一种用于研究番茄种子发育的有效清除方案(Solanum lycopersicum L.)
Summary
番茄种子是研究植物繁殖过程中遗传学和发育生物学的重要模型。该协议可用于清除不同发育阶段的番茄种子以观察更精细的胚胎结构。
Abstract
番茄(Solanum lycopersicum L.)是全球主要的经济作物之一。番茄种子是研究植物繁殖过程中遗传学和发育生物学的重要模型。番茄种子内更精细的胚胎结构的可视化通常受到种皮粘液、多细胞分层外皮和厚壁胚乳的阻碍,这需要通过费力的包埋切片来解决。更简单的替代方案是采用组织清除技术,使用化学试剂使种子几乎透明。虽然传统的清除程序可以深入了解具有较薄种皮的较小种子,但清除番茄种子在技术上仍然具有挑战性,尤其是在发育后期阶段。
这里介绍的是一种快速且省力的清除方案,用于观察开花后 3 至 23 天胚胎形态接近完成时的番茄种子发育。该方法将广泛用于 拟南芥 的水合氯醛澄清溶液与其他修饰相结合,包括省略福尔马林 – 乙酰醇(FAA)固定,添加次氯酸钠处理种子,去除软化的种皮粘液以及洗涤和真空处理。该方法可用于不同发育阶段番茄种子的高效清除,有助于全面监测突变种子的发育过程,具有良好的空间分辨率。该清除协议也可以应用于茄科其他商业重要物种的深度成像。
Introduction
番茄(番茄 )是世界上最重要的蔬菜作物之一,2020年产量为1.868亿吨肉质水果,面积达510万公顷1。它属于大型茄科,约有2,716种2,包括许多具有重要商业意义的作物,如茄子,辣椒,马铃薯和烟草。栽培番茄是一种二倍体物种(2n = 2x = 24),基因组大小约为900 Mb3。长期以来,通过从野生茄属中选择理想的性状,在番 茄 驯化和育种方面做出了巨大努力。番茄遗传学资源中心列出了 5,000 多种番茄种质,全球储存了 80,000 多种番茄种质4.番茄植株在温室中多年生,通过种子繁殖。成熟的番茄种子由三个主要隔室组成:一个成熟的胚胎、残留的细胞型胚乳和一个坚硬的种皮5,6 (图 1A)。双重受精后,细胞型胚乳的发育先于受精卵的发育。在开花后~5-6天(DAF),当胚乳由6至8个细胞核7组成时,首先观察到双细胞前胚。在 茄子松中,胚胎在20 DAF后接近其最终大小,种子在32 DAF8后可以发芽。随着胚胎的发育,胚乳逐渐被吸收,种子中只剩下少量胚乳。残留胚乳由胚根尖端周围的小胚乳和种子其余部分的侧胚乳组成9,10。外种皮由外皮增厚木质化的外表皮发育而来,与外皮残余物的死层形成硬壳以保护胚胎和胚乳5。
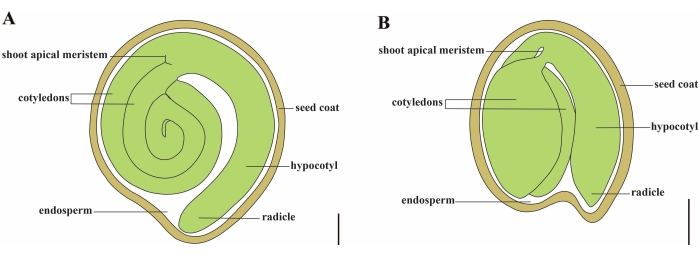
图1: Solanum lycopersicum 和 拟南芥中 成熟种子的示意图。 (A)成熟番茄种子的纵向解剖结构。(B)成熟 拟南芥 种子的纵向解剖。成熟的番茄种子的大小大约是 拟南芥 种子的 70 倍。比例尺 = (A) 400 μm, (B) 100 μm。 请点击此处查看此图的大图。
高质量番茄种子的生产取决于胚胎、胚乳和母体种子成分之间的协调11.剖析种子发育中的关键基因和网络需要对突变种子进行深入和完整的表型记录。传统的包埋切片技术,如半薄切片和石蜡切片,被广泛用于番茄种子,以观察胚胎的局部和更精细的结构12,13,14,15。然而,从薄片分析种子发育通常很费力,并且缺乏z轴空间分辨率。相比之下,组织清除是一种快速有效的方法,可以查明最有可能发生的胚胎缺陷的发育阶段16。清除方法通过用一种或多种生化剂均质化折射率来降低内部组织的不透明度16。全组织清除可以在不破坏其完整性的情况下观察植物组织结构,并且清除技术和三维成像的结合已成为获取植物器官形态和发育状态信息的理想解决方案17,18。多年来,种子清除技术已用于各种植物物种,包括拟南芥、大麦和普通贝塔19、20、21、22、23。其中,全镶样胚珠清除技术因其体积小、种皮细胞4-5层、核型胚乳24、25等特点,成为研究拟南芥种子发育的有效方法。随着不同清除混合物的不断更新,例如Hoyer溶液26的出现,尽管大麦胚珠的胚乳占种子的大部分,但大麦胚珠的内部结构以高度清晰的方式成像。甜菜的胚胎发生可以通过清除结合真空处理和盐酸软化来观察19。尽管如此,与上述物种不同,尚未报道通过清除番茄种子协议进行的胚胎学观察。这阻碍了对西红柿胚胎和种子发育的详细调查。
水合氯醛通常用作澄清溶液,其允许浸没的组织和细胞在不同的光学平面上显示,并基本上保留细胞或组织成分27,28,29。基于水合氯醛的清除方案已成功用于种子的全安装清除,以观察拟南芥21,28的胚胎和胚乳。然而,这种清除溶液在清除番茄种子方面效率不高,番茄种子比拟南芥种子更不透水。物理屏障包括:(1)番茄外皮在3至15 DAF 30,31处有近20个细胞层,(2)番茄胚乳是细胞型,而不是核型32,(3)番茄种子的大小约为70倍33,34和(4)产生大量的种皮粘液,这阻止了清除试剂的渗透并影响了胚胎细胞的可视化。
因此,本报告提出了一种优化的基于水合氯醛的清除方法,用于在不同阶段对番茄种子进行整体安装清除,该方法可以对胚胎发育过程进行深度成像(图2)。
Protocol
Representative Results
Discussion
与机械切片相比,清除技术对于三维成像更有利,因为它保留了植物组织或器官的完整性16。由于化学溶液更容易渗透,传统的澄清方案通常仅限于小样品。番茄种子是组织清除的一个有问题的样品,因为它的大小比拟南芥种子大约 70 倍,并且具有更多的渗透屏障。拟南芥种皮由四到五个活细胞层组成,这些活细胞层来自外皮和内皮24。然而,与<e…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
作者感谢乐杰博士和宋秀芬博士分别对微分干涉对比显微镜和常规清除方法的有益建议。这项研究由中国国家自然科学基金(31870299)和中国科学院青年创新促进会资助。图 2 是使用 BioRender.com 创建的。
Materials
| 1,000 µL pipette | GILSON | FA10006M | |
| 1,000 µL pipette tips | Corning | T-1000-B | |
| 2 ml centrifuge tube | Axygen | MCT-200-C | |
| 37% formaldehyde | DAMAO | 685-2013 | |
| 5,000 µL pipette | Eppendorf | 3120000275 | |
| 5,000 µL pipette tips | biosharp | BS-5000-TL | |
| 50 ml centrifuge tube | Corning | 430829 | |
| Absolute Ethanol | BOYUAN | 678-2002 | |
| Bottle glass | Fisher | FB800-100 | |
| Chloral Hydrate | Meryer | M13315-100G | |
| Coverslip | Leica | 384200 | |
| DIC microscope | Zeiss | Axio Imager A1 | 10x, 20x and 40x magnification |
| Disinfectant | QIKELONGAN | 17-9185 | |
| Dissecting needle | Bioroyee | 17-9140 | |
| Flower nutrient soil | FANGJIE | ||
| Forceps | HAIOU | 4-94 | |
| Glacial Acetic Acid | BOYUAN | 676-2007 | |
| Glycerol | Solarbio | G8190 | |
| Magnetic stirrer | IKA | RET basic | |
| Micro-Tom | Tomato Genetics Resource Center | LA3911 | |
| Orbital shaker | QILINBEIER | QB-206 | |
| Seeding substrate | PINDSTRUP | LV713/018-LV252 | Screening:0-10 mm |
| Single concave slide | HUABODEYI | HBDY1895 | |
| Slide | Leica | 3800381 | |
| Stereomicroscope | Leica | S8 APO | 1x to 4x magnification |
| Tin foil | ZAOWUFANG | 613 | |
| Tween 20 | Sigma | P1379 | |
| Vacuum pump | SHIDING | SHB-III | |
| Vortex meter | Silogex | MX-S |
Riferimenti
- . FAOSTAT Available from: https://www.fao.org/faostat/en/#data/QCL (2022)
- Olmstead, R. G., Bohs, L. A summary of molecular systematic research in Solanaceae: 1982-2006. Acta Horticulturae. 745, 255-268 (2007).
- Consortium, T. G. The tomato genome sequence provides insights into fleshy fruit evolution. Nature. 485 (7400), 635-641 (2012).
- Ebert, A. W., Chou, Y. Y. The tomato collection maintained by AVRDC – The World Vegetable Center: composition, germplasm dissemination and use in breeding. Acta Horticulturae. 1101, 169-176 (2015).
- Hilhorst, H., Groot, S., Bino, R. J. The tomato seed as a model system to study seed development and germination. Acta Botanica Neerlandica. 47, 169-183 (1998).
- Chaban, I. A., Gulevich, A. A., Kononenko, N. V., Khaliluev, M. R., Baranova, E. N. Morphological and structural details of tomato seed coat formation: A different functional role of the inner and outer epidermises in unitegmic ovule. Plants-Basel. 11 (9), 1101 (2022).
- Iwahori, S. High temperature injuries in tomato. V. Fertilization and development of embryo with special reference to the abnormalities caused by high temperature. Journal of The Japanese Society for Horticultural Science. 35 (4), 379-386 (1966).
- Xiao, H., et al. Integration of tomato reproductive developmental landmarks and expression profiles, and the effect of SUN. on fruit shape. BMC Plant Biology. 9 (1), 49 (2009).
- Karssen, C. M., Haigh, A. M., Toorn, P., Weges, R., Taylorson, R. B. Physiological mechanisms involved in seed priming. Recent advances in the development and germination of seeds. NATO ASI Series. 187, (1989).
- Nonogaki, H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Frontiers in Plant Science. 5, 233 (2014).
- Doll, N. M., Ingram, G. C. Embryo-endosperm interactions. Annual Review of Plant Biology. 73, 293-321 (2022).
- Serrani, J. C., Fos, M., Atarés, A., García-Martínez, J. L. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv micro-tom of tomato. Journal of Plant Growth Regulation. 26 (3), 211-221 (2007).
- Yang, C., et al. A regulatory gene induces trichome formation and embryo lethality in tomato. Proceedings of the National Academy of Sciences USA. 108 (29), 11836-11841 (2011).
- Goetz, S., et al. Role of cis-12-oxo-phytodienoic acid in tomato embryo development. Plant Physiology. 158 (4), 1715-1727 (2012).
- Ko, H. Y., Ho, L. H., Neuhaus, H. E., Guo, W. J. Transporter SlSWEET15 unloads sucrose from phloem and seed coat for fruit and seed development in tomato. Plant Physiology. 187 (4), 2230-2245 (2021).
- Richardson, D. S., Lichtman, J. W. Clarifying tissue clearing. Cell. 162 (2), 246-257 (2015).
- Kurihara, D., Mizuta, Y., Sato, Y., Higashiyama, T. ClearSee: A rapid optical clearing reagent for whole-plant fluorescence imaging. Development. 142 (23), 4168-4179 (2015).
- Vieites-Prado, A., Renier, N. Tissue clearing and 3D imaging in developmental biology. Development. 148 (18), (2021).
- Kwiatkowska, M., Kadłuczka, D., Wędzony, M., Dedicova, B., Grzebelus, E. Refinement of a clearing protocol to study crassinucellate ovules of the sugar beet (Beta vulgaris L., Amaranthaceae). Plant Methods. 15, 71 (2019).
- Ponitka, A., Ślusarkiewicz-Jarzina, A. Cleared-ovule technique used for rapid access to early embryo development in Secale cereale × Zea mays crosses. Acta Biologica Cracoviensia. Series Botanica. 46, 133-137 (2014).
- Ceccato, L., et al. Maternal control of PIN1 is required for female gametophyte development in Arabidopsis. PLoS One. 8 (6), 66148 (2013).
- Wilkinson, L. G., Tucker, M. R. An optimised clearing protocol for the quantitative assessment of sub-epidermal ovule tissues within whole cereal pistils. Plant Methods. 13, 67 (2017).
- Hedhly, A., Vogler, H., Eichenberger, C., Grossniklaus, U. Whole-mount clearing and staining of Arabidopsis flower organs and Siliques. Journal of Visualized Experiments. (134), e56441 (2018).
- Creff, A., Brocard, L., Ingram, G. A mechanically sensitive cell layer regulates the physical properties of the Arabidopsis seed coat. Nature Communications. 6, 6382 (2015).
- Yang, T., et al. The B3 domain-containing transcription factor ZmABI19 coordinates expression of key factors required for maize seed development and grain filling. Plant Cell. 33 (1), 104-128 (2021).
- Anderson, L. E. Hoyer’s solution as a rapid permanent mounting medium for bryophytes. Bryologist. 57 (3), 242-244 (1954).
- Herr, J. M. A new clearing-squash technique for the study of ovule development in angiosperms. American Journal of Botany. 58 (8), 785-790 (1971).
- Yadegari, R., et al. Cell differentiation and morphogenesis are uncoupled in Arabidopsis raspberry embryos. The Plant Cell. 6 (12), 1713-1729 (1995).
- Grini, P. E., Jurgens, G., Hulskamp, M. Embryo and endosperm development is disrupted in the female gametophytic capulet mutants of Arabidopsis. Genetica. 162 (4), 1911-1925 (2002).
- Kataoka, K., Uemachi, A., Yazawa, S. Fruit growth and pseudoembryo development affected by uniconazole, an inhibitor of gibberellin biosynthesis, in pat-2 and auxin-Induced parthenocarpic tomato fruits. Scientia Horticulturae. 98 (1), 9-16 (2003).
- de Jong, M., Wolters-Arts, M., Feron, R., Mariani, C., Vriezen, W. H. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant Journal. 57 (1), 160-170 (2009).
- Roth, M., Florez-Rueda, A. M., Paris, M., Stadler, T. Wild tomato endosperm transcriptomes reveal common roles of genomic imprinting in both nuclear and cellular endosperm. Plant Journal. 95 (6), 1084-1101 (2018).
- Orsi, C. H., Tanksley, S. D. Natural variation in an ABC transporter gene associated with seed size evolution in tomato species. PLoS Genetics. 5 (1), 1000347 (2009).
- Herridge, R. P., Day, R. C., Baldwin, S., Macknight, R. C. Rapid analysis of seed size in Arabidopsis for mutant and QTL discovery. Plant Methods. 7 (1), 3 (2011).
- Xu, T. T., Ren, S. C., Song, X. F., Liu, C. M. CLE19 expressed in the embryo regulates both cotyledon establishment and endosperm development in Arabidopsis. Journal of Experimental Botany. 66 (17), 5217-5227 (2015).
- Ghadiri Alamdari, N., Salmasi, S., Almasi, H. Tomato seed mucilage as a new source of biodegradable film-forming material: effect of glycerol and cellulose nanofibers on the characteristics of resultant films. Food and Bioprocess Technology. 14 (12), 2380-2400 (2021).
- Gardner, R. O. An overview of botanical clearing technique. Stain Technology. 50 (2), 99-105 (1975).
- Beresniewicz, M. M., Taylor, A. G., Goffinet, M. C., Terhune, B. T. Characterization and location of a semipermeable layer in seed coats of leek and onion (Liliaceae), tomato and pepper (Solanaceae). Seed Science and Technology. 23 (1), 123-134 (1995).
- Stebbins, G. L. A bleaching and clearing method for plant tissues. Science. 87 (2245), 21-22 (1938).
- Debenham, E. M. A modified technique for the microscopic examination of the xylem of whole plants or plant organs. Annals of Botany. 3 (2), 369-373 (1939).
- Morley, T. Accelerated clearing of plant leaves by NaOH in association with oxygen. Stain Technology. 43 (6), 315-319 (1968).

