High-Throughput Screening of Microbial Isolates with Impact on Caenorhabditis elegans Health
Summary
Gut microbes may positively or negatively impact the health of their host via specific or conserved mechanisms. Caenorhabditis elegans is a convenient platform to screen for such microbes. The present protocol describes high-throughput screening of 48 bacterial isolates for impact on nematode stress resistance, used as a proxy for worm health.
Abstract
With its small size, short lifespan, and easy genetics, Caenorhabditis elegans offers a convenient platform to study the impact of microbial isolates on host physiology. It also fluoresces in blue when dying, providing a convenient means of pinpointing death. This property has been exploited to develop high-throughput label-free C. elegans survival assays (LFASS). These involve time-lapse fluorescence recording of worm populations set in multiwell plates, from which population median time of death can be derived. The present study adopts the LFASS approach to screen multiple microbial isolates at once for the effects on C. elegans susceptibility to severe heat and oxidative stresses. Such microbial screening pipeline, which can notably be used to prescreen probiotics, using severe stress resistance as a proxy for host health is reported here. The protocol describes how to grow both C. elegans gut microbiota isolate collections and synchronous worm populations in multiwell arrays before combining them for the assays. The example provided covers the testing of 47 bacterial isolates and one control strain on two worm strains, in two stress assays in parallel. However, the approach pipeline is readily scalable and applicable to the screening of many other modalities. Thus, it provides a versatile setup to rapidly survey a multiparametric landscape of biological and biochemical conditions that impact C. elegans health.
Introduction
The human body harbors an estimated 10-100 trillion live microbial cells (bacteria, archaea fungi), which are primarily found in the gut, skin, and mucosal environments1. In a healthy state, these provide benefits to their host, including vitamin production, maturation of the immune system, stimulation of innate and adaptive immune responses to pathogens, regulation of fat metabolism, modulation of stress responses, and more, with an impact on growth and development, disease onset, and ageing2,3,4,5. The gut microbiota also evolves considerably throughout life. The most drastic evolution occurs during infancy and early childhood6, but significant changes also occur with age, including a decrease in Bifidobacterium abundance and an increase in Clostridium, Lactobacillus, Enterobacteriaceae, and Enterococcus species7. Lifestyle can further alter gut microbial composition leading to dysbiosis (loss of beneficial bacteria, overgrowth of opportunistic bacteria), resulting in various pathologies such as inflammatory bowel disease, diabetes, and obesity5, but also contributing to Alzheimer's and Parkinson's diseases8,9,10,11.
This realization has critically contributed to refining the concept of the gut-brain axis (GBA), where interactions between gut physiology (now including the microbes within it) and the nervous system are considered the main regulator of animal metabolism and physiological functions12. However, the precise role of microbiota in gut-brain signaling and the associated mechanisms of action are far from being fully understood13. With gut microbiota being a key determinant of healthy aging, how bacteria modulate the aging process has become a subject of intense research and controversy6,14,15.
With the demonstration that the roundworm Caenorhabditis elegans hosts a bonafide gut microbiota dominated-as in other species-by Bacteroidetes, Firmicutes, and Actinobacteria16,17,18,19,20, its rapid rise as an experimental platform to study host-gut commensal interactions21,22,23,24,25,26 has significantly expanded our investigative arsenal26,27,28,29. In particular, high-throughput experimental approaches available for C. elegans to study gene-diet, gene-drug, gene-pathogen, etc. interactions, can be adapted to rapidly explore how bacterial isolates and cocktails impact C. elegans health and aging.
The present protocol describes an experimental pipeline to screen at once arrays of bacterial isolates or mixtures set in multiwell plates for effects on C. elegans stress resistance as a proxy for health, which can be used to identify probiotics. It details how to grow large worm populations and handle bacterial arrays in 96- and 384-well plate formats before processing worms for automated stress resistance analysis using a fluorescence plate reader (Figure 1). The approach is based on label-free automated survival assays (LFASS)30 that exploit the phenomenon of death fluorescence31, whereby dying worms produce a burst of blue fluorescence that can be used to pinpoint the time of death. Blue fluorescence is emitted by glucosyl esters of anthranilic acid stored in C. elegans gut granules (a type of lysosome-related organelle), which burst when a necrotic cascade is triggered in the worm gut upon death31.
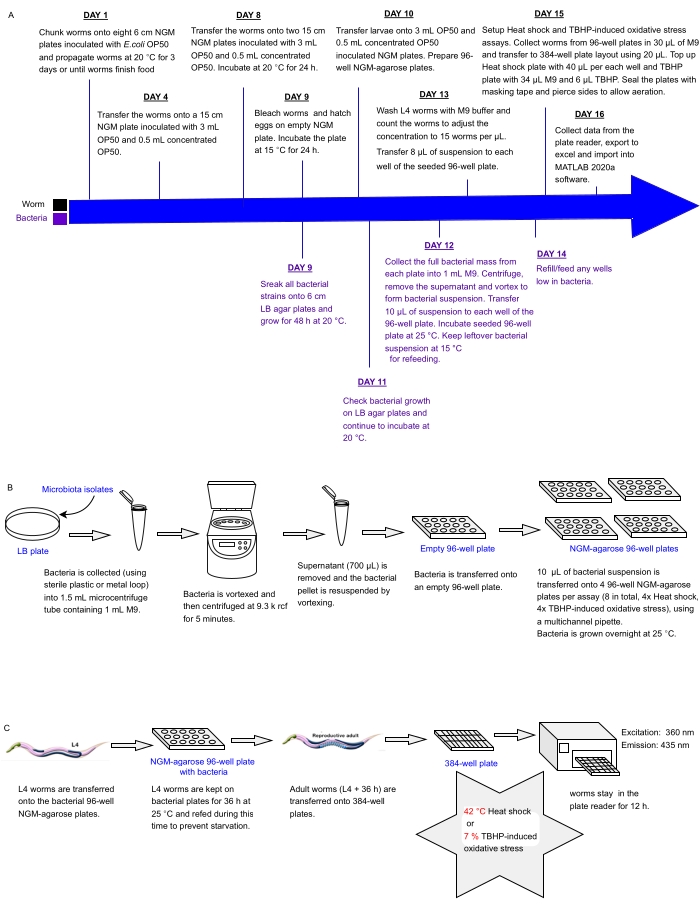
Figure 1: Experimental workflow for high-throughput screening of bacterial isolates with impact on C. elegans resistance to stress. (A) Timeline for worm and bacterial maintenance and assay setup. (B) 96-well bacterial plate array setup and handling. (C) 384-well worm plate setup. Please click here to view a larger version of this figure.
Protocol
Representative Results
Discussion
C. elegans offers many advantages for rapidly screening multiple experimental parameters at once, owing to its small size, transparency, fast development, short lifespan, inexpensiveness, and ease of handling. Its considerably simpler genome, body plan, nervous system, gut, and microbiome, yet complex and similar enough to humans, make it a powerful preclinical model, where mechanistic insight can be gained while testing for bioactive efficacy or toxicity. As interest is growing in developing microbial intervent…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank the CGC Minnesota (Madison, USA, NIH – P40 OD010440) for providing worm strains and OP50 and Pr. Hinrich Schulenburg (CAU, Kiel, Germany) for providing all the environmental microbial isolates depicted here. This work was funded by a UKRI-BBSRC grant to AB (BB/S017127/1). JM is funded by a Lancaster University FHM PhD scholarship.
Materials
| 10 cm diameter plates (Non-vented) | Fisher Scientific | 10720052 | Venting is not necessary for bacterial cultures |
| 15 cm diameter plates (Vented) | Fisher Scientific | 168381 | |
| 384-well black, transparent flat bottom plates | Corning | 3712 or 3762 | Not essential to be sterile for fast stress assays |
| 6 cm diameter plates (Vented) | Fisher Scientific | 150288 | Venting is necessary for worm cultures to avoid hypoxia |
| 96-well transparent plates (Biolite) | Thermo | 130188 | |
| Agar (<4% ash) | Sigma-Aldrich | 102218041 | Good quality agar is important for the structural integrity of the culture media, to avoid worm burrowing |
| Agarose | Fisher Scientific | BP1356 | |
| Avanti Centrifuge J-26 XP | Beckman coulter | ||
| Bleach | Honeywell | 425044 | |
| Calcium chloride | Sigma-Aldrich | C5080 | |
| Centrifuge 5415 R | Eppendorf | ||
| Centrifuge 5810 R | Eppendorf | ||
| Cholesterol | Sigma-Aldrich | C8667 | |
| LB agar | Difco | 240110 | |
| LB broth | Invitrogen | 12795084 | |
| LoBind tips | VWR | 732-1488 | Lo-bind reduce worm loss during transfers |
| LoBind tubes | Eppendorf | 22431081 | |
| Magnesium sulfate | Fisher Scientific | M/1100/53 | |
| Plate reader- infinite M nano+ | Tecan | Monochromator setup enables fluorescence tuning but adequate filter-based setups may be used | |
| Plate reader- Spark | Tecan | ||
| Potassium phosphate monobasic | Honeywell | P0662 | |
| Sodium chloride | Sigma-Aldrich | S/3160/63 | |
| Stereomicroscope setup with transillumination base | Leica | MZ6, or M80 | Magnification from 0.6-0.8x up to 40-60x is necessary, as is a good quality transillumination base with a deformable, titable or slidable mirror to adjust contrast |
| t-BHP (tert-Butyl hydroperoxide) | Sigma-Aldrich | 458139 | |
| Transparent adhesive seals Nunc | Fisher Scientific | 101706871 | It is important that it is transparent and that it can tolerate the temperatures involved in the assays. |
| Tryptophan | Sigma-Aldrich | 1278-7099 | |
| Yeast extract | Fisher Scientific | BP1422 |
Riferimenti
- Krishna, S., et al. Integrating microbiome network: establishing linkages between plants, microbes and human health. The Open Microbiology Journal. 13, 330-342 (2019).
- Amon, P., Sanderson, I. What is the microbiome. Archives of Disease in Childhood – Education & Practice Edition. 102 (5), 257-260 (2017).
- Belkaid, Y., Harrison, O. J. Homeostatic immunity and the microbiota. Immunity. 46 (4), 562-576 (2017).
- Cabreiro, F., Gems, D. Worms need microbes too: microbiota, health and aging in Caenorhabditis elegans. EMBO Molecular Medicine. 5 (9), 1300-1310 (2013).
- Vaga, S., et al. Compositional and functional differences of the mucosal microbiota along the intestine of healthy individuals. Scientific Reports. 10 (1), 14977 (2020).
- Nagpal, R., et al. Gut microbiome and aging: Physiological and mechanistic insights. Nutrition and Healthy Aging. 4 (4), 267-285 (2018).
- Mitsuoka, T. Establishment of intestinal bacteriology. Biosci Microbiota Food Health. 33 (3), 99-116 (2014).
- Bonfili, L., et al. Microbiota modulation as preventative and therapeutic approach in Alzheimer’s disease. The FEBS Journal. 288 (9), 2836-2855 (2021).
- Vendrik, K. E. W., et al. Fecal microbiota transplantation in neurological disorders. Frontiers in Cellular and Infection Microbiology. 10, 98 (2020).
- Wang, Q., et al. The role of gut dysbiosis in Parkinson’s disease: mechanistic insights and therapeutic options. Brain. 144 (9), 2571-2593 (2021).
- Zhu, X., et al. The relationship between the gut microbiome and neurodegenerative diseases. Neuroscience Bulletin. 37 (10), 1510-1522 (2021).
- Miller, I. The gut-brain axis: historical reflections. Microbial Ecology in Health and Disease. 29 (1), 1542921 (2018).
- Foster, J. A., Rinaman, L., Cryan, J. F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress. 7, 124-136 (2017).
- Coman, V., Vodnar, D. C. Gut microbiota and old age: Modulating factors and interventions for healthy longevity. Experimental Gerontology. 141, 111095 (2020).
- Conway, J., Duggal, N. A. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Research Reviews. 68, 101323 (2021).
- Berg, M., et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. The ISME Journal. 10 (8), 1998-2009 (2016).
- Dirksen, P., et al. CeMbio – The Caenorhabditis elegans Microbiome Resource. G3: Genes, Genomes, Genetics. 10 (9), 3025-3039 (2020).
- Dirksen, P., et al. The native microbiome of the nematode Caenorhabditis elegans: gateway to a new host-microbiome model. BMC Biology. 14, 38 (2016).
- Samuel, B. S., Rowedder, H., Braendle, C., Felix, M. A., Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proceedings of the National Academy of Sciences of the United States of America. 113 (27), 3941-3949 (2016).
- Zimmermann, J., et al. The functional repertoire contained within the native microbiota of the model nematode Caenorhabditis elegans. The ISME Journal. 14 (1), 26-38 (2020).
- Dinic, M., et al. Host-commensal interaction promotes health and lifespan in Caenorhabditis elegans through the activation of HLH-30/TFEB-mediated autophagy. Aging. 13 (6), 8040-8054 (2021).
- Goya, M. E., et al. Probiotic Bacillus subtilis protects against alpha-Synuclein aggregation in C. elegans. Cell Reports. 30 (2), 367-380 (2020).
- Hacariz, O., Viau, C., Karimian, F., Xia, J. The symbiotic relationship between Caenorhabditis elegans and members of its microbiome contributes to worm fitness and lifespan extension. BMC Genomics. 22 (1), 364 (2021).
- Shin, M. G., et al. Bacteria-derived metabolite, methylglyoxal, modulates the longevity of C. elegans through TORC2/SGK-1/DAF-16 signaling. Proceedings of the National Academy of Sciences of the United States of America. 117 (29), 17142-17150 (2020).
- Zhang, F., et al. Natural genetic variation drives microbiome selection in the Caenorhabditis elegans gut. Current Biology. 31 (12), 2603-2618 (2021).
- Zhang, F., et al. High-throughput assessment of changes in the Caenorhabditis elegans gut microbiome. Methods in Molecular Biology. 2144, 131-144 (2020).
- Chan, J. P., et al. Using bacterial transcriptomics to investigate targets of host-bacterial interactions in Caenorhabditis elegans. Scientific Reports. 9 (1), 5545 (2019).
- Hartsough, L. A., et al. Optogenetic control of gut bacterial metabolism to promote longevity. Elife. 9, 56849 (2020).
- Pryor, R., et al. Host-microbe-drug-nutrient screen identifies bacterial effectors of Metformin therapy. Cell. 178 (6), 1299-1312 (2019).
- Benedetto, A., et al. New label-free automated survival assays reveal unexpected stress resistance patterns during C. elegans aging. Aging Cell. 18 (5), 12998 (2019).
- Coburn, C., et al. Anthranilate fluorescence marks a calcium-propagated necrotic wave that promotes organismal death in C. elegans. PLOS Biology. 11 (7), 1001613 (2013).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Ceron, J. Basic Caenorhabditis elegans methods: synchronization and observation. Journal of Visualized Experiments. (64), e4019 (2012).
- Stiernagle, T. Maintenance of C. elegans. WormBook. , 1-11 (2006).
- Naomi, R., et al. Probiotics for Alzheimer’s disease: a systematic review. Nutrients. 14 (1), 20 (2021).
- Zheng, S. Y., et al. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Research Reviews. 69, 101347 (2021).
- Gill, M. S., Olsen, A., Sampayo, J. N., Lithgow, G. J. An automated high-throughput assay for survival of the nematode Caenorhabditis elegans. Free Radical Biology and Medicine. 35 (6), 558-565 (2003).
- Park, H. -. E. H., Jung, Y., Lee, S. -. J. V. Survival assays using Caenorhabditis elegans. Molecules and Cells. 40 (2), 90-99 (2017).
- Partridge, F. A., et al. An automated high-throughput system for phenotypic screening of chemical libraries on C. elegans and parasitic nematodes. International Journal for Parasitology: Drugs and Drug Resistance. 8 (1), 8-21 (2018).
- Rahman, M., et al. NemaLife chip: a micropillar-based microfluidic culture device optimized for aging studies in crawling C. elegans. Scientific Reports. 10 (1), 16190 (2020).
- Stroustrup, N., et al. The Caenorhabditis elegans lifespan machine. Nature Methods. 10 (7), 665-670 (2013).
- Xian, B., et al. WormFarm: a quantitative control and measurement device toward automated Caenorhabditis elegans aging analysis. Aging Cell. 12 (3), 398-409 (2013).
- Brown, A. E., Schafer, W. R. Unrestrained worms bridled by the light. Nature Methods. 8 (2), 129-130 (2011).
- Churgin, M. A., et al. Longitudinal imaging of Caenorhabditis elegans in a microfabricated device reveals variation in behavioral decline during aging. Elife. 6, 26652 (2017).
- Jushaj, A., et al. Optimized criteria for locomotion-based healthspan evaluation in C. elegans using the WorMotel system. PLoS One. 15 (3), 0229583 (2020).
- Nambyiah, P., Brown, A. E. X. Quantitative behavioural phenotyping to investigate anaesthesia induced neurobehavioural impairment. Scientific Reports. 11 (1), 19398 (2021).
- Squiban, B., Belougne, J., Ewbank, J., Zugasti, O. Quantitative and automated high-throughput genome-wide RNAi screens in C. elegans. Journal of Visualized Experiments. (60), e3448 (2012).
- Zugasti, O., et al. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nature Immunology. 15 (9), 833-838 (2014).
- Zugasti, O., et al. A quantitative genome-wide RNAi screen in C. elegans for antifungal innate immunity genes. BMC Biology. 14, 35 (2016).

