Semi-Automated Phenotypic Analysis of Functional 3D Spheroid Cell Cultures
Summary
We present a protocol for growing high-reproducible spheroids and their phenotypic characterization using image capture and proteomics.
Abstract
We present a protocol that describes the properties and advantages of using a standalone clinostat incubator for growing, treating, and monitoring 3D cell cultures. The clinostat mimics an environment where cells can assemble as highly reproducible spheroids with low shear forces and active nutrient diffusion. We demonstrate that both cancer and non-cancer hepatocytes (HepG2/C3A and THLE-3 cell lines) require 3 weeks of growth prior to achieving functionalities comparable to liver cells. This protocol highlights the convenience of utilizing incubators for 3D cells with cameras monitoring the cell growth, as snapshots can be taken to count and measure spheroids upon treatment. We describe the comparison of THLE-3 and HepG2/C3A cell lines, showing how non-cancerous cell lines can be grown as well as immortalized cancer cells. We demonstrate and illustrate how proteomics experiments can be conducted from a few spheroids, which can be collected without perturbing cell signaling, i.e., no trypsinization required. We show that proteomics analysis can be used to monitor the typical liver phenotype of respiratory chain metabolism and the production of proteins involved in metal detoxification and describe a semi-automated system to count and measure the spheroid’s area. Altogether, the protocol presents a toolbox that comprises a phenotypic characterization via image capture and a proteomics pipeline to experiment on 3D cell culture models.
Introduction
In vitro cell cultures have been proven to be necessary and invaluable in establishing fundamental knowledge in biology. Much of the scientific understanding in biology and cancer specifically has come from the 2D culture system that is cells growing in a monolayer. Although 2D culture has been the dominant cell culture system, it has many disadvantages that can potentially stifle further biological progress. For example, 2D cultures lack cell-cell interactions important for cell signaling and proliferation1. To date, 3D culture systems have been shown to better model differentiation, drug response, tumor invasion, and biology2,3,4,5. 3D modeling of malignant cancers is especially vital due to the rise in the aging population and cancer mortality. Hepatocellular carcinoma (HCC) is one of the leading causes of cancer-related mortality worldwide and frequently has an abysmal prognosis6. HCC is known to have a low cure rate, poor drug response, and high rate of reoccurrence6,7,8. Several 3D models for normal liver and HCC have been developed that mimic the physiology of in vivo normal and malignant liver tissue9,10.
Some of the current 3D systems include liquid overlays, bioreactors, hydrogel, scaffolds, and 3D-printed structures. Spheroids generated in bioreactors specifically provide unique advantages because they mimic tumor distribution of nutrient exposure, gas exchange, and cell proliferation/quiescence11. Bioreactors are especially suitable for cancer models due to their ease of use, large scalability, nutrient diffusion, and accessibility11. In addition, bioreactors can allow for high throughput experiments, greater reproducibility, and decreased human error. The bioreactor used in this study is unique because it simulates a system of reduced gravity, which minimizes disruptive shearing forces applied in typical bioreactors allowing for better reproducibility12. The omnidirectional gravity and reduction in shearing forces allow for the cells to develop in a more physiological manner. As evidence, HepG2/C3A cells grown under this methodology develop spherical organelles that produce in vivo levels of ATP, adenylate kinase, urea, and cholesterol13,14. In addition, drug treatments in this 3D system are more advanced and automated in comparison to 2D cultures. In 2D cultures, drug treatments must often have a short time course due to the need to trypsinize and maintain cell health. Yet, in our case, we can perform long-term drug treatments of spheroids without the need to disrupt the structure and physiology of the cells. Therefore, a shift from 2D to 3D cultures is necessary to better model in vivo biological phenomena and further scientific development.
This paper presents a methodology for growing high-reproducible spheroids (Figure 1 and Figure 2) and shows a semi-automated system to phenotypically characterize 3D structures (Figure 3). At the image level, we provide information on counting and measuring spheroids' area (Figure 3). By using mass spectrometry methods, we show how proteomics can be used to assess specific biological functions (Figure 4). By collecting and analyzing this data, we hope to improve the understanding of the biology behind 3D cell culture systems.
Protocol
1. Buffers and reagents
- Cell growth media for HepG2/C3A cells: Prepare Dulbecco's modified Eagle's medium (DMEM, 4.5g/L glucose) containing 10% fetal bovine serum (FBS), non-essential amino acids (1% v/v), L-glutamine (1% v/v) and penicillin/streptomycin (0.5% v/v). Store the growth media at 4 °C.
- Cell growth media for THLE-3 cells: Prepare bronchial epithelial cell growth medium [BEGM] containing its supplements (bovine pituitary extract [BPE], hydrocortisone, human epidermal growth factor [hEGF], epinephrine, transferrin, insulin, retinoic acid, triiodothyronine, gentamicin sulfate-amphotericin [GA]) as well as 10% FBS, 5 ng/mL hEGF and 70 ng/mL phosphoetanolamine.
- 500 mM DL-Dithiothreitol (DTT): To prepare 10 mL, resuspend 0.771 g of DTT in 10 mL of HPLC grade water. Store 100 µL aliquots at -20 °C.
- 200 mM iodoacetamide: To prepare 1 mL, resuspend 36 mg of iodoacetamide in 1 mL of 50 mM ammonium bicarbonate. Do not store resuspended iodoacetamide.
- 5% sodium dodecyl sulfate (SDS): To prepare 50 mL, resuspend 2.5 g of SDS in 50 mL of 50 mM ammonium bicarbonate. Store at 4 °C.
- 12% phosphoric acid: To prepare 100 mL, dilute 14.1 mL of 85% phosphoric acid in 85.9 mL of HPLC grade water. Store in a glass bottle at room temperature (RT).
- Binding buffer: To prepare 1 L, add 900 mL of concentrated methanol to 100 mL of 10 mM ammonium bicarbonate or TEAB.
- 0.1% Trifluoroacetic acid (TFA) solution: Add 1 mL of concentrated TFA to 999 mL of HPLC grade water. Store at 4 °C.
- 60% acetonitrile/0.1% TFA solution: Add 600 mL of HPLC grade acetonitrile (60% v/v) to 399 mL of HPLC grade water. Next, add 1 mL of concentrated TFA to this solution, then store at 4 °C.
- Mobile Phase A (MPA) – 0.1% formic acid: Add 1 mL of concentrated formic acid to 999 mL of HPLC-grade water and mix well.
- Mobile Phase B (MPB) – 80% HPLC-grade acetonitrile + 0.1% formic acid: Add 800 mL of HPLC-grade acetonitrile to 199 mL of HPLC-grade water. Next, add 1 mL of concentrated formic acid to this solution and mix.
2. Preparation of spheroids
NOTE: Figure 1A represents the initial steps for preparing and culturing 3D spheroids from cell lines.
- Thaw frozen HepG2/C3A and THLE-3 cells and grow them as a monolayer using standard growth media in a tissue culture flask or dish until they reach approximately 80% confluency.
NOTE: When cells reach confluency, check the general cellular morphology and growth patterns using a microscope. It is not recommended to use cells at high passage numbers. - Wash cells twice with Hank's Balanced Salt Solution (HBSS, use 5 mL for a T75 cm2 flask or 10 cm dish).
- Add 5 mL of 0.05% trypsin-EDTA diluted in HBSS (1:2 dilution) and incubate for 5 min at 37 °C with 5% CO2. Use a microscope to assess cell detachment and add 3 mL of FBS or growth media (containing 10% FBS) to neutralize trypsin reaction.
- Transfer the cell suspension to a 15 mL tube. Spin down at 270 x g at RT for 5 min.
- Aspirate the supernatant and resuspend cells in 5 mL of complete growth media.
NOTE: If there are too many cells, dilute the cell suspension before counting. - Count the number of cells and dilute the cell suspension in complete growth media to obtain 1 x 106 in a maximum volume of 1.5 mL.
- Equilibrate an ultra-low attachment 24-well round bottom plate containing microwells.
- Wash the wells with 0.5 mL of growth media.
- Centrifuge the plate at 3,000 x g for 5 min (this removes air bubbles from the surface of wells).
- Transfer the cell suspension (prepared in step 1.4) into the plate and centrifuge for 3 min at 120 x g.
NOTE: The cell suspension volume may vary depending on the cell count, but it is important to limit it to 1.5 mL. - Incubate the plate for 24 h at 37 °C with 5% CO2 to begin spheroid formation.
3. Spheroids culture into bioreactors (Figure 2)
NOTE: To preserve the structure of spheroids, use wide bore tips when handling 3D spheres.
- Equilibrate the bioreactor by filling the humidity chamber with 25 mL of sterile water and the cell chamber with 9 mL of growth media 24 h before transferring the spheroids to it (Figure 2A). Be sure to use a 10 mL syringe coupled to a long needle when filling humidity and cell chambers.
- Incubate the bioreactor, rotating (15 rpm) in the 3D incubator (Figure 2D,F), for 24 h at 37 °C with 5% CO2.
- Using 1 mL wide bore tips, gently pipette up and down to detach spheroids from the ultra-low attachment plate and transfer the spheroids to a tissue culture dish.
- Wash the ultra-low attachment plate with 0.5 mL of pre-warmed growth media to capture leftover spheroids and transfer them to the same dish from step 3.3.
- Assess spheroids' size, compactness, and roundness using a light microscope (4x magnification) and select the ones that are sufficiently formed.
NOTE: Cells are sufficiently formed when they are compact and do not fall apart during handling. It is also important that spheroids are a single unit and not clumped together. A spheroid size between 100-200 µm is preferred when transferred to the bioreactor. - Transfer spheroids into the equilibrated bioreactor filled with 5 mL of fresh growth media. After transferring the spheroids, completely fill the bioreactor with fresh growth media, and be sure to avoid adding bubbles to the bioreactor when filling it with media.
- Place the bioreactor in the 3D incubator and adjust the rotation speed using the control unit (Figure 2C). For HepG2/C3A spheroids, set the rotation speed to 10-11 rpm; for THLE-3 spheroids, set it to 11-12 rpm.
NOTE: The rotation speed is correctly set when the spheroids are evenly distributed in the center of the bioreactor and not touching its walls. Figure 2E shows a rotating bioreactor. - Exchange growth media every 2-3 days by removing 10 mL of old media and replacing it with 10 mL of fresh media; be sure not to remove spheroids when exchanging media (Figure 2B).
NOTE: It is recommended to have a routine of media exchange (for example, 48 h/48 h/72 h) and keep a detailed record. - Adjust rotation speed every time media is changed. Increase the speed as spheroids grow in size and number.
- After 15 days in culture, split spheroids into two new bioreactors.
NOTE: Depending on the cell line and growth rate, the time may vary and should be optimized individually. - After 20 days, spheroids are ready for collection.
4. Image capture and counting of spheroids
NOTE: The simplified pipeline for spheroids count is shown in Figure 3A. For spheroids count and area determination (section 5), it is critical to evaluate the compactness of the 3D structures. This will contribute to a more enhanced color contrast, which is necessary for the method to be accurate.
- Open the 3D app installed on the tablet.
- Select the bioreactor and take a snapshot.
NOTE: Alternatively, a picture can be taken. The following parameters should be considered.- Place a black background behind the bioreactor (black support is provided in the bioreactor box).
- Ensure there is no light reflection on the cell chamber.
- Take the picture as close to the bioreactor as possible.
- Open one picture in FIJI (ImageJ).
- Prepare the picture for analysis:
- Select the Oval tool. Draw a circle around the plug.
- Click Delete on the keyboard to make everything inside the circle black. Draw a circle around the cell chamber. Make sure that all spheroids are inside the circle.
- Run macro to count spheroids:
- Click Plugins > Macro > Record. Copy macro text from Supplementary File 1 into the recorder.
- Press Create, and a new window will open. Press Run to analyze the picture opened.
- A new window will open with the results (count, total area, average size, and percentage area).
- Close the image and repeat steps 4.4 and 4.5 for each image for which the spheroid count is needed. For easier and quicker processing, save the macro and run it by clicking Plugins > Macro > Run and select the saved macro.
5. Planimetric determination of the spheroid area
NOTE: The simplified pipeline for determining the spheroid area is shown in Figure 3B.
- Take images as described in step 3.5.
- Before starting, set a global scale to measure the area of the spheroids.
- Open in FIJI a picture with a scale bar with the desired magnification. Select the line tool. Draw over the scale bar (the line needs to be as long as the scale bar is).
- Open Analyze > Set Scale. Write the know distance (the length of the line is automatically entered in the Distance in Pixels. Make sure to tick Global.
- Press OK. Now the scale is set for the picture that will be analyzed.
- To start the planimetric determination, run the macro (Supplementary File 2).
- Click Process > Batch > Macro. Choose the folder that contains the pictures to be analyzed. This folder will be the Input.
- Choose the folder created in step 5.3.1 as Output. Press Process.
- As a quality check, evaluate if the spheroid area measured corresponds to the original image.
NOTE: Macro excludes spheroids on the edge where only a part of the spheroid can be measured.
6. Collection of spheroids
NOTE: It is strongly advised that spheroids are collected using wide bore tips to preserve their 3D structure. The collection can be done by using the plug in the front of the bioreactor (Figure 2A).
The size of spheroids at collection may vary depending on the cell line, starting number of cells, and splitting process (days in culture, number of spheroids per bioreactor, and splitting ratio).
- To collect spheroids, remove 5 mL of media from the bioreactor through the top port using a syringe coupled to a long needle. Be sure to let spheroids sink to the bottom center of the bioreactor (near the bottom port).
- Open the front port and collect spheroids using a 1 mL wide bore tip. Place the spheroids in microcentrifuge tubes. Centrifuge the spheroids at 500 x g for 5 min and discard media.
- Wash the spheroids with 200 µL of HBSS to remove the FBS. Centrifuge at 500 x g for 5 min and discard the supernatant.
- Snap-freeze the spheroid pellet with liquid nitrogen and store at -80 °C until processing.
7. Viability of spheroids
NOTE: Spheroid viability was determined by measuring the activity of the adenylate kinase (AK) released by damaged cells (Figure 4A). Due to the diffusion gradient, AK measurement is efficient when spheroids are smaller than 900 µm in diameter12. If the spheroids become larger, or if there is any doubt regarding the viability measurement, an ATP assay can be performed15.
- Collect the spheroid supernatant and transfer 20 µL to a 96-well white-walled plate (flat bottom clear). Add 100 µL of adenylate kinase detection reagent to each well and homogenize by gently pipetting up and down.
- Remove bubbles by centrifugation in the speed vacuum for 2 min at RT. Incubate the plates for 20 min at RT.
- Place the plate in the luminometer (plate reader, luminescence mode) and initiate the program. Set the parameters according to the kit manufacturer's instructions.
NOTE: In bioluminescent viability assays, the direct luminometer light output (commonly RLUs) may be used to calculate the cell response. As adenylate kinase will only leak from cells whose cell integrity has been compromised, it is possible to achieve a total adenylate kinase control by using a lysis reagent.
8. Protein extraction
NOTE: Figure 1B represents the workflow for spheroids processing and protein extraction.
- Collect spheroids (section 6) on day 36. Resuspend spheroids in 25 µL of 5% SDS to lyse the cells.
- After adding 5% SDS, homogenize the pellet by pipetting up and down. In some cases when it is difficult to lyse the spheroids, use a small pestle.
- Incubate the samples with 20 mM DTT for 1 h to reduce proteins. Mix by pipetting up and down.
- Incubate samples with 40 mM iodoacetamide for 30 min protected from light to alkylate proteins. Mix by pipetting up and down.
- Add 12% phosphoric acid solution (10x) to the samples at a final concentration of 1.2%.
- Dilute samples in six volumes of binding buffer and gently mix.
- Load the sample to an S-Trap filter plate and spin down at 500 x g for 30 s.
NOTE: If the total volume of the sample in step 8.4 exceeds the column volume capacity, load samples in batches and repeat step 8.6 until everything is passed through the column. - Wash the samples twice with 150 µL of binding buffer. After each wash, spin down at 500 x g for 30 s and discard the flow through.
- Incubate the samples with 1 µg of sequencing-grade trypsin diluted in 50 mM ammonium bicarbonate overnight at 37 °C.
NOTE: At least 20 µg of protein is recommended as starting material for comprehensive proteome analysis. For this, 1 µg of trypsin is the ideal amount for efficient digestion. Remove any bubbles between the buffer and the column bed. - Elute peptides with 40 µL of 50 mM ammonium bicarbonate and pool into the same collection tube.
- Spin down at 500 x g for 30 s. Elute peptides with 40 µL of 0.1% TFA and pool them into the same collection tube.
- Spin down at 500 x g for 30 s. Elute the peptides with 40 µL of 60% acetonitrile and 0.1 % TFA and pool them into the same collection tube.
- Spin down at 500 x g for 30 s. Dry pooled eluates in the speed vacuum and store the samples at -80 °C until processing.
9. Sample cleanup
NOTE: Before proceeding to the proteomics analysis, it is necessary to remove the salt present in the samples. Salts can interfere with high-performance liquid chromatography-mass spectrometry (HPLC-MS) analysis as they ionize during electrospray, suppressing the signal from peptides. The setup for de-salting used in this study was previously demonstrated by Joseph-Chowdhury and colleagues16.
- Before starting, ensure there is a clean 96-well collection plate to collect the flow through during the following steps. Mix the C18 resin (50 mg/mL in 100% acetonitrile) on a magnetic stir plate.
- Add 70 µL of the C18 resin suspension per well of the 96-well filter plate, do this swiftly to ensure that C18 resin does not pool at the bottom of the pipette.
- Turn on the vacuum gently to prevent splashing. Discard the flow-through collected on the 96-well collection plate.
- Wash the resin with 100 µL of 0.1% TFA. Turn on the vacuum gently to prevent splashing and mixing of samples and discard the flow through.
- Resuspend each sample in 100 µL of 0.1% TFA. Check and ensure that the pH is between 2-3.
- Load each sample into each well of the filter plate. Turn on the vacuum gently to prevent splashing and mixing of samples and discard the flow through.
- Wash with 100 µL of 0.1% TFA. Turn on the vacuum gently to prevent splashing and mixing of samples. Discard the flow through.
- Replace the 96-well collection plate with a new 96-well plate to collect the de-salted samples.
- Add 60 µL of acetonitrile/0.1% TFA per well. To elute the samples from the C18 resin, turn on the vacuum gently to prevent splashing.
- Collect the flow through and dry in a speed vacuum at RT. Proceed to LC-MS/MS or store the plate at -80 °C until ready to process the samples.
10. Proteomics analysis via liquid chromatography coupled with mass spectrometry
NOTE: To generate the data for this manuscript, a nLC-MS/MS system with a two-column system setup with a 300 µm ID x 0.5 cm C18 trap column and a 75 µm ID x 25 cm C18-AQ (3 µm) analytical nano-column packed in-house was used.
- Prepare the mobile phases to run on the HPLC:
Mobile Phase A (MPA): 0.1% formic acid in HPLC-grade water
Mobile Phase B (MPB): 0.1% formic acid in HPLC-grade acetonitrile - Program the HPLC method as follows: 4%-34% MPB over 120 min, 34%-90% MPB over 5 min, isocratic 90% MPB over 5 min; flow rate: 300 nL/min.
- Set up the MS acquisition method to perform data-independent acquisition (DIA) to generate MS/MS spectra of the detected peptide signals.
- Resuspend 1 µg of samples in 10 µL of 0.1% TFA prior to placing the samples in the nLC autosampler.
- Run the nLC-MS method as programmed for canonical proteomics acquisition:
- Set full MS scan to 300-1100 m/z in the orbitrap, with a resolution of 120,000 (at 200 m/z) and an automatic gain control (AGC) target of 125.
- Set MS/MS in the orbitrap with sequential isolation window of 50 m/z with an AGC target of 400 and a Higher-energy collisional dissociation (HCD) energy of 30.
11. Data analysis
- Import the nLC-MS/MS raw data files into a peak detection software compatible with the MS platform used.
- Select the proper database (human, mouse, etc).
- Set N-terminal acetylation as variable modification and carbamidomethyl cysteine as fixed modification.
- Specify trypsin as the digestive enzyme with two missed cleavage allowed.
- Set mass tolerance accordingly to the mass analyzer utilized for the spectra acquisition.
- Export the analysis as a spreadsheet and process the data further as needed.
Representative Results
In this protocol, we describe the properties of an innovative stress-free 3D cell incubator, a system designed specifically for the culturing of 3D spheroids (Figure 2). We optimized the protocol for 3D culturing of THLE-3 and HepG2/C3A cell lines. The protocol described here is simple to use and allows for the reproducibility and cost-effective culturing of > 100 spheroids per bioreactor. Once in the bioreactor, spheroids are treated similarly to cells maintained in 2D culture. Optimal growth conditions are achieved by exchanging the media two to three times a week (Figure 2B) and adjusting the rotation speed according to spheroids' growth and size (Figure 2C). This system, in which 3D spheroids are cultured in rotating bioreactors (Figure 2E,F), provides an optimal growth environment for 3D structures by exposing spheroid to an equal and very low amount of shear force.
We have previously shown how spheroids can be used for the analysis of chromatin modification16. Here, we demonstrate in detail how to obtain liver spheroids and how proteomics experiments can be conducted for the analysis of the full proteome (Figure 1). In brief, the protocol was initiated by using THLE-3 or HepG2/C3A flat cells until the culture reached 80% confluency. To culture cells as spheroids, approximately 2,000 cells were plated in an ultra-low attachment plate containing microwells to allow them to self-aggregate, and then, formed spheroids were transferred to a bioreactor (Figure 1A). Although they are functionally active after 3 weeks in culture, as previously demonstrated17, we show results from spheroids collected after 36 days in culture for this protocol. After collection, spheroids were spun down, and both pellet and supernatant were stored for analysis. Cell viability was assessed from the supernatant for the quantification of adenylate kinase released by damaged cells, as described previously17. Cellular proteins were extracted from the cell pellet, and the full proteome was analyzed by high-resolution mass spectrometry (Figure 1B).
This protocol also demonstrates a semi-automated method for spheroids counting (Figure 3A) using the public image processing program FIJI (Fiji Is Just ImageJ)18. A good-quality picture of the spheroid should be taken for the analysis, and some parameters should be considered as mentioned in section 5. Then, after preparing the picture for analysis, a macro script (Supplementary File 1) is used for counting the spheroids. The macro works by first making a folder called FIJI Spheroids counting, inside the folder where the spheroid pictures are located. In this folder, all information from the analysis is saved; this includes a picture of the spheroids that were counted, with an ID number on each spheroid. It also includes an Excel file called spheroid counting. This file contains the pixel area and ID number for each spheroid that was counted. The data corresponding to one analyzed picture is presented in each tab of the file. The tab is labeled according to the name of the picture analyzed. As spheroid size can be impaired by many factors, including the number of structures within a vessel and drug treatment, it is also important to monitor their surface area (planimetry). The macro script presented here (Supplementary File 2) works by measuring the black areas, which correspond to spheroids in the picture (Figure 3B). The output is gathered in a file called planimetry.xlsx, which contains the measured area, perimeter, and diameter of each spheroid. There is also a measurement called Feret, used to calculate the diameter. Feret is the longest possible diameter, while minFeret is the shortest. The diameter is the average of these two. Inside the output folder, besides the planimetry.xlsx file, there is also a picture of the spheroids that were measured.
Before proceeding to the proteome analysis, spheroids' viability was evaluated over culture time. Levels of AK increase up to day 17, reaching approximately 7% of cell death, and then the death decrease to levels below 5% (Figure 4A), which is in accordance with previously published work17. This protocol also shows the full proteome analysis for monitoring the cell phenotype. Firstly, the proteomes of THLE-3 and HepG2/C3A flat cells and spheroids were compared. By analyzing the first principal component (PC1), it is evident that there is a strict separation of spheroid samples from flat cell cultures, and it seems that the correlation of cell type (THLE-3 and HepG2/C3A) is not relevant (Figure 4B). Although THLE-3 and HepG2/C3A spheroids do not cluster together, they share similar profiles consistent with liver function. We demonstrate in this protocol the example of metallothioneins, which have a role in metal detoxification performed by the liver. We identified in the proteomics analysis 2 isoforms overexpressed in spheroids in comparison to flat cells (MT1E and MT1X) (Figure 4C). We also show the Gene Ontology (GO) enrichment of both cell lines grown as spheroids. The carbohydrate metabolic process, which comprises the tricarboxylic acid cycle (TCA cycle), the electron transport chain (cellular respiration), and pyruvate metabolism, is a frequent term and is enriched in both HepG2/C3A and THLE-3 spheroids (Figure 4D,E). Cellular detoxification, fatty acid, and cholesterol metabolism are other functions enriched in both spheroids. Together, these functions are known to be crucial for the liver function.
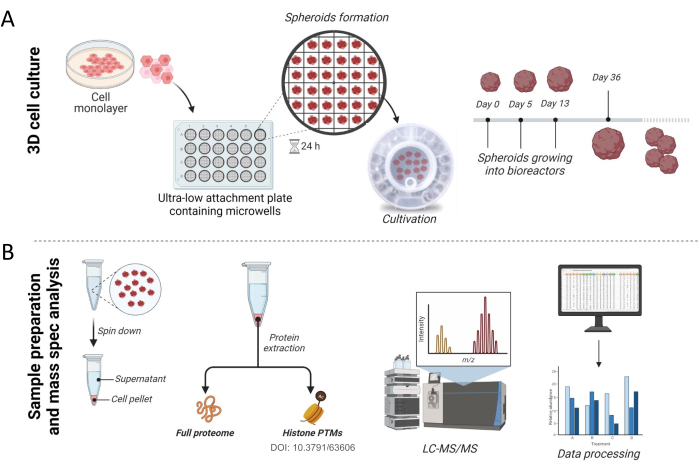
Figure 1: Workflow for spheroids culture and sample preparation. (A) 3D cell culture experimental approach. Flat cell cultures at desired confluency were trypsinized and seeded on an ultra-low attachment 24 well plate containing microwells, where cells self-assemble into spheroids. After 24 h, spheroids were transferred to a bioreactor and cultivated until they are ready for analysis. (B) After collection, spheroids were pelleted and both pellet and culture supernatant were stored until processing. Histones16and non-histones proteins were extracted, digested into peptides, and analyzed by high-resolution mass spectrometry. Raw files obtained from the mass spectrometry were searched against the human database, and the data were further processed. Please click here to view a larger version of this figure.
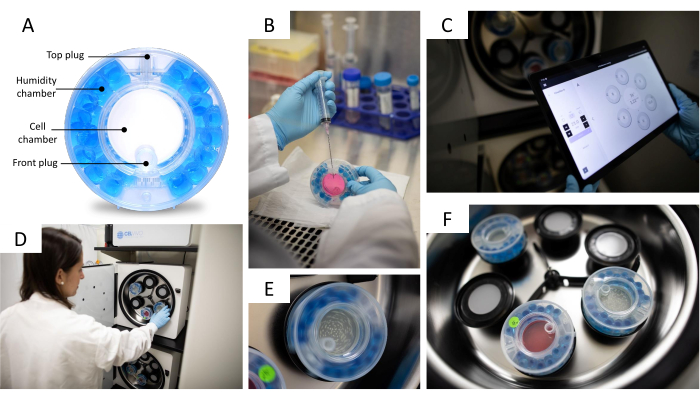
Figure 2: 3D cell culture system. (A) Bioreactor parts. The bioreactor is composed of a gas exchange and humidification chamber containing water beads and an openable cell chamber with two plugs for media exchange and spheroids collection. (B) Bioreactor media exchange. The bioreactor is filled with 10 mL of growth media by using a syringe with a needle. (C) The system control app. The rotation speed, CO2 level, temperature, alarm log, and other functionalities can be controlled using the control unit. (D) Placing the bioreactor in the 3D incubator. Each bioreactor has an associated motor which can spin the bioreactor slowly. (E) Bioreactor in movement with the speed (rpm) controlled by a (C) tablet. The speed (rpm) is adjusted according to the spheroids' size. (F) Bioreactors inside de 3D incubator. The 3D incubator can fit up to 6 individually controlled bioreactors. Photo courtesy of Jason Torres Photography. Please click here to view a larger version of this figure.
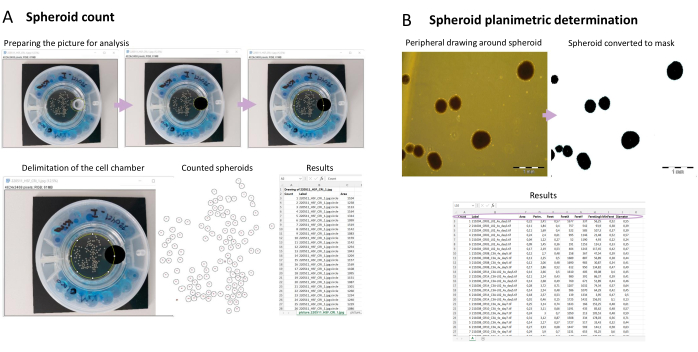
Figure 3: Phenotypic characterization of spheroids via image capture. (A) Semi-automated spheroid count. After taking snapshots of the spheroids in the bioreactor, the image is prepared for analysis in FIJI. Each spheroid is counted, and an ID number is provided for each of them. A macro is used, and the results are displayed showing the ID for the counted spheroid, the label (name of the picture that was analyzed), and the area (the number of pixels counted in the spheroid). (B) Planimetric determination of spheroid area. Using a macro, the area, perimeter, and diameter of a specific spheroid are determined. Please click here to view a larger version of this figure.
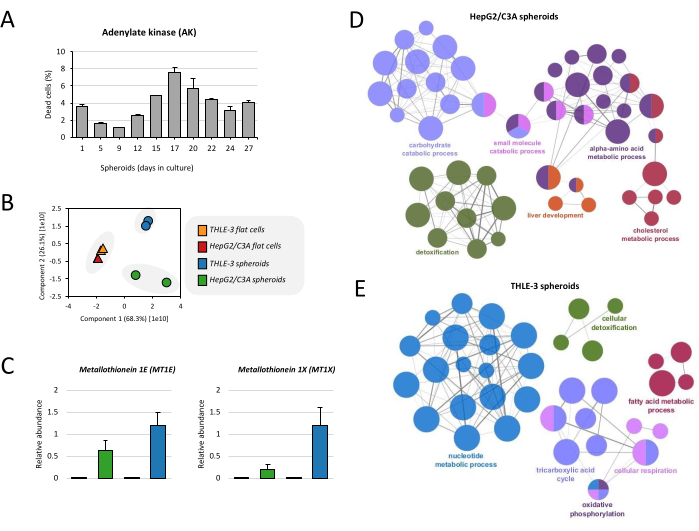
Figure 4: Proteome analysis of liver spheroids. (A) Viability of spheroids was calculated based on the release of adenylate kinase (AK) on culture supernatant. Results are the means of duplicate data points ± SD. (B) Principal component analysis (PCA) was performed to compare the proteome of THLE-3 and HepG2/C3A flat cells and spheroids.(C) Relative abundance of metallothioneins, which are proteins expressed by the human liver. Data are represented as means ± SEM. (D) Functionally grouped network show GO enrichment for HepG2/C3A spheroids and (E) THLE-3 spheroids, where only the label of the most significant term per group is shown. The network was constructed using ClueGo19. The node size represents the term enrichment significance. Please click here to view a larger version of this figure.
Supplementary File 1: Macro script for spheroid counting. Please click here to download this File.
Supplementary File 2: Macro for spheroids planimetric determination. Please click here to download this File.
Discussion
Understanding the biology behind three-dimensional (3D) cellular structures is extremely important for a more comprehensive knowledge of their functionalities. There is a growing interest in using 3D models for studying complex biology and performing toxicity screening. When cultivating cells in 3D many factors need to be considered, including the phenotypic assessment of the model system. A phenotype is defined as a group of observable characteristics of a specific organism, such as morphology, behavior, physiological and biochemical properties20.
In this protocol, we demonstrate how proteomics experiments can be conducted from a few spheroids and can be used to monitor the typical liver phenotype. Mass spectrometry has become an extensively applied method for 3D cell characterization, allowing the investigation of a variety of biological questions12,16,21,22. For a comprehensive proteome analysis, it is recommended to use at least 20 µg of protein starting material, from which 1 µg is injected into the mass spectrometer. It is important to mention that adding less sample might lead to loss of sensitivity, and adding more would gradually worsen the quality of the chromatography and eventually lead to blocking the column. In this study, we showed that the HepG2/C3A and THLE-3 spheroids are enriched with important proteins from glycolysis and TCA cycle, which are specific liver pathways and are critical for maintaining blood glucose levels and for energy production23,24. Actually, mass spectrometry analysis provides not only information at the protein level but also allows the investigation of protein post-translational modifications, as shown previously by our group16.
Another aspect to be considered in 3D phenotypic studies is the number and size of spheroids. Besides making experiments more reproducible, counting the number of spheroids and determining their size is essential to determine when to split the culture into multiple bioreactors, as the number of 3D structures within a vessel can impact spheroids' size and metabolic activity levels. However, it is important to highlight that the number and size of spheroids depend on the cell line, starting number of cells, splitting process, and time of collection. Details of HepG2/C3A spheroid culture, such as number of cells per spheroids, protein content, and size as a function of age, were provided by Fey, Korzeniowska, and Wrzesinski25. For accurate and successful analysis using the semi-automated method described here, the most critical step is a good spheroids' picture. For simplicity, the picture can be taken with a phone or tablet, but its resolution should be kept as high as possible. As images are quick to acquire, they allow large-scale screening experiments to visualize specific phenotypic features or investigate responses to drug treatment. Therefore, due to the increasing number of cell-based assays, a number of open-source software have been developed over the past 10 years for image analysis26. In this protocol, we describe a semi-automated system using the software FIJI18 to count and measure spheroids' size. We presented scripts (simple programming commands) to define a sequence of algorithmic operations that can be applied to an image collection, making the analysis an easy and quick process. However, depending on the characteristic of the spheroid, a manual measurement should be employed. For instance, if the spheroids are too translucent, the FIJI script will be imprecise. By the way, one of the most important criteria for this method to work is the compactness of the spheroids. This characteristic will contribute to a more enhanced color contrast between the spheroids and the background, which is necessary for the method to be accurate.
In summary, besides presenting a methodology for growing high-reproducible spheroids, a semi-automated system coupled with phenotypic characterization via image capture and proteomics was also described. We expect this toolbox for analyzing 3D cells to become more robust with full-automated image analysis software and next-generation mass spectrometers.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The Sidoli lab gratefully acknowledges the Leukemia Research Foundation (Hollis Brownstein New Investigator Research Grant), AFAR (Sagol Network GerOmics award), Deerfield (Xseed award), Relay Therapeutics, Merck, and the NIH Office of the Director (1S10OD030286-01).
Materials
| 1.5 mL microcentrifuge tubes | Bio-Rad | 2239480 | |
| 10 mL syringe | Fisher Scientific | 1481754 | Luer lock tip, graduated to 12 mL |
| 1000 µL wide bore pipet tips | Fisher Scientific | 14222703 | |
| 200 µL wide bore pipet tips | Fisher Scientific | 14222730 | |
| 96-well Orochem filter plate | Orochem | OF1100 | |
| 96-well skirted plate | Axygen | PCR-96-FS-C | |
| 96-well vacuum manifold | Millipore | MAVM0960R | |
| Ammonium bicarbonate | Sigma | A6141-25G | |
| Bronchial Epithelial Cell Growth Medium (BEGM) | Lonza | CC-3170 | |
| Cell culture grade water | Corning | 25-055-CV | |
| ClinoReactor | CelVivo | N/A | Bioreactor for 3D cell culture |
| ClinoStar incubator | CelVivo | N/A | CO2 incubator for 3D cell culture |
| DTT | Sigma | D0632-5G | |
| Dulbecco's Modified Eagle's Medium (DMEM) | Fisher Scientific | MT17205CV | |
| Elplasia 24-well round bottom ultra-low attachment plate containing microwells | Corning | 4441 | |
| Fetal Bovine Serum | Fisher Scientific | MT35010CV | |
| Formic acid | Thermo | 28905 | |
| Hank's Balanced Salt Solution (HBSS) | Fisher Scientific | MT21022CV | |
| hEGF | Corning | 354052 | |
| HERAcell vios 160i | Thermo | 51033557 | CO2 incubator for 2D cell culture |
| HPLC grade acetonitrile | Fisher Scientific | A955-4 | |
| HPLC grade methanol | Fisher Scientific | A452-1 | |
| HPLC grade water | Fisher Scientific | W5-4 | |
| Iodoacetamide | Sigma | I1149-5G | |
| L-glutamine | Fisher Scientific | MT25015CI | |
| Non-essential amino acids | Fisher Scientific | MT25025CI | |
| Oasis HLB Resin 30 µm | Waters | 186007549 | |
| Orbitrap Fusion Lumos Tribrid mass spectrometer | Thermo | IQLAAEGAAPFADBMBHQ | High resolution mass spectrometer |
| PAULA microscope | Leica | ||
| Penicillin-Streptomycin | Fisher Scientific | MT3002CI | |
| PerkinElmer Victor X2 multilabel microplate reader | PerkinElmer | ||
| pH paper | Hydrion | 93 | |
| Phosphoetanolamine | Sigma | P0503 | |
| Phosphoric acid | Fisher Scientific | A260-500 | |
| Pipette gun | Eppendorf | Z666467 (Milipore Sigma) | |
| Refrigerated centrifuge | Thermo | 75-217-420 | |
| Reprosil-Pur resin | MSWIL | R13.AQ.003 | 120 Å pore size, C18-AQ phase, 3 μM bead size |
| SDS | Bio-Rad | 1610301 | |
| Sequencing grade modified trypsin | Promega | V511A | |
| SpeedVac vacuum concentrator (96-well plates) | Thermo | 15308325 | Savant SPD1010 |
| Sterile hood | Thermo | 1375 | |
| Sterile serological pipettes | Fisher Scientific | 1367549 | |
| S-trap | Protifi | C02-micro-80 | |
| Syringe needle (18 G) | Fisher Scientific | 14817100 | 3" length, 0.05" diameter |
| Trifluoroacetic acid (TFA) | Thermo | 28904 | |
| Trypsin-EDTA | Gibco | 25300-054 | |
| Vortex | Sigma | Z258415 | |
| Water bath | Fisher Scientific | FSGPD10 |
Riferimenti
- Ravi, M., Paramesh, V., Kaviya, S. R., Anuradha, E., Solomon, F. D. 3D cell culture systems: advantages and applications. Journal of Cellular Physiology. 230 (1), 16-26 (2015).
- Nirmalanandhan, V. S., Duren, A., Hendricks, P., Vielhauer, G., Sittampalam, G. S. Activity of anticancer agents in a three-dimensional cell culture model. Assay and Drug Development Technologies. 8 (5), 581-590 (2010).
- Erickson, I. E., Huang, A. H., Chung, C., Li, R. T., Burdick, J. A., Mauck, R. L. Differential maturation and structure-function relationships in mesenchymal stem cell- and chondrocyte-seeded hydrogels. Tissue Engineering Part A. 15 (5), 1041-1052 (2009).
- Vinci, M., et al. Advances in establishment and analysis of three-dimensional tumor spheroid-based functional assays for target validation and drug evaluation. BMC Biology. 10 (29), (2012).
- Liu, J., Abate, W., Xu, J., Corry, D., Kaul, B., Jackson, S. K. Three-dimensional spheroid cultures of A549 and HepG2 cells exhibit different lipopolysaccharide (LPS) receptor expression and LPS-induced cytokine response compared with monolayer cultures. Innate Immunity. 17 (3), 245-255 (2011).
- Khafaga, A. F., Mousa, S. A., Aleya, L., Abdel-Daim, M. M. Three-dimensional (3D) cell culture: a valuable step in advancing treatments for human hepatocellular carcinoma. Cancer Cell International. 22 (1), 243 (2022).
- Llovet, J. M., Burroughs, A., Bruix, J. Hepatocellular carcinoma. The Lancet. 362 (9399), 1907-1917 (2003).
- Sia, D., Llovet, J. M. Liver cancer: Translating ‘-omics’ results into precision medicine for hepatocellular carcinoma. Nature Reviews Gastroenterology & Hepatology. 14 (10), 571-572 (2017).
- Tang, J., et al. A three-dimensional cell biology model of human hepatocellular carcinoma in vitro. Tumour Biology. 32 (3), 469-479 (2011).
- van Zijl, F., Mikulits, W. Hepatospheres: Three dimensional cell cultures resemble physiological conditions of the liver. World Journal of Hepatology. 2 (1), 1-7 (2010).
- Chaicharoenaudomrung, N., Kunhorm, P., Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World Journal of Stem Cells. 11 (12), 1065-1083 (2019).
- Wrzesinski, K., Fey, S. J. Metabolic reprogramming and the recovery of physiological functionality in 3D cultures in micro-bioreactors. Bioengineering(Basel, Switzerland). 5 (1), 22 (2018).
- Breslin, S., O’Driscoll, L. Three-dimensional cell culture: The missing link in drug discovery. Drug Discovery Today. 18 (5-6), 240-249 (2013).
- Kapalczynska, M., et al. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Archives of Medical Science: AMS. 14 (4), 910-919 (2018).
- Wrzesinski, K., Frandsen, H. S., Calitz, C., Gouws, C., Korzeniowska, B., Fey, S. J. Clinostat 3D cell culture: Protocols for the preparation and functional analysis of highly reproducible, large, uniform spheroids and organoids. Methods in Molecular Biology. 2273, 17-62 (2021).
- Joseph-Chowdhury, J. N., et al. Global level quantification of histone post-translational modifications in a 3D cell culture model of hepatic tissue. Journal of Visualized Experiments: JoVE. 183, 63606 (2022).
- Wrzesinski, K., Fey, S. J. After trypsinisation, 3D spheroids of C3A hepatocytes need 18 days to re-establish similar levels of key physiological functions to those seen in the liver. Toxicology Research. 2, 123-135 (2013).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Bindea, G., et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 25 (8), 1091-1093 (2009).
- Houle, D., Govindaraju, D. R., Omholt, S. Phenomics: The next challenge. Nature Reviews. Genetics. 11 (12), 855-866 (2010).
- Gonneaud, A., Asselin, C., Boudreau, F., Boisvert, F. M. Phenotypic analysis of organoids by proteomics. Proteomics. 17 (20), (2017).
- Avelino, T. M., et al. Mass spectrometry-based proteomics of 3D cell culture: A useful tool to validate culture of spheroids and organoids. SLAS Discovery. 27 (3), 167-174 (2022).
- Chiang, J., McManus, L. M., MitchellIn, R. N. . Liver physiology: Metabolism and Detoxification. Pathobiology of Human Disease. , 1770-1782 (2014).
- Begriche, K., Massart, J., Robin, M. A., Borgne-Sanchez, A., Fromenty, B. Drug-induced toxicity on mitochondria and lipid metabolism: Mechanistic diversity and deleterious consequences for the liver. Journal of Hepatology. 54 (4), 773-794 (2011).
- Fey, S. J., Korzeniowska, B., Wrzesinski, K. Response to and recovery from treatment in human liver-mimetic clinostat spheroids: a model for assessing repeated-dose drug toxicity. Toxicology Research. 9 (4), 379-389 (2020).
- Smith, K., et al. Phenotypic image analysis software tools for exploring and understanding big image data from cell-based assays. Cell Systems. 6 (6), 636-653 (2018).

