterium oxide (D, 99.9%) | Eurisotop | DLM-4DR-PK | |
| Dow Corning high-vacuum silicone grease | Sigma-Aldrich | Z273554-1EA | |
| Ethanol 96%, EMSURE Reag. Ph Eur | Sigma-Aldrich | 1.5901 | |
| Glass dessicator | VWR | 75871-660 | |
| Glass dessicator plate, 140 mm | VWR | 89038-068 | |
| Indium wire, 1.0 mm (0.04 in) dia, Puratronic, 99.999% | Alfa Aesar | 00470.G1 | |
| Lysozyme from chicken egg white dialyzed, lyophilized, powder, ~100,000 U/mg | Sigma-Aldrich | 62970 | |
| nPDyn | v3.x | see github.com/kpounot/nPDyn, model functions fot fitting also included in the software | |
| OHAUS AX324 Adventurer balance, internal calibration | Dutscher | 92641 | |
| Phosphorus pentoxide, ReagentPlus, 99% | Sigma-Aldrich | 214701 | |
| Pipette ErgoOne 0.5-10 μL | Starlab | S7100-0510 | |
| Pipette ErgoOne 100-1,000 μL | Starlab | S7100-1000 | |
| Pipette ErgoOne 20-200 μL | Starlab | S7100-2200 | |
| Pipette tip TipOne 1,000 μL | Starlab | S1111-6001 | |
| Pipette tip TipOne 10 μL | Starlab | S1111-3200 | |
| Pipette tip TipOne 200 μL | Starlab | S1111-0206 | |
| Sodium deuteroxide solution, 40 wt. % in D2O, 99.5 atom % D | Sigma-Aldrich | 372072 |
Riferimenti
- Jacrot, B. Des neutrons pour la science: Histoire de l’Institut Laue-Langevin. Des neutrons pour la science. EDP Sciences. , (2021).
- Mahieu, E., Gabel, F. Biological small-angle neutron scattering: recent results and development. Acta Crystallographica Section D. 74 (8), 715-726 (2018).
- Grimaldo, M., Roosen-Runge, F., Zhang, F., Schreiber, F., Seydel, T. Dynamics of proteins in solution. Quarterly Reviews of Biophysics. 52, 7 (2019).
- Martel, A., et al. Membrane permeation versus amyloidogenicity: A multitechnique study of islet amyloid polypeptide interaction with model membranes. Journal of the American Chemical Society. 139 (1), 137-148 (2017).
- Pounot, K., et al. Tracking internal and global diffusive dynamics during protein aggregation by high-resolution neutron spectroscopy. The Journal of Physical Chemistry Letters. 11 (15), 6299-6304 (2020).
- Grimaldo, M., et al. Protein short-time diffusion in a naturally crowded environment. The Journal of Physical Chemistry Letters. 10 (8), 1709-1715 (2019).
- Jasnin, M., Stadler, A., Tehei, M., Zaccai, G. Specific cellular water dynamics observed in vivo by neutron scattering and NMR. Physical Chemistry Chemical Physics. 12 (35), 10154-10160 (2010).
- Frick, B. The neutron backscattering spectrometer IN16 at ILL-high energy resolution with high intensity and excellent signal-to-noise ratio. Neutron News. 13 (2), 15-22 (2002).
- Frick, B., Mamontov, E., van Eijck, L., Seydel, T. Recent backscattering instrument developments at the ILL and SNS. Zeitschrift für Physikalische Chemie. 224 (1-2), 33-60 (2010).
- Frick, B., Combet, J., van Eijck, L. New possibilities with inelastic fixed window scans and linear motor Doppler drives on high resolution neutron backscattering spectrometers. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 669, 7-13 (2012).
- Appel, M., Frick, B., Magerl, A. A flexible high speed pulse chopper system for an inverted neutron time-of-flight option on backscattering spectrometers. Scientific Reports. 8 (1), 13580 (2018).
- Squires, G. L. . Introduction to the theory of thermal neutron scattering. , (1996).
- Singwi, K. S., Sjölander, A. Diffusive motions in water and cold neutron scattering. Physical Review. 119 (3), 863-871 (1960).
- Sears, V. F. Theory of cold neutron scattering by homonuclear diatomic liquids: i. free rotation. Canadian Journal of Physics. 44 (6), 1279-1297 (1966).
- Sears, V. F. Theory of cold neutron scattering by homonuclear liquid: ii. hindered rotation. Canadian Journal of Physics. 44 (6), 1299-1311 (1966).
- Schirò, G., et al. Translational diffusion of hydration water correlates with functional motions in folded and intrinsically disordered proteins. Nature Communications. 6, 6490 (2015).
- Grimaldo, M., et al. Hierarchical molecular dynamics of bovine serum albumin in concentrated aqueous solution below and above thermal denaturation. Physical Chemistry Chemical Physics. 17 (6), 4645-4655 (2015).
- Eanes, E. D., Glenner, G. G. X-ray diffraction studies on amyloid filaments. Journal of Histochemistry & Cytochemistry. 16 (11), 673-677 (1968).
- Bonar, L., Cohen, A. S., Skinner, M. M. Characterization of the Amyloid Fibril as a Cross-β Protein. Proceedings of the Society for Experimental Biology and Medicine. 131 (4), 1373-1375 (1969).
- Chiti, F., Dobson, C. M. Protein Misfolding, Amyloid Formation, and Human Disease: A Summary of Progress Over the Last Decade. Annual Review of Biochemistry. 86 (1), 27-68 (2017).
- Knowles, T. P. J., Vendruscolo, M., Dobson, C. M. The amyloid state and its association with protein misfolding diseases. Nature Reviews Molecular Cell Biology. 15 (6), 384-396 (2014).
- Maji, S. K., et al. Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science. 325 (5938), 328-332 (2009).
- Li, J., et al. The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell. 150 (2), 339-350 (2012).
- Knowles, T. P. J., Mezzenga, R. Amyloid fibrils as building blocks for natural and artificial functional materials. Advanced Materials. 28 (31), 6546-6561 (2016).
- Stephens, A. D., Kaminski Schierle, G. S. The role of water in amyloid aggregation kinetics. Current Opinion in Structural Biology. 58, 115-123 (2019).
- Adamcik, J., Mezzenga, R. Amyloid polymorphism in the protein folding and aggregation energy landscape. Angewandte Chemie International Edition. 57 (28), 8370-8382 (2018).
- Liu, Z., et al. Entropic contribution to enhanced thermal stability in the thermostable P450 CYP119. Proceedings of the National Academy of Sciences of the United States of America. 115 (43), 10049-10058 (2018).
- Coquelle, N., et al. Chromophore twisting in the excited state of a photoswitchable fluorescent protein captured by time-resolved serial femtosecond crystallography. Nature Chemistry. 10 (1), 31-37 (2018).
- Henzler-Wildman, K. A., et al. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 450 (7171), 913-916 (2007).
- Fichou, Y., et al. Hydration water mobility is enhanced around tau amyloid fibers. Proceedings of the National Academy of Sciences of the United States of America. 112 (20), 6365-6370 (2015).
- Burns, J., Pennock, C. A., Stoward, P. J. The specificity of the staining of amyloid deposits with thioflavine T. The Journal of Pathology and Bacteriology. 94 (2), 337-344 (1967).
- Iqbal, K., Liu, F., Gong, C. -. X., Grundke-Iqbal, I. Tau in Alzheimer disease and related tauopathies. Current Alzheimer Research. 7 (8), 656-664 (2010).
- Krȩżel, A., Bal, W. A formula for correlating pKa values determined in D2O and H2O. Journal of Inorganic Biochemistry. 98 (1), 161-166 (2004).
- Dolman, M., Halling, P. J., Moore, B. D., Waldron, S. How dry are anhydrous enzymes? Measurement of residual and buried 18O-labeled water molecules using mass spectrometry. Biopolymers. 41 (3), 313-321 (1997).
- Pounot, K. kpounotnPDyn: v3.0.0. Zenodo. , (2021).
- Yi, Z., Miao, Y., Baudry, J., Jain, N., Smith, J. C. Derivation of mean-square displacements for protein dynamics from elastic incoherent neutron scattering. Journal of Physical Chemistry B. 116 (16), 5028-5036 (2012).
- Peters, J., Kneller, G. R. Motional heterogeneity in human acetylcholinesterase revealed by a non-Gaussian model for elastic incoherent neutron scattering. The Journal of Chemical Physics. 139 (16), 165102 (2013).
- Zeller, D., Telling, M. T. F., Zamponi, M., García Sakai, V., Peters, J. Analysis of elastic incoherent neutron scattering data beyond the Gaussian approximation. The Journal of Chemical Physics. 149 (23), 234908 (2018).
- Roosen-Runge, F., Seydel, T. A generalized mean-squared displacement from inelastic fixed window scans of incoherent neutron scattering as a model-free indicator of anomalous diffusion confinement. EPJ Web of Conferences. 83, 02015 (2015).
- Ortega, A., Amorós, D., García de la Torre, J. Prediction of hydrodynamic and other solution properties of rigid proteins from atomic- and residue-level models. Biophysical Journal. 101 (4), 892-898 (2011).
- Hennig, M., Frick, B., Seydel, T. IUCr Optimum velocity of a phase-space transformer for cold-neutron backscattering spectroscopy. Journal of Applied Crystallography. 44 (3), 467-472 (2011).
- Paalman, H. H., Pings, C. J. Numerical evaluation of X-ray absorption factors for cylindrical samples and annular sample cells. Journal of Applied Physics. 33 (8), 2635-2639 (1962).
- Ow, S. -. Y., Dunstan, D. E. The effect of concentration, temperature and stirring on hen egg white lysozyme amyloid formation. Soft Matter. 9 (40), 9692-9701 (2013).
- Tominaga, T., Sahara, M., Kawakita, Y., Nakagawa, H., Yamada, T. Evaluation of sample cell materials for aqueous solutions used in quasi-elastic neutron scattering measurements. Journal of Applied Crystallography. 54 (6), 1631-1640 (2021).
- Beck, C., et al. Following protein dynamics in real time during crystallization. Crystal Growth & Design. 19 (12), 7036-7045 (2019).
- Smith, A. A., Testori, E., Cadalbert, R., Meier, B. H., Ernst, M. Characterization of fibril dynamics on three timescales by solid-state NMR. Journal of Biomolecular NMR. 65 (3-4), 171-191 (2016).
- Wang, T., Jo, H., DeGrado, W. F., Hong, M. Water distribution, dynamics, and interactions with Alzheimer’s β-amyloid fibrils investigated by solid-state NMR. Journal of the American Chemical Society. 139 (17), 6242-6252 (2017).
- Rezaei-Ghaleh, N., Giller, K., Becker, S., Zweckstetter, M. Effect of zinc dinding on β-amyloid structure and dynamics: Implications for Aβ aggregation. Biophysical Journal. 101 (5), 1202-1211 (2011).
- Vugmeyster, L., et al. Fast motions of key methyl groups in amyloid-β fibrils. Biophysical Journal. 111 (10), 2135-2148 (2016).
- Yang, X., Wang, B., Hoop, C. L., Williams, J. K., Baum, J. NMR unveils an N-terminal interaction interface on acetylated-α-synuclein monomers for recruitment to fibrils. Proceedings of the National Academy of Sciences of the United States of America. 118 (18), (2021).
- Tuttle, M. D., et al. Solid-state NMR structure of a pathogenic fibril of full-length human α-synuclein. Nature Structural & Molecular Biology. 23 (5), 409-415 (2016).
- Karamanos, T. K., Kalverda, A. P., Thompson, G. S., Radford, S. E. Mechanisms of amyloid formation revealed by solution NMR. Progress in Nuclear Magnetic Resonance Spectroscopy. 88-89, 86-104 (2015).
- Lai, Y. -. C., Kuo, Y. -. H., Chiang, Y. -. W. Identifying protein conformational dynamics using spin-label ESR. Chemistry – An Asian Journal. 14 (22), 3981-3991 (2019).
- Franck, J. M., Han, S. Overhauser dynamic nuclear polarization for the study of hydration dynamics, explained. Methods in Enzymology. 615, 131-175 (2019).
- Pavlova, A., et al. Protein structural and surface water rearrangement constitute major events in the earliest aggregation stages of tau. Proceedings of the National Academy of Sciences of the United States of America. 113 (2), 127-136 (2016).
- Lin, Y., et al. Liquid-liquid phase separation of tau driven by hydrophobic interaction facilitates fibrillization of tau. bioRxiv. , (2020).
- Decatur, S. M. Elucidation of residue-level structure and dynamics of polypeptides via isotope-edited infrared spectroscopy. Accounts of Chemical Research. 39 (3), 169-175 (2006).
- Chatani, E., Tsuchisaka, Y., Masuda, Y., Water Tsenkova, R. molecular system dynamics associated with amyloidogenic nucleation as revealed by real time near infrared spectroscopy and aquaphotomics. PLoS One. 9 (7), 101997 (2014).
- Goret, G., Aoun, B., Pellegrini, E. MDANSE: An interactive analysis environment for molecular dynamics simulations. Journal of Chemical Information and Modeling. 57 (1), 1-5 (2017).
- Fujiwara, S., et al. Internal dynamics of a protein that forms the amyloid fibrils observed by neutron scattering. Journal of the Physical Society of Japan. 82, (2013).
- Schiró, G., et al. Neutron scattering reveals enhanced protein dynamics in concanavalin a amyloid fibrils. Journal of Physical Chemistry Letters. 3 (8), 992-996 (2012).
- Pounot, K., et al. Zinc determines dynamical properties and aggregation kinetics of human insulin. Biophysical Journal. 120 (5), 886-898 (2021).
- Fujiwara, S., et al. Dynamic properties of human α-synuclein related to propensity to amyloid fibril formation. Journal of Molecular Biology. 431 (17), 3229-3245 (2019).
- Sanz, A., et al. High-pressure cell for simultaneous dielectric and neutron spectroscopy. Review of Scientific Instruments. 89 (2), 023904 (2018).
- Adams, M. A., et al. Simultaneous neutron scattering and Raman scattering. Applied Spectroscopy. 63 (7), 727-732 (2009).

