Topographical EEG Recordings of Visual Evoked Potentials in Mice using Multichannel Thin-film Electrodes
Summary
The present protocol describes a simple procedure to acquire and analyze the topography of epicranial visual evoked potentials with 32-multichannel thin-film electrodes in the mouse.
Abstract
Visual evoked potentials (VEP) allow the characterization of visual function in preclinical mouse models. Various methods exist to measure VEPs in mice, from non-invasive EEG, subcutaneous single-electrodes, and ECoG to fully invasive intracortical multichannel visual cortex recordings. It can be useful to acquire a global, topographical EEG-level characterization of visual responses previous to local intracortical microelectrode measurements in acute experimental settings. For example, one use case is to assess global cross-modal changes in VEP topography in deafness models before studying its effects on a local intracortical level. Multichannel epicranial EEG is a robust method to acquire such an overview measure of cortical visual activity. Multichannel epicranial EEG provides comparable results through a standardized, consistent approach to, for example, identify cross-modal, pathological, or age-related changes in cortical visual function. The current study presents a method to obtain the topographical distribution of flash-evoked VEPs with a 32-channel thin-film EEG electrode array in anesthetized mice. Combined with analysis in the time and frequency domain, this approach allows fast characterization and screening of the topography and basic visual properties of mouse cortical visual function, which can be combined with various acute experimental settings.
Introduction
Mice are a preclinical model of degenerative processes of vision and ophthalmological diseases1,2,3,4. Visual evoked potentials (VEPs) are commonly used to measure cortical visual function and, for example, to assess visual degeneration in pathological models5,6. The VEP latency, conduction time, amplitude, multifocal characteristics, or spatial acuity of cortical visual evoked potentials provide diagnostic information on the functional integrity of the visual system7,8,9.
In mice, cortical visual evoked potentials can be measured across various spatial scales with methods of different complexity from non-invasive EEG, subdermal needle electrodes, and skull-implanted screws, to fully invasive intracranial approaches with epicortical ECoG, to intracortical electrode recordings10,11,12,13,14,15,16,17. These methods have different strengths and weaknesses. For example, a low number of electrodes only provides limited information on cortical VEP distribution, whereas subcutaneous needle electrodes often fail to ensure consistent recording locations. Moreover, implanted screws or fully invasive methods require damaging, penetrating, or removing of the skull and often provide only local information.
In acute experiments, a first global overview of cortical visual function is often desired, which is eventually followed by further experimental steps and compared to local intracortical recordings. For example, one potential use case is utilized first to investigate the EEG-level effects of visual cross-modal reorganization of deafness or hearing loss on VEP topography and cortical visual activity18,19 before studying the impacts on a local intracortical level.
Multichannel EEG recordings with thin-film multi-electrode arrays can provide a systematized VEP topography from the mouse skull20,21,22,23,24. Such epicranial recordings can have advantages over ECoG recordings by leaving the integrity of the skull intact and avoiding direct manipulation of the cortical surface. In addition, thin-film multi-electrodes provide a standardized electrode configuration, allowing the comparison of visual evoked spatio-temporal brain activity between experiments similar to a standardized EEG system in humans25. A standardized framework also facilitates using common EEG analysis toolboxes (e.g., Fieldtrip, Chronux, EEGLAB, and ERPLAB) to analyze mouse EEG in the time and frequency domain or in terms of connectivity26,27,28,29,30,31.
The present protocol describes a procedure for topographical VEP recordings in mice using a 32-channel thin-film electrode. This can be used as part of acute experiments followed by additional experimental steps, such as intracortical microelectrode recordings from specific brain areas. It is demonstrated here how to reliably record epicranial flash evoked VEPs with 32-channel thin-film electrodes from the mouse. In addition, exemplary analysis of topographical VEP recordings in the time and frequency domain is presented.
Protocol
All animals were handled and housed according to German (TierSchG, BGBl. I S. 1206, 1313) and European Union (ETS 123; Directive 2010/63/EU) guidelines for animal research. The animal experiments were approved by German state authorities (Lower Saxony State Office for Consumer Protection and Food Safety, LAVES) and were monitored by the university animal welfare officer. A 3-month-old male C57BL/6J mouse was used for the present study.
1. Animal details
- Perform the VEP measures in mice adequate for the particular research question.
- Be aware of mouse strain peculiarities in physiology that can impact the experiment by altered anesthetic sensitivities, seizure susceptibility, age, or genetic background.
NOTE: Mouse strains with different genetic backgrounds may often develop other sensory impairments (e.g., C57BL/6 progressive hearing loss), which might go unnoticed but indirectly affect visual processing (e.g., cross-modal influences19).
2. Induction of general anesthesia
- Prepare a surgical field at the recording site. Determine the pre-surgical weight of the mouse.
- Induce anesthesia with ketamine/xylazine, i.p., with a dosage of 100 mg/kg ketaminehydrochloride, 4 mg/kg xylazinhydrochloride, and 5mg/kg carprofen (see Table of Materials).
- Immediately after injection, place the mouse back in the cage.
- Place an infrared heating lamp (see Table of Materials) at an adequate distance and wait for full induction of general anesthesia for at least 5 min.
- Observe the stopping of spontaneous movements and loss of the righting reflex. Check for loss of the toe-pinch reflex.
3. Physiological monitoring and maintenance of general anesthesia
- Transfer the mouse to the recording site onto a heating pad.
- Use a rectal temperature probe attached to a temperature control system to keep the core body temperature at 37.6-37.8 °C.
- Ensure optimal body temperature of the mouse. In addition to the heating pad, minimize heat loss by increasing room temperature if required.
- Place two subcutaneous silver wire electrodes (0.2 mm diameter, see Table of Materials) near the right shoulder and left hind leg for ECG monitoring.
- Attach the silver wire ECG electrodes to the ECG amplifier.
- Monitor heart rate and ECG waves on an oscilloscope (see Table of Materials) during the experiment.
NOTE: During adequate ketamine/xylazine anesthesia, heart rate can range between 160-250 bpm32,33. Heart rate can vary depending on mouse strain, age, and physiological status. - Check the toe-pinch reflex and vital signs. The absence of the toe-pinch reflex response indicates a sufficient level of anesthesia.
- Monitor the status and quality of the eyes during the procedure closely, and apply ophthalmic drops or gel.
- Monitor adequate anesthesia levels by regularly checking physiological parameters, heart rate, reflexes, and EEG activity.
- If the general anesthesia level gets too light, apply an additional dose of ketamine/xylazine (i.p.).
- Proceed fast with the experimental steps to minimize the duration of general anesthesia and the number of subsequent doses of additional ketamine/xylazine used to maintain general anesthesia.
4. Electrode placement and recording setup
- At the recording site, reassess adequate anesthetic depth by checking the toe-pinch reflex.
- Shave the top of the head with an electric shaver.
- To allow a free visual field, do not fixate the mice in a stereotactic frame during the experiment.
- Apply lidocaine (if additionally required by your local institutional guidelines for animal care).
- Make a 1 cm midline incision of the scalp.
- Retract the skin to both sides using small vessel clamps to hold the skin in place and to expose the skull.
- Clean the surface of the skull with a cotton swab and saline NaCl (0.9%).
- Attach a silver wire reference electrode (diameter 0.2 mm) for the EEG subcutaneously above the nose (nose reference) or behind the ear (mastoid).
- Connect the nose reference via the probe connector to the headstage (see Table of Materials) reference output.
- Next, attach the EEG electrode to the headstage using an adapter attached to the headstage via a probe connector and attached to an electrode holder (see Table of Materials).
- Place the multichannel thin-film EEG electrode (see Table of Materials) on the skull with bregma as a reference position to ensure a standardized electrode position for each experiment.
- Use a drop of saline to adjust the electrode's position. While the skull surface is still wet, the electrode is easily movable. Then, dry the skull carefully with a cotton swab.
- Wait until the saline solution dries and the electrode firmly attaches to the skull.
- Cover the electrode with a small drop of silicone oil to avoid the adherence of the electrode to the overlying skin. This step helps to easily remove the electrode after the recording.
NOTE: Be careful not to apply too much silicone oil to avoid deteriorating the contact of the electrode with the skull. - Close the skin over the electrode for protection and to minimize electrical noise.
- Apply a small drop of tissue adhesive (see Table of Materials) to the skin to prevent the reopening of the skin incision.
5. EEG recording and VEP measurement
- Filter EEG signals during acquisition with a wide filter between 1-9000 Hz. Use wide filter settings for the control of artifacts. Perform noise and adequate filtering later during offline processing.
- Check the signal quality of the ongoing EEG activity by checking for high-frequency electrical noise, 50/60 Hz artifacts, and ECG artifacts. Apply adequate ground and reference connections to the animal and equipment or apply adequate shielding.
- Check anesthesia depth by observing EEG activity. Try to avoid a 'too deep' anesthesia state which is characterized by the occurence of burst-suppression EEG activity. Consider the experiment's time course and ensure that anesthesia does not get too light during the recording.
- Use an LED stroboscope (or other types of visual stimulation) to produce ultra-short flashes (see Table of Materials) (Figure 1).
- Place the stroboscope at a 30 cm distance for binocular visual stimulation in front of the mouse.
NOTE: The mouse and stroboscope are placed in a dark and soundproof chamber during the experiment. - Adapt the mice to the dark for 5 min before stimulation.
- Elicit stroboscope flashes by triggering the stroboscope with a TTL pulse.
NOTE: A stimulation artifact can occur due to the high voltage of the stroboscope. This can be attenuated by increasing the distance or shielding the electrode. - Generate hardware TTL triggers with a multifunction I/O device (see Table of Materials) controlled by a stimulation PC to trigger the light source (i.e., stroboscope).
- Send TTL signal in parallel to the acquisition system to provide high-precision timestamps for the stimulus onset.
- Send TTL output, which is synchronized with the flash, from the LED stroboscope as feedback and as a second control for accurate stimulus onset trigger timestamps. Register this together with the recordings.
- Set the stimulus rate (e.g., inter-stimulus interval = 2 s). Set the number of stimulus repetitions (e.g., n = 150 repetitions)
- Adjust the intensities manually on the stroboscope (e.g., flash duration of 512 µs at a fixed value of 3000 lm).
NOTE: This, however, has to be adapted to the specific experimental question. - Start the stimulus presentation, record the EEG signals with recording software (see Table of Materials), and save the data on the recording PC.
6. Completion of the experiment, electrode removal, and electrode cleaning
- If the recording is part of a longer protocol, continue with further experimental steps.
- At the end of the experiment, euthanize the animal according to institutional guidelines23,34.
- Remove the EEG electrode with care (since they can be reused).
- Slowly with the application of fluid remove the electrode from the skull.
NOTE: The electrodes can stick to the surface of the skull; avoid damage to the platinum sites during removal. - Clean the electrode after the experiment with isotonic saline NaCl (0.9%) and immerse it in a protein remover and disinfecting solution (see Table of Materials) for 30 min.
- Store the electrode array in a dry, protected place.
7. Basic VEP signal processing: Time and frequency domain
- Import the raw data files into the analysis software (see Table of Materials).
- Filter EEG signals with a Butterworth bandpass zero-phase filter between 1-100 Hz.
NOTE: Select adequate filter types and parameters depending on the respective research question27,35,36. - Downsample the signals to 1000 Hz.
- Segment the EEG signals into trials and average the trials to yield visual evoked potentials and determine the N1 peak latency.
NOTE: Re-referencing can be performed offline if necessary (e.g., common average referencing, bipolar referencing, local average "Laplacian" referencing)23. - Determine the voltage values at specific time points of interest and plot voltage distribution maps using the analysis software.
- Calculate global field power37 by calculating the spatial standard deviation of all the electrode channels.
- Calculate a multi-taper time-frequency spectrogram with Chronux Toolbox28,31. Use a bandwidth product TW = 3 and K = 5 tapers with a 300 ms window size and 30 ms step size.
Representative Results
Recording visual evoked potentials with multichannel EEG allows the assessment of the topography of VEP amplitudes, latencies, or frequency components in mice. Figure 2A shows an example of a flash evoked VEP topography recorded with an epicranial 32-channel EEG from a 3-month-old male C57BL/6J mouse. The strongest visual evoked activity occurs in the occipital region above the visual cortex.
Figure 2B shows the voltage distribution over the skull at different time points pre- and post flash onset. The strongest N1 amplitude of the VEP occurs at 50 ms poststimulus above the central occipital regions. This is followed by a potential reversal and a positive peak across the occipital and frontal areas at 300 ms poststimulus. The butterfly plot in Figure 1B shows the peak latency of the VEP N1 component at 46 ms and a positive peak at around 300 ms post onset.
Figure 2C shows the global field power calculated from all the EEG channels37,38. This can be used to analyze the multichannel EEG data and determine the peak latency (46 ms), though it does not show changes at 300 ms poststimulus in the shown example. Moreover, it can evaluate time points of interest where topographical signals spatially change from a common modification.
Figure 2D shows a time-frequency analysis of the response and gamma power in the 30-45 Hz power distribution at different time points. The topographies for gamma power are also centered above the central occipital regions and shift toward more frontal regions at later time points. Below, the time-frequency plot for an occipital single EEG channel with a multi-taper spectrogram shows fast activity during the response.
VEP characteristics are comparable between C57Bl/6J mice of 2-3 months old (Figure 3A). The topography shows consistent spatiotemporal patterns above the occipital and frontal areas at different time points in three example mice (Figure 3B). The spatiotemporal negative potentials occur above the occipital regions at 50 ms post stimulus onset, followed by positivity around 300 ms shifted toward the frontal regions in all mice.
The latencies are also consistent between mice (Figure 3B), with N1 component latencies (46 ms, 44 ms, 57 ms) and amplitudes (0.14 mV, 0.12 mV, 0.13 mV). However, amplitudes differ more frequently because they can be affected by variables, electrode impedance, electrode contacts, and visual status. Similarly, global field power (i.e., the analysis of multichannel EEG data) reflects consistency between animals, with peak latencies (46 ms, 44 ms, 57 ms) reflecting intracortical visual response latencies39.
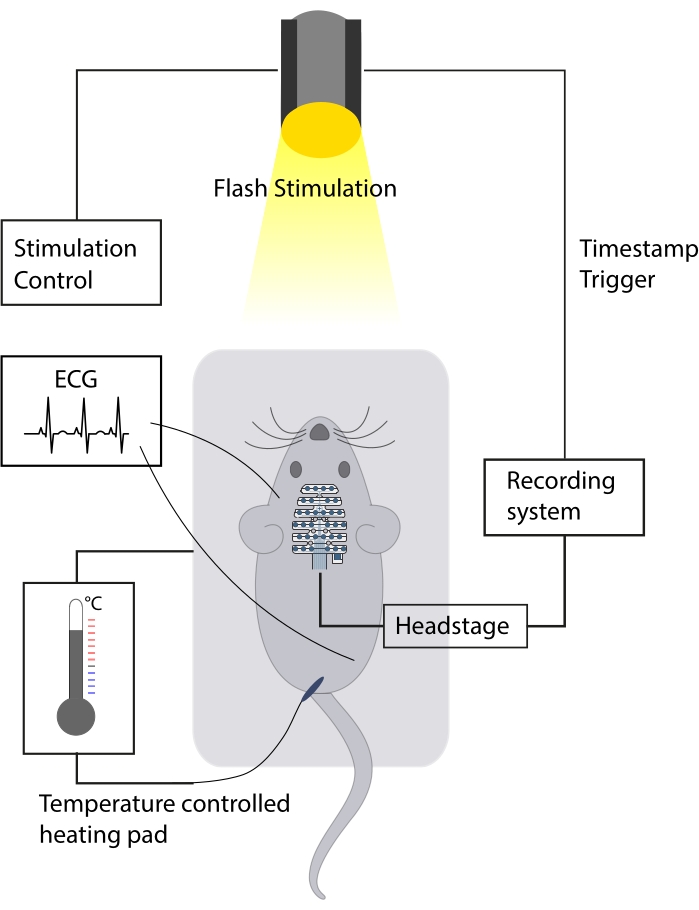
Figure 1: Schematic of the experimental setup. The mouse is preferably placed inside a soundproof chamber. A temperature-controlled heating pad is used to maintain adequate body temperature throughout the experiment. ECG recordings are assessed with silver wire ECG electrodes, which allow for heart rate monitoring. EEG signals are recorded with a multichannel EEG electrode during visual stimulation elicited with a LED stroboscope. Please click here to view a larger version of this figure.
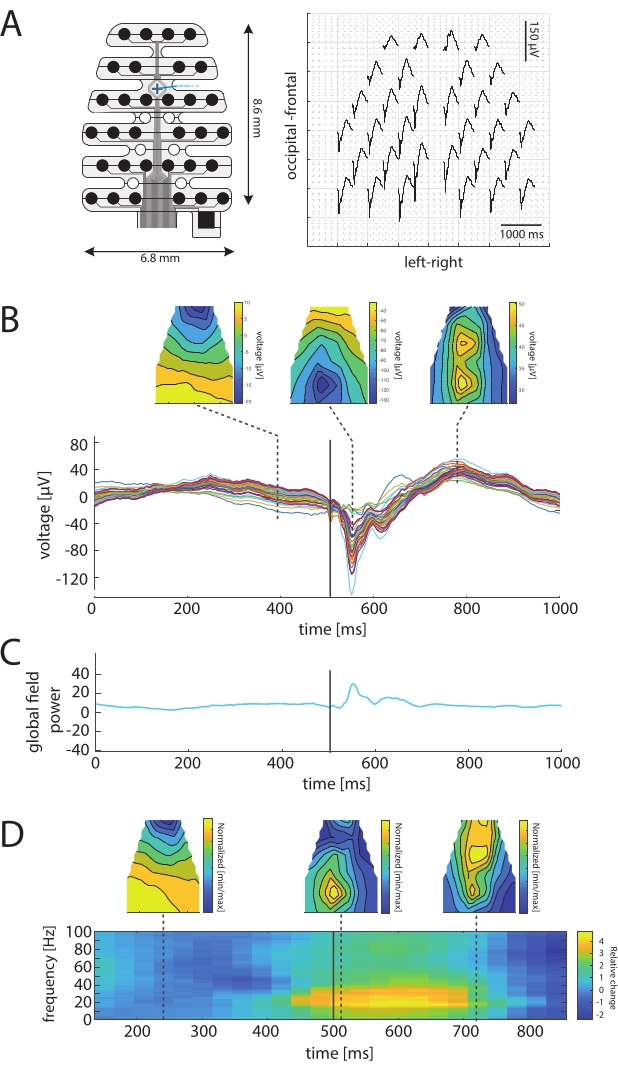
Figure 2: VEP topography and global field power in the mouse EEG. (A) 32-channel EEG electrode (left) and the topography of VEPs (right) from a 500 ms interval post stimulus onset (right). (B) VEP topographies at different time points after flash stimulation (150 repetitions) to both eyes (top). The distribution of visual evoked potentials shows the concentration of the largest responses in the occipital regions. Below: individual 32 EEG traces for an interval of 500 ms pre- and 500 ms post stimulus onset with negative peak N1. A deflection generated by the stimulus artifact is visible at stimulus onset. (C) Global field power of the EEG signals. (D) Time-frequency representation of visually evoked activity for one occipital channel (bottom). Topographical power distribution in the gamma range (30-40 Hz) at different time points. Please click here to view a larger version of this figure.
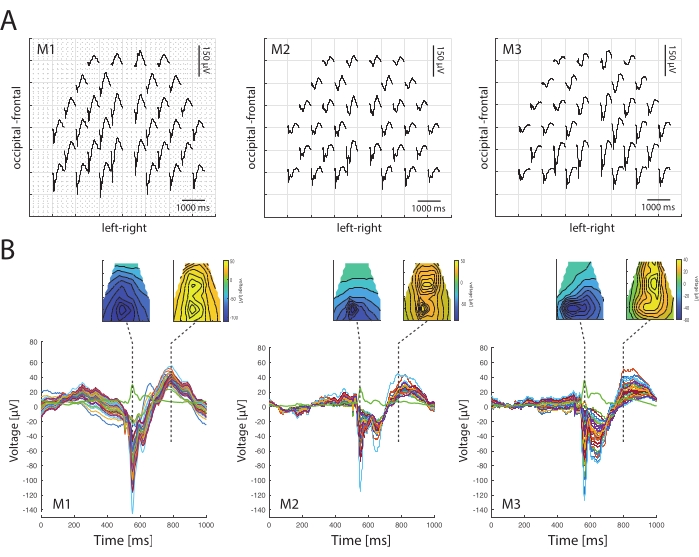
Figure 3: Comparison of spatiotemporal profiles of visual evoked potentials between three different mice. (A) VEP topographies of three mice (M1, M2, M3) after flash stimulation to both eyes (average of 150 repetitions). Traces are shown for a duration of 500 ms post stimulus onset. The distribution of visual evoked potentials shows the concentration of the largest responses in the occipital regions. (B) Spatiotemporal profiles are shown at different time points for the three example mice at post 50 ms and post 300 ms relative to stroboscope flash; below are butterfly plots of the responses above for the three mice. VEPs show a prominent negative peak N1. The green line shows the global field power of responses and reflects the latency of EEG activity. Please click here to view a larger version of this figure.
Discussion
This article describes a method for recording epi-cranial multichannel EEG with thin-film electrodes and how to acquire a consistent topographical representation of visual evoked potentials in the mouse. Here, we exemplarily showed binocular flash stimulation, but this approach can also be applied with other types of visual stimuli (i.e., monocular, spatial gratings, focal visual field) using, for example, a larger display.
A critical step in the protocol is the positioning of the electrode. Precise and consistent electrode positioning must be ensured to allow comparability between experiments. Importantly, the electrode position needs to be documented for later comparison (i.e., operating microscope images). Although different types of thin-film electrodes and configurations (from other distributors) can be used, it is advised to decide on one defined electrode configuration and stick to it for back-comparability in experiments. A second critical aspect is good electrode contact with the skull. The quality of contact influences the electrode impedance and signal quality40. Thus, similar contact to the skull of all the electrode sites is important, especially for analyzing spatial response distributions. If an electrode channel shows a noisy signal (i.e., high-frequency components), it must be ensured that sufficient contact with the skull is provided, and impedances must be tested during the experiments. However, the present VEP recording is quite robust for overall signal quality.
If the researcher wants to reuse the same electrode multiple times, removing the electrode is another important step. It is important to carefully and slowly separate the electrode from the skull by using liquid (e.g., saline) to avoid damaging the contacts during removal. Monitoring animal health and physiological status is an important aspect of all animal experiments. Physiological status can influence comparability. It is advised to proceed fast (i.e., similar timing between experiments) with the protocol. Ensuring physiological body temperature is especially important (i.e., it helps to increase the surrounding temperature and to work in a warm room). One should take special care of the eyes of the mouse, as they reflect the physiological state, but leaving them untouched is often the best way. In case the eyes of the mouse become opaque, this is a sign that the physiological condition of the mouse is degrading. This state is reversible41; however, the animal's physiological condition requires special attention (it must be ensured that the core body temperature is in the physiological range, the heart rate and ECG waves should be checked, and excessively deep anesthesia levels should be avoided).
Monitoring adequate anesthesia depth is essential. It is important to avoid too light and too deep anesthesia, characterized by the presence of burst-suppression in the EEG signal. This can lead to unwanted effects in terms of sensory responsiveness42,43,44. Also, visual response properties change depending on anesthesia depth and the type of anesthetic used45.
An unresolved limitation of this method is that off-the-shelf thin-film electrodes currently do not cover the lateral, temporal regions of the mouse brain. This limits the assessment of EEG signals from temporal regions (e.g., auditory cortex), sensory interactions, and source localization. Specifically, customized EEG arrays can provide a solution here. Another limit is that, depending on the recording system, it may be difficult to assess electrode impedances during the experiment to allow for comparable electrode properties. A third limitation of the method is that it is not fully non-invasive and requires an incision of the skin and general anesthesia. To resolve the latter, one can implant the grid chronically21,22 or apply true non-invasive scalp EEG13.
Regarding the usability in acute experiments, the described epicranial EEG measures represent a good trade-off between different methods. The procedure leaves the cortex and skull intact and allows less manipulation, avoiding potential functional damage to the cortical surface. This potentially provides better consistency in the screening of cortical visual activity.
In contrast to simpler recording methods, using multichannel thin-film EEG recordings significantly improves the information content of experimental data due to the comparability of electrode positions. It provides a repeatable, topographic, and more complete overview compared to single-electrode approaches. Additionally, this approach allows the use of EEG toolboxes (EEGLAB, ERPLAB, Fieldtrip, Chronux), utilizing electrode configuration, and ultimately facilitating data analysis26,27,28,29,30.
Potential applications of multichannel thin-film EEG are visual phenotyping of mice strains and assessment of visual function previous to intracortical recordings. Also, and most importantly, the multichannel EEG approach can easily be extended to assess auditory or somatosensory potentials, respectively.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, Cluster of Excellence 2177 "Hearing4all", Project number 390895286).
Materials
| Bepanthen 5% Dexpantheol | Bayer | Ophtamic gel | |
| Cheetah software 5.11 | Neuralnyx | Version 5.11 | Recording software for neurophysiologcal signals |
| Digital Lynx SX | Neuralynx | Digital Lynx 16SX | Recording system |
| ECG differential amplifier | Otoconsult | WDA2 V1.0 | |
| Electric shaver | Aesculap | GT420 | |
| Electrode Holder | TSE Systems | 430005-HE | |
| Examination light | Heine | HL 5000 | Cold light source lamp |
| Heating Pad + Temperature Control system | CWE | TC-1000 Mouse | |
| Histoacryl 0.5 mL | B.Braun | Tissue adhesive | |
| Infrared heat lamp | Sanitas | SIL 06 | |
| Ketamine 10% | WDT | Ketaminhydrochlorid | |
| LED stroboscope | Monarch | Nova Strobe PBL | Visual stimulation |
| Matlab 2021a | The Mathworks | 2021a | Stimulus control and analysis |
| Moria Vessel Clamp | Fine Science Tools | 18320-11 | |
| Mouse EEG electrode | NeuroNexus | H32 (Reticular) | 32-channel EEG electrode. Thickness: 20 μm; length: 8.6 mm; width 6.8 mm. Platinum sites: 500 μm diameter |
| Mouse Frame | custom made | Information available on request | |
| Multifunction I/O device | National Instruments | PCIe-6353 with BNC 2090A | Analog stimulus generation, output, and trigger |
| NaCl 0.9% | B.Braun | Isotonic, sterile, nonpyrogenic | |
| Neuralynx HS36 | Neuralynx | HS-36 | Headstage |
| Neuronexus probe connector | Neuralynx | ADPT-HS36-N2T-32A | Electrode connector |
| Oscilloscope | Tektronix | TDS 2014B | |
| Progent Intensive Cleaner | Menicon | Protein remover and disinfecting solution for rigid gas permeable lenses | |
| Recording PC | HP | HP Z800 | Recording PC |
| Rimadyl (Carprofen) | Zoetis | Carprofen | |
| Silicon Oil M 1000 | Carl Roth | 4045.1 | |
| Silver wire | Science Products | AG-8W | Diameter 203 µm; ECG and reference electrode |
| Sound proof chamber | IAC acoustics | ||
| Stereotactic Micromanipulator | TSE Systems | 430005-M/P | For EEG electrode placement |
| Stimulation PC | Dell | Dell Precision T5810 | Stimulation PC |
| Surgical microscope | Zeiss | Op-Mi Focus | |
| Surgical tape | 3M | 1527-0 | 1.25 cm x 9.1 m |
| Thilo-Tears 3 mg/g | Alcon Pharma GmbH | Ophtamic gel | |
| Vaselin Lichtenstein | Winthrop | White vaselin ointment | |
| Xylazin 2% | Bernburg | Xylazinehydrochlorid | |
| Xylocaine Spray (10 mg/puff) | Aspen | Lidocaine |
Riferimenti
- Haider, N. B., Ikeda, A., Naggert, J. K., Nishina, P. M. Genetic modifiers of vision and hearing. Human Molecular Genetics. 11 (10), 1195-1206 (2002).
- Joiner, M. A., Lee, A. Voltage-gated Cav1 channels in disorders of vision and hearing. Current Molecular Pharmacology. 8 (2), 143-148 (2015).
- Krebs, M. P., et al. Mouse models of human ocular disease for translational research. PLOS One. 12 (8), 0183837 (2017).
- Won, J., et al. Mouse model resources for vision research. Journal of Ophthalmology. 2011, 1-12 (2011).
- Peachey, N. S., Ball, S. L. Electrophysiological analysis of visual function in mutant mice. Documenta Ophthalmologica. 107 (1), 13-36 (2003).
- Tokashiki, N., et al. Reliable detection of low visual acuity in mice with pattern visually evoked potentials. Scientific Reports. 8 (1), 15948 (2018).
- Creel, D. J. Visually evoked potentials. Handbook of Clinical Neurology. 160, 501-522 (2019).
- Sutter, E. E. Imaging visual function with the multifocal m-sequence technique. Vision Research. 41 (10-11), 1241-1255 (2001).
- Porciatti, V., Pizzorusso, T., Maffei, L. The visual physiology of the wild type mouse determined with pattern VEPs. Vision Research. 39 (18), 3071-3081 (1999).
- Makowiecki, K., Garrett, A., Clark, V., Graham, S. L., Rodger, J. Reliability of VEP recordings using chronically implanted screw electrodes in mice. Translational Vision Science & Technology. 4 (2), 15 (2015).
- Tomiyama, Y., et al. Measurement of Electroretinograms and Visually Evoked Potentials in Awake Moving Mice. PLOS One. 11 (6), 0156927 (2016).
- You, Y., Klistorner, A., Thie, J., Graham, S. L. Improving reproducibility of VEP recording in rats: electrodes, stimulus source and peak analysis. Documenta Ophthalmologica. 123 (2), 109-119 (2011).
- Yeon, C., Kim, D., Kim, K., Chung, E. Visual evoked potential recordings in mice using a dry non-invasive multichannel scalp EEG sensor. Journal of Visualized Experiments. (131), e56927 (2018).
- Liu, S., et al. An optimized procedure to record visual evoked potential in mice. Experimental Eye Research. 218, 109011 (2022).
- Ryu, S. B., et al. Spatially confined responses of mouse visual cortex to intracortical magnetic stimulation from micro-coils. Journal of Neural Engineering. 17 (5), 056036 (2020).
- Słowiński, P., et al. Background EEG connectivity captures the time-course of epileptogenesis in a mouse model of epilepsy. eNeuro. 6 (4), (2019).
- Troncoso, E., Muller, D., Czellar, S., Zoltan Kiss, J. Epicranial sensory evoked potential recordings for repeated assessment of cortical functions in mice. Journal of Neuroscience Methods. 97 (1), 51-58 (2000).
- Land, R., et al. Cross-modal plasticity in higher-order auditory cortex of congenitally deaf cats does not limit auditory responsiveness to cochlear implants. Journal of Neuroscience. 36 (23), 6175-6185 (2016).
- Land, R., Radecke, J. -. O., Kral, A. Congenital deafness reduces, but does not eliminate auditory responsiveness in cat extrastriate visual cortex. Neuroscienze. 375, 149-157 (2018).
- Choi, J. H., Koch, K. P., Poppendieck, W., Lee, M., Shin, H. S. High resolution electroencephalography in freely moving mice. Journal of Neurophysiology. 104 (3), 1825-1834 (2010).
- Lee, M., Kim, D., Shin, H. S., Sung, H. G., Choi, J. H. High-density EEG recordings of the freely moving mice using polyimide-based microelectrode. Journal of Visualized Experiments. (47), e2562 (2010).
- Jonak, C. R., Lovelace, J. W., Ethell, I. M., Razak, K. A., Binder, D. K. Reusable multi-electrode array technique for electroencephalography in awake freely moving mice. Frontiers in Integrative Neuroscience. 12, 53 (2018).
- Land, R., Kapche, A., Ebbers, L., Kral, A. 32-channel mouse EEG: Visual evoked potentials. Journal of Neuroscience Methods. 325, 108316 (2019).
- Kim, D., Yeon, C., Kim, K. Development and experimental validation of a dry non-invasive multichannel mouse scalp EEG sensor through visual evoked potential recordings. Sensors. 17 (2), 326 (2017).
- Megevand, P., Quairiaux, C., Lascano, A., Kiss, J., Michel, C. A mouse model for studying large-scale neuronal networks using EEG mapping techniques. Neuroimage. 42 (2), 591 (2008).
- Lee, C., et al. Dipole source localization of mouse electroencephalogram using the Fieldtrip toolbox. PLOS ONE. 8 (11), 79442 (2013).
- Oostenveld, R., Fries, P., Maris, E., Schoffelen, J. M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational Intelligence and Neuroscience. 2011, 156869 (2011).
- Bokil, H., Andrews, P., Kulkarni, J. E., Mehta, S., Mitra, P. P. Chronux: A platform for analyzing neural signals. Journal of Neuroscience Methods. 192 (1), 146-151 (2010).
- Delorme, A., Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 134 (1), 9-21 (2004).
- Lopez-Calderon, J., Luck, S. J. ERPLAB: An open-source toolbox for the analysis of event-related potentials. Frontiers in Human Neuroscience. 8, 213 (2014).
- Bokil, H., Tchernichovsky, O., Mitra, P. P. Dynamic phenotypes: Time series analysis techniques for characterizing neuronal and behavioral dynamics. Neuroinformatics. 4 (1), 119-128 (2006).
- Ho, D., et al. Heart rate and electrocardiography monitoring in mice. Current Protocols in Mouse Biology. 1 (1), 123-139 (2011).
- Hart, C. Y. T., Burnett, J. C., Redfield, M. M. Effects of avertin versus xylazine-ketamine anesthesia on cardiac function in normal mice. American Journal of Physiology – Heart and Circulatory Physiology. 281 (5), 1938-1945 (2001).
- Land, R., Kral, A. Temporal acuity is preserved in the auditory midbrain of aged mice. Neurobiology of Aging. 110, 47-60 (2022).
- Widmann, A., Schröger, E., Maess, B. Digital filter design for electrophysiological data – A practical approach. Journal of Neuroscience Methods. 250, 34-46 (2015).
- Widmann, A., Schröger, E. Filter effects and filter artifacts in the analysis of electrophysiological data. Frontiers in psychology. 3, 233 (2012).
- Skrandies, W. Global field power and topographic similarity. Brain Topography. 3 (1), 137-141 (1990).
- Hamburger, H. L., Michelle, M. A. G. Global Field Power measurement versus classical method in the determination of the latency of evoked potential components. Brain Topography. 3 (3), 391-396 (1991).
- Land, R., Engler, G., Kral, A., Engel, A. K. Response properties of local field potentials and multiunit activity in the mouse visual cortex. Neuroscienze. 254, 141-151 (2013).
- Kappenman, E. S., Luck, S. J. The effects of electrode impedance on data quality and statistical significance in ERP recordings. Psychophysiology. 47 (5), 888-904 (2010).
- Calderone, L., Grimes, P., Shalev, M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Experimental Eye Research. 42 (4), 331-337 (1986).
- Land, R., Engel, A., Kral, A. Auditory influences in V1 of mice induced by varying anesthesia level. i-Perception. 2 (8), 755 (2011).
- Land, R., Engler, G., Kral, A., Engel, A. K. Auditory evoked bursts in mouse visual cortex during isoflurane anesthesia. PLOS One. 7 (11), 49855 (2012).
- Hudetz, A. G., Imas, O. A. Burst activation of the cerebral cortex by flash stimuli during isoflurane anesthesia in rats. Anesthesiology. 107 (6), 983-991 (2007).
- Imas, O. A., Ropella, K. M., Ward, B. D., Wood, J. D., Hudetz, A. G. Volatile anesthetics enhance flash-induced gamma oscillations in rat visual cortex. Anesthesiology. 102 (5), 937-947 (2005).

.
