In Vitro Myelination of Peripheral Axons in a Coculture of Rat Dorsal Root Ganglion Explants and Schwann Cells
Summary
In the coculture system of dorsal root ganglia and Schwann cells, myelination of the peripheral nervous system can be studied. This model provides experimental opportunities to observe and quantify peripheral myelination and to study the effects of compounds of interest on the myelin sheath.
Abstract
The process of myelination is essential to enable rapid and sufficient signal transduction in the nervous system. In the peripheral nervous system, neurons and Schwann cells engage in a complex interaction to control the myelination of axons. Disturbances of this interaction and breakdown of the myelin sheath are hallmarks of inflammatory neuropathies and occur secondarily in neurodegenerative disorders. Here, we present a coculture model of dorsal root ganglion explants and Schwann cells, which develops a robust myelination of peripheral axons to investigate the process of myelination in the peripheral nervous system, study axon-Schwann cell interactions, and evaluate the potential effects of therapeutic agents on each cell type separately. Methodologically, dorsal root ganglions of embryonic rats (E13.5) were harvested, dissociated from their surrounding tissue, and cultured as whole explants for 3 days. Schwann cells were isolated from 3-week-old adult rats, and sciatic nerves were enzymatically digested. The resulting Schwann cells were purified by magnetic-activated cell sorting and cultured under neuregulin and forskolin-enriched conditions. After 3 days of dorsal root ganglion explant culture, 30,000 Schwann cells were added to one dorsal root ganglion explant in a medium containing ascorbic acid. The first signs of myelination were detected on day 10 of coculture, through scattered signals for myelin basic protein in immunocytochemical staining. From day 14 onward, myelin sheaths were formed and propagated along the axons. Myelination can be quantified by myelin basic protein staining as a ratio of the myelination area and axon area, to account for the differences in axonal density. This model provides experimental opportunities to study various aspects of peripheral myelination in vitro, which is crucial for understanding the pathology of and possible treatment opportunities for demyelination and neurodegeneration in inflammatory and neurodegenerative diseases of the peripheral nervous system.
Introduction
In the peripheral nervous system (PNS), rapid information transduction is mediated by myelin-enwrapped axons. The myelination of axons is essential to enable the fast propagation of electric impulses, since the conduction velocity of the nerve fibers correlates to the axon diameter and myelin thickness1. Sensory signaling from the periphery to the central nervous system (CNS) relies on the activation of first-order sensory neurons that reside in enlargements of the dorsal root, termed dorsal root ganglia (DRG). For the formation and maintenance of myelin, continuous communication between axons and Schwann cells, which are the myelinating Glia cells in the PNS, is mandatory2.
Many diseases of the PNS disturb the transduction of information by either primary axonal or demyelinating damage, resulting in hypesthesia or dysesthesia. First-order sensory neurons have the ability to regenerate to an extent after neuronal damage, by a complex interaction between the neuron and surrounding Schwann cells3. In this case, Schwann cells can undergo cellular reprogramming to clear axonal as well as myelin debris and promote axonal regeneration, resulting in remyelination4. Understanding the mechanisms of myelination in health and disease is important, in order to find possible treatment options for demyelinating disorders of the PNS. Myelin can also be damaged by acute neurotrauma, and approaches to promote myelination to advance functional recovery after peripheral nerve injury are under investigation5.
Our knowledge of peripheral myelination has benefited largely from myelinating cocultures of Schwann cells and sensory neurons. Since the first approaches were applied6,7,8, myelination has been studied intensely with the use of different coculture systems9,10,11. Here, we provide a rapid and facile protocol for robust in vitro myelination of dorsal root ganglion axons. The protocol for Schwann cell preparation is based on the protocol by Andersen et al.12, previously published in Pitarokoili et al.13. We use Schwann cells derived from juvenile rats and embryonic DRG explant cultures for the coculture, in which myelination occurs at around day 14. The goal of the method is to provide a system to investigate the formation of myelin as a result of direct axon-Schwann cell interaction, and to study modulators of PNS myelination. In comparison to dissociated neuronal cell cultures, DRG explants are more anatomically preserved and form long axonal processes. Quantification of the myelinated axon area provides a sufficient readout for myelination in the coculture. The method is a valuable tool to screen therapeutic compounds for their potential effect on PNS myelination, and can also be utilized in addition to in vivo studies in animal models14.
Protocol
All procedures were performed in accordance with the European Communities Council Directive for the care and use of laboratory animals.
1. Schwann cell culture
- Coating for Schwann cell culture
- Coat the cell culture dishes under sterile conditions. Apply 2 mL of 0.01% poly-L-lysine (PLL) to two 60 mm tissue culture (TC) dishes each and incubate overnight at 4 °C.
- Remove the PLL, wash the TC dishes 2x with distilled water, and incubate with 2 mL of 1 µg/cm2 laminin overnight at 4 °C. Wash the TC dishes 2x with aqua dest, and let the plates air-dry.
- Medium preparation for Schwann cell culture
- Prepare 50 mL of Schwann cell medium by adding 10% heat-inactivated fetal calf serum (FCS), 2 µM forskolin, 10 nM neuregulin, and 50 µg/mL gentamycin to Dulbecco′s Modified Eagle′s Medium (DMEM)/F-12 (high glucose) under sterile conditions.
- Prepare 70 mL of Leibovitz's L-15 medium with 50 µg/mL gentamycin under sterile conditions.
- Sciatic nerve preparation
NOTE: All sciatic nerve preparation steps are performed under a clean bench.- Prepare one 100 mm TC dish with 5 mL of ice-cold Dulbecco's phosphate-buffered saline (DPBS) without Ca2+ and Mg2+, one 100 mm TC dish with 5 mL of ice-cold Leibovitz's L-15 medium, and one 100 mm TC dish with 5 mL of ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin.
- Clean all the instruments by autoclaving. Spray the instruments and working area with 70% ethanol.
- Euthanize five 3-week-old male Sprague Dawley rats using CO2 inhalation and decapitation. Spray the rat's torso with 70% ethanol.
- Open the dorsal lower left limb with scissors and remove the biceps femoris muscle carefully. Loosen the sciatic nerve by smooth elevation with curved forceps, ensuring not to bruise the nerve.
- Hold the most proximal part of the nerve with the curved forceps to straighten the nerve, and clip the nerve as high as possible using scissors. Then, clip the nerve close to the sacral plexus and the paw with scissors. Repeat steps 1.3.4-1.3.5 for the right side.
NOTE: While opening the limb, take care not to bruise any blood vessels. - Using forceps, put the left and right sciatic nerves into a 100 mm TC dish with ice-cold DPBS.
- Sciatic nerve refurbishment
- Use forceps to transfer all the nerves to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin. Continue using a stereomicroscope and remove fat, muscle, and blood vessels from the nerves with two pairs of fine forceps. Grab the nerves with forceps and transfer them to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium.
- Identify the proximal and distal ends of the sciatic nerve. Remove the epineurium with one pair of fine forceps in a proximal to distal direction, while holding the proximal nerve end with the second pair of fine forceps.
- Transfer the purified nerves to a 100 mm TC dish with ice-cold Leibovitz's L-15 medium and 50 µg/mL gentamycin. Tease the isolated nerve fascicles to separate and isolate single nerve fibers using two pairs of fine forceps.
- Transfer the nerve fibers to a 50 mL tube using a 10 mL serological pipette and take up as little medium as possible. Add 50 mL of Leibovitz's L-15 medium with 50 µg/mL gentamycin to the nerve fibers and slew a few times in the 50 mL tube.
- Enzymatic digestion of the sciatic nerve
NOTE: The next steps (steps 1.5-1.8, 2.1, and 2.2) are performed under sterile conditions.- Prepare the enzymatic digestion solution containing 0.25% dispase II, 0.05% type I collagenase, and 50 µg/mL gentamycin in 10 mL of DMEM (high glucose).
- Centrifuge the tube at 188 x g for 5 min at 4 °C, remove the supernatant with a 25 mL serological pipette ,and transfer the pellet with the remaining Leibovitz's L-15 medium into a 60 mm TC dish using a 1,000 mL pipette.
- Rinse the 50 mL tube with 10 mL of the enzymatic digestion solution and add it to the dish containing the nerve fibers. Distribute the tissue in the dish carefully with the tip of a pipette to maximize the accessible surface for digestion.
- Incubate at 37 °C and 5% CO2 for 18 h, and stop the digestion by adding 10 mL of 40% FCS in Hanks' balanced salt solution, without Ca2 and Mg2+ (HBSS).
- Cell separation
- Transfer the digested nerves into a 50 mL tube using a serological pipette and centrifuge at 188 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet in 10 mL of DMEM containing 10% FCS and 50 µg/mL gentamycin. Resuspend the pellet 20 times subsequently, using a 10 mL, 5 mL, 2 mL, 1 mL, and 200 µL pipette tip.
- Filter the cell suspension through a 100 µm cell strainer and centrifuge at 188 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet with 4 mL of DMEM containing 10% FCS and 50 µg/mL gentamycin.
- Add 2 mL of the cell suspension to each of the two PLL- and laminin-coated 60 mm TC dishes, and incubate at 37 °C and 5% CO2. Leave the plates untouched for 2 days in the incubator to protect the cells from mechanical stress and to support adherence.
- Schwann cell differentiation
- After 2 days, remove the medium and carefully rinse the plates 2x with DMEM (high glucose), 10% FCS, and 50 µg/mL gentamycin. Afterward, add 2 mL of Schwann cell medium. Replace the Schwann cell medium every 2nd day, and observe the cell appearance and confluency using a microscope.
NOTE: Schwann cells and DRG explants need to be prepared in a timely, coordinated manner for the coculture. Make sure a rat with embryos at E 13.5 is available for DRG preparation when the Schwann cells are close to a confluency of 80%.
- After 2 days, remove the medium and carefully rinse the plates 2x with DMEM (high glucose), 10% FCS, and 50 µg/mL gentamycin. Afterward, add 2 mL of Schwann cell medium. Replace the Schwann cell medium every 2nd day, and observe the cell appearance and confluency using a microscope.
- Cell trypsinization and magnetic separation
NOTE: For exemplary pictures of different Schwann cell culture stages, see Supplementary Figure 1.- When the cells reach a confluency of about 80% (6-12 days of culture), carefully wash the plates 2x with 3 mL of DPBS and incubate with 2 mL of 0.05% Trypsin/EDTA (prewarmed to 37 °C) for 3 min. When the cells detach from the plate bottom, inactivate digestion by the addition of 2 mL of DMEM with 10% FCS and 50 µg/mL gentamycin.
NOTE: Stick to a trypsinization time of strictly 3 min, and proceed rapidly afterward. - Resuspend the cell pellet in 2 mL of magnetic cell separation buffer containing DPBS with 0.5% bovine serum albumin (BSA) and 2 nM EDTA. Combine 10 µL of the cell suspension with 10 µL of trypan blue and count the cells using a staining chamber.
- Centrifuge the cell suspension at 188 x g for 10 min at 4 °C and resuspend the cell pellet in 90 µL of magnetic cell separation buffer per 1 x 107 cells. Add 10 µL of Thy-1 microbeads per 1 x 107 cells. Resuspend the solution a few times and incubate for 15 min in the dark at 8 °C.
- Add 2 mL of the magnetic cell separation buffer to the cell suspension and centrifuge at 300 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet in 500 µL of magnetic cell separation buffer.
- Moisten the magnetic cell separation column with 1 mL of the magnetic cell separation buffer. Place the magnetic cell separation column in the magnetic cell separator. Apply the cells to the magnetic cell separation column. Collect the flow through and centrifuge at 300 x g at 4 °C for 10 min.
NOTE: Fibroblasts are positively selected and remain in the column, while Schwann cells pass the column. Fibroblasts can be collected with a stamp (e.g., as a negative control for Schwann cell staining protocols). - Discard the supernatant and resuspend the pellet in 1 mL of the coculture medium (see step 3.1.1). Count the cells after staining with trypan blue and a staining chamber.
- When the cells reach a confluency of about 80% (6-12 days of culture), carefully wash the plates 2x with 3 mL of DPBS and incubate with 2 mL of 0.05% Trypsin/EDTA (prewarmed to 37 °C) for 3 min. When the cells detach from the plate bottom, inactivate digestion by the addition of 2 mL of DMEM with 10% FCS and 50 µg/mL gentamycin.
2. DRG explant culture
- DRG growth medium preparation
- Prepare DRG growth medium by adding 2% B27, 2% horse serum, 1% L-glutamine, 0.5% penicillin/streptomycin, and 10 ng/mL nerve growth factor (NGF) to the neurobasal medium. Store the growth medium at 4 °C.
NOTE: The growth medium can be used for 2 days.
- Prepare DRG growth medium by adding 2% B27, 2% horse serum, 1% L-glutamine, 0.5% penicillin/streptomycin, and 10 ng/mL nerve growth factor (NGF) to the neurobasal medium. Store the growth medium at 4 °C.
- Coating for DRG explants
- Incubate the coverslips in 70% ethanol for 1 h and place in the wells of 4-well dishes using curved forceps. After the ethanol has dried, apply 300 µL of 0.2 mg/mL poly-D-lysine (PDL) per well and incubate overnight at 37 °C and 5% CO2.
- Wash the coverslips 3x for 5 min each with DPBS consecutively. Take off the DPBS, apply 300 µL of 1 µg/mL laminin to the coverslips, and incubate overnight at 37 °C and 5% CO2.
- After three washing steps with DPBS for 5 min, replace the DPBS with 190 µL of DRG growth medium. Place the 4-well plates into the incubator at 37 °C and 5% CO2.
- DRG preparation
NOTE: Harvest the DRG of embryonic rats under a clean bench.- Before preparation, clean all the instruments with 70% ethanol. Fill 10 (two per embryo) 35 mm TC dishes with 2 mL of ice-cold HBSS per dish, and two 100 mm TC dishes with 5 mL of ice-cold HBSS per dish.
- Euthanize the pregnant rats (adult female Sprague Dawley rats, E13.5) by CO2 inhalation and decapitation.
- Spray the body with 70% ethanol and open the ventral torso of the rat. Carefully remove the uterus and place it into a 100 mm TC dish with ice-cold HBSS.
- Hold the uterus using curved forceps and open the uterus wall with fine forceps. Remove one amniotic sac and open it carefully by pinching a hole with fine forceps.
- Remove the embryo from the surrounding tissues, cut the umbilical cord, and decapitate the embryo using fine forceps. Place the torso into a 100 mm TC dish filled with HBSS using curved forceps and a spatula.
- Quickly remove all the embryos from the uterus and transfer them into one 100 mm TC dish filled with HBSS. If there are more than five embryos, prepare an additional 35 mm TC dish with 2 mL of HBSS, according to step 2.3.1.
- Gently place one embryo torso into a 35 mm TC dish filled with HBSS using a spatula and curved forceps. Under a stereomicroscope, open the dorsal part of the torso to divide the embryo into two halves using fine forceps and micro scissors. Turn one half to the side and identify the strand of DRG located in a line at the dorsal part of the embryo.
- Cut out the DRG as a whole strand using fine forceps and micro scissors. Place the DRG in a fresh 35 mm TC dish filled with 2 mL of HBSS, and separate a single DRG from the remaining tissue using fine forceps and micro scissors.
- DRG cell culture
- Take 4-well plates containing 190 µL of DRG growth medium from the incubator to the clean bench where the DRG are to be prepared. Transfer a single DRG carefully into one well of a 4-well culture plate using fine forceps and a spatula. Place the DRG into the center of each well, since a central position is important for the attachment of the DRG.
- Work under sterile conditions from now on. Place the explant cultures in the incubator at 37 °C and 5% CO2. The next day, add 50 µL of the DRG growth medium carefully to each well using a 100 mL pipette.
- Observe DRG explant adherence and axon outgrowth using a microscope daily, and discard DRG explants that have detached from the coverslip or failed to outgrow axons on day 3 of culture.
NOTE: DRG explants are very fragile in the first days of culture and need to be handled with care, especially when moving the plates in and out of the incubator when the medium is changed, or even when closing the incubator door. The precise volume of 190 µL of medium per well is crucial to keep the DRG explants in place during the first day of culture. Loose DRG explants can be identified easily during the daily control, as they swim in the medium instead of adhering to the coverslip.
3. Coculture
- Transfer of Schwann cells to the DRG explant culture
- Prepare the coculture medium by adding 0.1% ascorbic acid to the DRG growth medium.
- On day 3 of the DRG explant culture, carefully replace the DRG growth medium with 250 µL of the coculture medium containing 30,000 Schwann cells (from step 1.8) per well.
- Keep the coculture of the DRG explants and Schwann cells for up to 22 days. Replace 250 µL of the coculture medium carefully every other day, and observe the appearance of the cells using a microscope.
NOTE: For an example of DRG axons and Schwann cells in the coculture in the first days of culture, see Figure 1.
- Immunocytochemical staining
NOTE: Handle the coculture samples extremely carefully during the staining procedure, since they can be damaged easily.- To fix the cells on the coverslips, remove the medium slowly, wash 3x with DPBS carefully, and incubate in 4% paraformaldehyde (PFA) for 10 min. Replace the PFA with DPBS, and store the fixed cells at 4 °C for up to 1 week.
CAUTION: When handling 4% PFA, wear the recommended personal protective equipment. - Wash the coverslips 3x with DPBS for 5 min, then block with blocking solution (10% goat serum, 10% bovine serum albumin [BSA], 0.1% gelatin, and 0.05% Triton X-100) in DPBS for 1 h. Dilute the primary antibodies βIII-tubulin (1:7,500) and myelin basic protein (MBP) (1:750) in the blocking solution, and incubate overnight at 4 °C.
- Wash the cells 3x with DPBS for 5 min. Use fluorescent dye-conjugated secondary antibodies in a dilution of 1:1,000 in blocking solution, and incubate for 2 h at room temperature (RT).
- Wash the cells 3x with DPBS for 5 min, and mount the cells on microscopic slides with fluorescence mounting medium, including 4′,6-diamidino-2-phenylindole (DAPI). Store the slides in the dark at 4 °C until microscopic documentation.
CAUTION: When handling fluorescence mounting medium, wear the recommended personal protective equipment.
- To fix the cells on the coverslips, remove the medium slowly, wash 3x with DPBS carefully, and incubate in 4% paraformaldehyde (PFA) for 10 min. Replace the PFA with DPBS, and store the fixed cells at 4 °C for up to 1 week.
- Analysis
- Take pictures of eight defined regions surrounding the DRG explant in the center using an inverted microscope.
- Use an image analysis software application to quantify the areas of βIII-tubulin positive axons and myelination stained by MBP.
- Calculate the percentage of myelination as a ratio of the myelinated axons and non-myelinated axons.
Representative Results
Myelination in the coculture was assessed on days 10, 12, 14, 16, 18, and 20. The DRG explants and Schwann cells were stained for MBP, βIII-tubulin, and DAPI. The axonal network in the coculture was dense and did not change visibly in the time course of the observation. The first signs of myelin, in the form of small fragments, were detectable on day 10 and increased on day 12 (Figure 2). The MBP-positive areas increased over time until day 20 of culture. The myelination was quantified as a ratio of the MBP and βIII-tubulin positive areas. Myelination increased significantly on days 18 and 20 compared to day 10 (Figure 3; **p ≤ 0.01; ***p ≤ 0.001).
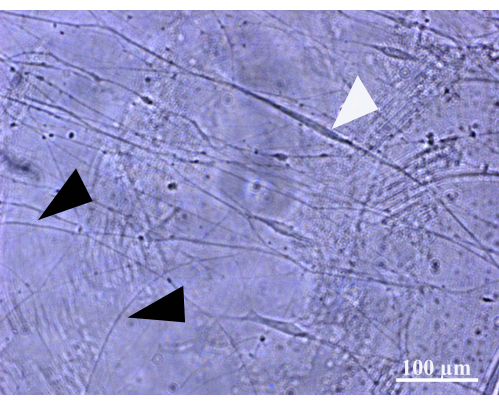
Figure 1: Appearance of Schwann cells and axons in the coculture. Exemplary picture of the coculture on day 3. Black arrows show thin DRG axons, and the white arrow points to a Schwann cell with an elongated and spindle-shaped morphology attached to an axon. Scale bar: 100 µm. Please click here to view a larger version of this figure.
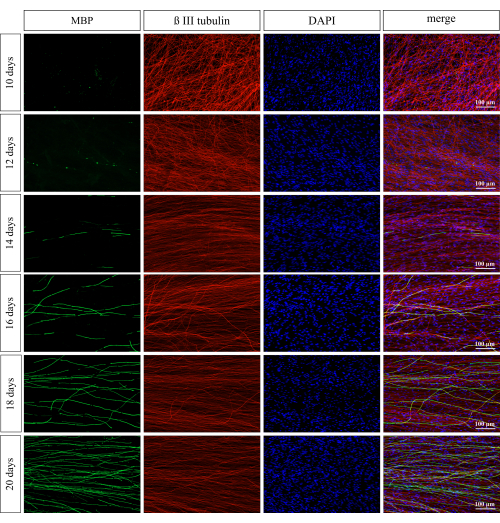
Figure 2: Myelination in a coculture of DRG explants and Schwann cells. Staining for the neuronal marker βIII-tubulin and MBP was performed on days 10, 12, 14, 16, 18, and 20, after joining both cultures to investigate the development of myelination in the coculture. First signs of myelination were visible on days 10 and 12 of coculture. From day 14 on, the MBP signal was more pronounced, and myelin-enwrapped axons were detected. The myelination increased with the time of coculture until day 20. Scale bars: 100 µm. Please click here to view a larger version of this figure.
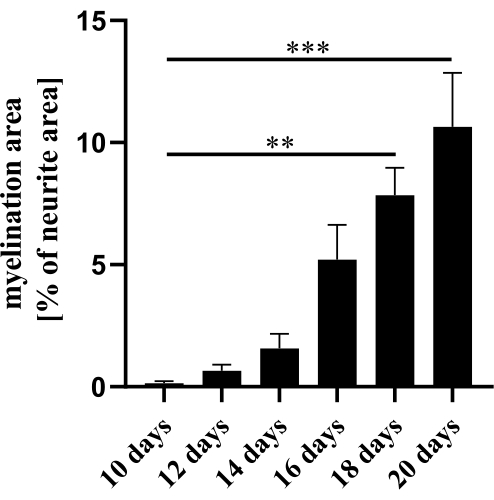
Figure 3: Quantification of myelination. On days 10, 12, 14, 16, 18, and 20 of coculture, myelination was assessed by staining axons with βIII-tubulin, and myelin with MBP. The percentage of myelinated axons was determined by calculation of the ratio of the MBP positive and βIII-tubulin stained areas. Significant differences were detected on days 18 and 20 in comparison to day 10 of coculture. Data are expressed as mean ± SEM; n = 3. One-way ANOVA with Kruskal-Wallis and Dunn's multiple comparison post-hoc test. Please click here to view a larger version of this figure.
Supplementary Figure 1: Stages of Schwann cell culture. Exemplary brightfield pictures of cultured Schwann cells (A) before and (B) after magnetic cell separation. Before magnetic cell separation, the culture includes Schwann cells with an elongated and spindle shape morphology, flat and spread-out appearing fibroblasts, and remnants of connective tissue. To achieve a purer culture of Schwann cells, magnetic cell separation is performed. Scale bars: 200 µm. Please click here to download this File.
Supplementary Figure 2: Myelination of DRG explants with and without additional Schwann cells. The MBP stained area was used to measure myelination in the coculture on day 14. The myelination of DRG explants was significantly increased if Schwann cells were added to the culture (unpaired t-test, **p ≤ 0.01). n = 3. Please click here to download this File.
Supplementary Figure 3: Gene expression analysis in the coculture. Relative gene expression levels of MBP, PMP22, MAG, Oct6, Egr2, and Olig1 were analyzed in coculture samples on day 22 by qPCR (A). MBP and PMP22 were the targets with the highest gene expression levels in the coculture. An exemplary picture of qPCR amplification products applied to an agarose gel demonstrates the qualitative abundance of the targets (B). The first and last lanes on the gel represent a DNA ladder (100 base pairs). n = 5. Please click here to download this File.
Discussion
Here, we present a rapid and facile protocol for the generation of in vitro myelination by merging two separate cell type cultures, Schwann cells and dorsal root ganglion explants.
A critical step of the protocol is the cultivation of DRG explants, especially in the first days of culture. DRG are very fragile before a strong axonal network is built and must be handled very carefully, for example, when taken out of the incubator or during a change of medium. DRG that detach from the bottom of the well and are found swimming in the medium show unsuccessful cultivation. For Schwann cells, changing proliferation rates due to different cultivation conditions and supplements (e.g., serum in the medium) can represent a challenge for the coculture. If the culture is overgrown by an excessive number of Schwann cells, it detaches from the bottom of the well and becomes unusable. A titration of Schwann cell number in the culture is therefore recommended. During the establishment of the Schwann cell protocol, purity tests should be performed, using SOX10 or S100 immunostaining. In general, handling of the culture, including change of medium as well as washing and fixation steps, must be carried out carefully to ensure a successful outcome. It is important to select the time point of observation according to the research interest; day 14 represents the starting point for the first dense myelinated axons, and so can be selected as the time point of observation for the initiation of myelination, while later time points offer more complete myelin sheaths.
As this in vitro method of myelination only comprises DRG and Schwann cells, it does not exactly resemble the process of myelination in the organism. Other contributing factors, such as surrounding tissue, the microenvironment, immune cells, and signaling from distant structures, are not represented in this model. However, this method provides a suitable model to investigate myelination of the PNS by the separate analysis and manipulation of both cell types9,15,16. Schwann cells or DRG for the coculture can be harvested from diseased or treated animals to decipher the contribution of the specific cell type17,18. When using the late stages of this setup, it is highly recommended to include a control condition without additionally adding Schwann cells, to rule out the possible effects of DRG-derived Schwann cells. In our experience, myelination in DRG explants without Schwann cells is detected to a significantly lesser extent than in the coculture (Supplementary Figure 2).
The usage of DRG explants provides the advantage of intact structural architecture in comparison to dissociated neuronal cultures. Immunofluorescent staining of myelin basic protein (MBP) allows for quantifying the myelinated axon area, and provides a sufficient readout for myelination in the coculture. Gene expression analysis of coculture samples on day 22 revealed the presence of MBP, peripheral myelin protein (PMP22), myelin-associated glycoprotein (MAG), octamer-binding factor 6 (Oct6), ETS-related gene 2 (Erg2), and oligodendrocyte transcription factor 1 (Olig1), with the highest expression levels for MBP and PMP22 (Supplementary Figure 3). Hence, the described protocol provides a method with several target options for myelination markers in the coculture.
Potential applications of this method include basic research approaches on the process of myelination in the periphery, as well as the validation of therapeutic compounds for diseases of the PNS, including the damage of myelin.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Prof. Dr. Ralf Gold and PD Dr. Gisa Ellrichmann for their advice and support.
Materials
| Anti-MBP, rabbit | Novus Biologicals, Centannial, USA | ABIN446360 | |
| Anti-ßIII-tubulin, mouse | Biolegend, San Diego, USA | 657402 | |
| Ascorbic acid | Sigma Aldrich GmbH, Steinheim, Germany | A4403-100MG | |
| B27-supplement | Thermo Fisher Scientific, Schwerte, Germany | 17504-044 | |
| Biosphere Filter Tip, 100 µL | Sarstedt, Nümbrecht, Germany | 70760212 | |
| Biosphere Filter Tip, 1250 µL | Sarstedt, Nümbrecht, Germany | 701186210 | |
| Biosphere Filter Tip, 20 µL | Sarstedt, Nümbrecht, Germany | 701114210 | |
| Biosphere Filter Tip, 300 µL | Sarstedt, Nümbrecht, Germany | 70765210 | |
| Bovine serum albumin | Carl Roth, Karlsruhe, Germany | 8076.4 | |
| Cell strainer, 100 µM | BD Bioscience, Heidelberg, Germany | 352360 | |
| Centrifuge 5810-R | Eppendorf AG, Hamburg, Germany | 5811000015 | |
| CO2 Incubator Heracell | Heraeus Instruments, Hanau, Germany | 51017865 | |
| Coverslips 12 mm | Carl Roth, Karlsruhe, Germany | P231.1 | |
| Curved fine forceps | Fine Science Tools GmbH, Heidelberg, Germany | 11370-42 | |
| DAPI fluoromount-G(R) | Biozol, Eching, Germany | SBA-0100-20 | |
| Dispase II | Sigma Aldrich GmbH, Steinheim, Germany | 4942078001 | |
| Distilled water (Water Purification System) | Millipore, Molsheim, France | ZLXS5010Y | |
| DMEM/F-12, GlutaMAX | Thermo Fisher Scientific, Schwerte, Germany | 31331093 | |
| DPBS (no Ca2+ and no Mg2+) | Sigma Aldrich GmbH, Steinheim, Germany | D8537-6X500ML | |
| Ethanol | VWR, Radnor, USA | 1009862500 | |
| FCS | Sigma Aldrich GmbH, Steinheim, Germany | F7524 | FCS must be tested for Schwann cell culture |
| Fine forceps (Dumont #5) | Fine Science Tools GmbH, Heidelberg, Germany | 11252-20 | |
| Forceps | Fine Science Tools GmbH, Heidelberg, Germany | 11370-40 | |
| Forskolin | Sigma Aldrich GmbH, Steinheim, Germany | F6886-10MG | |
| Gelatin | Sigma Aldrich GmbH, Steinheim, Germany | G1393-20ML | |
| Gentamycin | Thermo Fisher Scientific, Schwerte, Germany | 5710064 | |
| Goat anti-mouse IgG Alexa Fluor 488 | Thermo Fisher Scientific, Schwerte, Germany | A11036 | |
| Goat anti-rabbit IgG Alexa Fluor 568 | Thermo Fisher Scientific, Schwerte, Germany | A11001 | |
| HBSS (no Ca2+ and no Mg2+) | Thermo Fisher Scientific, Schwerte, Germany | 14170138 | |
| HERAcell Incubator | Heraeus Instruments, Hanau, Germany | 51017865 | |
| Heraguard ECO 1.2 | Thermo Fisher Scientific, Schwerte, Germany | 51029882 | |
| Horse serum | Pan-Biotech, Aidenbach, Germany | P30-0712 | |
| Image J Software | HIH, Bethesda, USA | ||
| Laminin | Sigma Aldrich GmbH, Steinheim, Germany | L2020-1MG | |
| Leibovitz´s L-15 Medium | Thermo Fisher Scientific, Schwerte, Germany | 11415064 | |
| L-Glutamine 200 mM | Thermo Fisher Scientific, Schwerte, Germany | 25030024 | |
| MACS Multistand | Miltenyi Biotec, Bergisch Gladbach, Germany | 130042303 | |
| Microscissors | Fine Science Tools GmbH, Heidelberg, Germany | 15000-08 | |
| Microscope | Motic, Wetzlar, Germany | Motic BA 400 | |
| Microscope Axio observer 7 | Zeiss, Oberkochen, Germany | 491917-0001-000 | |
| Microscope slide | VWR, Radnor, USA | 630-1985 | |
| MiniMACS separator | Miltenyi Biotec, Bergisch Gladbach, Germany | 130091632 | |
| MS columns | Miltenyi Biotec, Bergisch Gladbach, Germany | 130-042-201 | |
| Neubauer counting chamber | Assistant, Erlangen, Germany | 40441 | |
| Neuregulin | Peprotech, Rocky Hill, USA | 100-03 | |
| Neurobasal medium | Thermo Fisher Scientific, Schwerte, Germany | 21103049 | |
| NGF | Sigma Aldrich GmbH, Steinheim, Germany | N1408 | |
| Normal goat serum | Biozol, Eching, Germany | S-1000 | |
| Nunclon Δ multidishes, 4 well | Sigma Aldrich GmbH, Steinheim, Germany | D6789 | |
| Paraformaldehyde | Acros Organics, New Jersey, USA | 10342243 | |
| Penicillin/Streptomycin | Thermo Fisher Scientific, Schwerte, Germany | 15140-122 | |
| Pipetboy | Eppendorf AG, Hamburg, Germany | 4430000018 | |
| Pipettes | Eppendorf AG, Hamburg, Germany | 2231300004 | |
| Poly-D-Lysin | Sigma Aldrich GmbH, Steinheim, Germany | P6407-5MG | |
| Poly-L-Lysin | Sigma Aldrich GmbH, Steinheim, Germany | P4707-50ML | |
| Reaction tubes, 15 mL | Sarstedt, Nümbrecht, Germany | 62554502 | |
| Reaction tubes, 50 mL | Sarstedt, Nümbrecht, Germany | 62547254 | |
| Reaction vessels, 1.5 mL | Sarstedt, Nümbrecht, Germany | 72690001 | |
| Safety Cabinet S2020 1.8 | Thermo Fisher Scientific, Schwerte, Germany | 51026640 | |
| Scissors | Fine Science Tools GmbH, Heidelberg, Germany | 14083-08 | |
| Serological pipette, 10 mL | Sarstedt, Nümbrecht, Germany | 861254025 | |
| Serological pipette, 25 mL | Sarstedt, Nümbrecht, Germany | 861685001 | |
| Serological pipette, 5 mL | Sarstedt, Nümbrecht, Germany | 861253001 | |
| Spatula | Fine Science Tools GmbH, Heidelberg, Germany | 10094-13 | |
| Stereomicroscope Discovery.V8 | Zeiss, Oberkochen, Germany | 495015-0012-000 | |
| Surgical scissors | Fine Science Tools GmbH, Heidelberg, Germany | 14007-14 | |
| TC dish 100, cell + | Sarstedt, Nümbrecht, Germany | 833902300 | |
| TC dish 35, cell + | Sarstedt, Nümbrecht, Germany | 833900300 | |
| TC dish 60, cell + | Sarstedt, Nümbrecht, Germany | 833901300 | |
| Thy-1 Microbeads (MACS Kit) | Miltenyi Biotec, Bergisch Gladbach, Germany | 130-094-523 | |
| Triton X-100 | Sigma Aldrich GmbH, Steinheim, Germany | X100-500ML | |
| Trypan Blue Solution 0.4% | Thermo Fisher Scientific, Schwerte, Germany | 15250061 | |
| Trypsin (2.5%), no phenol red | Thermo Fisher Scientific, Schwerte, Germany | 15090-046 | |
| Trypsin-EDTA (0.05%), phenol red | Thermo Fisher Scientific, Schwerte, Germany | 25300-054 | |
| Type I Collagenase | Sigma Aldrich GmbH, Steinheim, Germany | C1639 | |
| Water bath type 1008 | GFL, Burgwedel, Germany | 4285 |
Riferimenti
- Lee, K. H., Chung, K., Chung, J. M., Coggeshall, R. E. Correlation of cell body size, axon size, and signal conduction velocity for individually labelled dorsal root ganglion cells in the cat. The Journal of Comparative Neurology. 243 (3), 335-346 (1986).
- Taveggia, C. Schwann cells-axon interaction in myelination. Current Opinion in Neurobiology. 39, 24-29 (2016).
- Gordon, T. Peripheral nerve regeneration and muscle reinnervation. International Journal of Molecular Sciences. 21 (22), 8652 (2020).
- Nocera, G., Jacob, C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cellular and Molecular Life Sciences. 77 (20), 3977-3989 (2020).
- Modrak, M., Talukder, M. A. H., Gurgenashvili, K., Noble, M., Elfar, J. C. Peripheral nerve injury and myelination: Potential therapeutic strategies. Journal of Neuroscience Research. 98 (5), 780-795 (2020).
- Salzer, J. L., Bunge, R. P., Glaser, L. Studies of Schwann cell proliferation. III. Evidence for the surface localization of the neurite mitogen. The Journal of Cell Biology. 84 (3), 767-778 (1980).
- Wood, P. M., Bunge, R. P. Evidence that sensory axons are mitogenic for Schwann cells. Nature. 256 (5519), 662-664 (1975).
- Eldridge, C. F., Bunge, M. B., Bunge, R. P., Wood, P. M. Differentiation of axon-related Schwann cells in vitro. I. Ascorbic acid regulates basal lamina assembly and myelin formation. The Journal of Cell Biology. 105 (2), 1023-1034 (1987).
- Paivalainen, S., et al. Myelination in mouse dorsal root ganglion/Schwann cell cocultures. Molecular and Cellular Neuroscience. 37 (3), 568-578 (2008).
- Clark, A. J., et al. Co-cultures with stem cell-derived human sensory neurons reveal regulators of peripheral myelination. Brain. 140 (4), 898-913 (2017).
- Taveggia, C., Bolino, A. DRG neuron/Schwann cells myelinating cocultures. Methods in Molecular Biology. 1791, 115-129 (2018).
- Andersen, N. D., Srinivas, S., Pinero, G., Monje, P. V. A rapid and versatile method for the isolation, purification and cryogenic storage of Schwann cells from adult rodent nerves. Scientific Reports. 6, 31781 (2016).
- Pitarokoili, K., et al. Intrathecal triamcinolone acetonide exerts anti-inflammatory effects on Lewis rat experimental autoimmune neuritis and direct anti-oxidative effects on Schwann cells. Journal of Neuroinflammation. 16 (1), 58 (2019).
- Grüter, T., et al. Immunomodulatory and anti-oxidative effect of the direct TRPV1 receptor agonist capsaicin on Schwann cells. Journal of Neuroinflammation. 17 (1), 145 (2020).
- Lehmann, H. C., Höke, A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS & Neurological Disorders – Drug Targets. 9 (6), 801-806 (2010).
- Joshi, A. R., et al. Loss of Schwann cell plasticity in chronic inflammatory demyelinating polyneuropathy (CIDP). Journal of Neuroinflammation. 13 (1), 255 (2016).
- Klimas, R., et al. Dose-dependent immunomodulatory effects of bortezomib in experimental autoimmune neuritis. Brain Communications. 3 (4), (2021).
- Szepanowski, F., et al. LPA1 signaling drives Schwann cell dedifferentiation in experimental autoimmune neuritis. Journal of Neuroinflammation. 18 (1), 293 (2021).

