Exercise Test for Evaluation of the Functional Efficacy of the Pig Cardiovascular System
Summary
The present protocol describes a large animal exercise test model to assess the functional capacity of the cardiovascular system for evaluating the efficiency of new therapies in the preclinical setting. It is comparable to a clinical exercise test.
Abstract
Despite the progress in treatments, cardiovascular diseases are still one of the biggest causes of mortality and morbidity worldwide. Gene therapy-based therapeutic angiogenesis is a promising approach for treating patients with significant symptoms, despite optimal pharmacological therapy and invasive procedures. However, many promising cardiovascular gene therapy techniques have failed to accomplish expectations in clinical trials. One explanation is a mismatch between preclinical and clinical endpoints used to measure efficacy. In animal models, the emphasis has usually been on easily quantifiable endpoints, such as the number and area of the capillary vessels calculated from histological sections. Apart from mortality and morbidity, endpoints in clinical trials are subjective, such as exercise tolerance and quality of life. However, the preclinical and clinical endpoints likely measure different aspects of the applied therapy. Nevertheless, both types of endpoints are required to develop successful therapeutic approaches. In clinics, the main goal is always to alleviate patients’ symptoms and improve their prognosis and quality of life. To achieve better predictive data from preclinical studies, endpoint measurements must be better matched to those in clinical studies. Here, we introduce a protocol for a clinically relevant treadmill exercise test in pigs. This study aims to: (1) provide a reliable exercise test in pigs that can be used to evaluate the safety and functional efficacy of gene therapy and other novel therapies, and (2) better match the endpoints between preclinical and clinical studies.
Introduction
Chronic cardiovascular diseases are significant causes of mortality and morbidity worldwide1,2. Although current treatments are effective for the majority of patients, many still cannot benefit from the current therapies due to, for example, diffuse chronic disease or comorbidities. In addition, in some patients, cardiac symptoms are not relieved by the available treatments, and their cardiovascular disease progresses despite optimal medical therapy3. Thus, there is a clear need to develop novel treatment options for severe cardiovascular diseases.
During the past several years, new molecular pathways and ways to manipulate these targets have been discovered, making gene therapy, cell therapy, and other novel therapies a realistic option for treating severe cardiovascular diseases4. However, after promising preclinical results, many cardiovascular applications have failed to fulfill expectations in clinical trials. In spite of the poor efficacy in clinical trials, several trials have established good safety profiles of novel therapies5,6,7,8,9. Thus, bringing new cardiovascular therapies to patients will require improved approaches and better preclinical models, study settings, and endpoints in preclinical studies that can predict clinical efficacy.
In animal models, the emphasis has usually been on easily quantifiable endpoints, such as the number and area of capillary vessels calculated from histological sections or parameters from left ventricle imaging at rest and under pharmacological stress. In clinical trials, many endpoints have been more subjective, such as exercise tolerance or symptom relief4. Thus, it is likely that the endpoints in preclinical studies and clinical trials measure different aspects of the applied therapy. For example, an increase in the quantity of blood vessels does not always correlate with better perfusion, cardiac function, or exercise tolerance. Nevertheless, both types of endpoints are required to develop successful therapeutic approaches10. Still, the main goal is always to alleviate symptoms and to improve the patient's prognosis and quality of life. To achieve this, endpoint measurements must be better matched between preclinical and clinical studies4.
Cardiorespiratory fitness reflects the ability of the circulatory and respiratory systems to provide oxygen during sustained physical activity, and thus it quantifies the functional capacity of an individual. Functional capacity is a key prognostic marker as it is a strong independent predictor for the risk of cardiovascular and all-cause mortality11. Improvements in cardiorespiratory fitness are associated with a reduced risk of mortality12. Exercise tests are suitable for evaluating aerobic performance and treatment responses in cardiovascular diseases. Depending on the availability, tests are performed on a bicycle ergometer or a treadmill. A gradual increase in the workload per minute is usually used, and abrupt increases are avoided; this leads to a linear physiological response. The most important variables in the exercise tests include the total exercise time, metabolic equivalents (METs) achieved, heart rate, and changes on an electrocardiogram (ECG) line between the QRS complex (Q, R, and S waves) and T-wave (ST segment). Clinical stress tests have low costs and are easily accessible13. For these reasons, stress tests, such as the 6 min walking test, have been widely used in clinics and should also be used in the preclinical evaluation of new therapies.
To our knowledge, there are no well-described large animal models for evaluating the functional efficacy of gene therapy or other novel therapies. Therefore, the clinically relevant exercise test provides an excellent perspective for evaluating the efficiency of these new therapies in the preclinical setting.
Protocol
All experiments are approved by the Animal Experiment Board of the University of Eastern Finland. This protocol describes a clinically relevant treadmill exercise test for pigs to evaluate the safety and efficacy of novel therapies for heart diseases. Female domestic pigs weighing 25-80 kg were used for the present study. The animals were obtained from a commercial source (see Table of Materials).
1. Setting up the running track
- Set up the running track so that animals can only move one way. Use gates and hatches to prevent the animals from moving back. The floor plan of the running track is shown in Figure 1, and an example of a running track is in Figure 2.
- Ensure the treadmill (see Table of Materials) has enough space to allow incline changes.
- Ensure the treadmill has an adjustable width to prevent the animal from turning during the run.
- Use transparent plastic to make the front wall of the treadmill. This prevents the animal from running away from the treadmill, but still allows the animal to see through the wall.
NOTE: It is essential that the animals can see through the front wall, as our experience suggests that pigs are more motivated to run if they see their fellow pigs on the other side of the wall. - Place an ECG monitor and defibrillator (see Table of Materials) next to the treadmill.
NOTE: Fatal arrhythmias may occur during the stress test, especially if the pig has myocardial ischemia14,15,16. - Ensure the running track includes a water point where the animals can drink and cool off after the run.
2. Acclimation period of the pigs before the test
- House the animals for 2 weeks before starting the experiments.
- During the 1st week of acclimation, ensure the animals get accustomed to their handlers and new housing environment, excluding the running track.
- During the 2nd week of the acclimation period, ensure the animals get accustomed to the running track.
- Start accustoming so that the animals get familiar with the running track. First, keep all the gates open, so the animals can walk freely on the track and explore the environment.
- When the animals are more familiar with the track, turn on the treadmill and let the animal run for short periods at a time, such as 7 min. The length of the running times must be extended daily.
NOTE: Remember to reward the animals during the acclimation period. For example, the pigs were rewarded with unsalted popcorn in the present study.
3. The exercise test
NOTE: Pigs should be fasted at least 2 h before the exercise test or given only a tiny portion of food before the run.
- Turn on the treadmill and set the incline to 5%-10%.
- As soon as the animal is on the treadmill, start the treadmill with a starting speed of 2 km/h.
- Increase the speed by 0.5 km/h every 60 s until 5 km/h is reached. The total running time is 15 min.
- In case the animal cannot run the entire time at the maximum speed, perform the steps below.
- If the pig is not running as fast as a selected speed, gently push it from the back, as this may give the animal the feeling that it needs to run faster without slowing down.
- Try gently pushing the animal a maximum of three times; after that, slow down the speed by 0.5 km/h at a time until the pig can handle the speed. Do not slow down to below 2 km/h.
- If the animal refuses to run even at a slow speed, turn off the treadmill and stop the test.
4. ECG monitoring during the exercise test
- Place ECG electrodes (see Table of Materials) in anatomical locations which have minimal movement during the run, such as scapulas or the chest.
NOTE: Use ECG electrodes designed for exercise tests to achieve better adhesion to the skin. Remember to shave hair from the area where the ECG electrodes will be placed. - Record the heart rate changes during the run.
NOTE: Our experience suggests that ST segment analyses are often complicated due to movement and other artifacts. Rhythm monitoring can also be done using an implantable loop recorder or pacemaker.
5. Data collection
- Record the run distance, total time, and speed every time the speed is changed.
NOTE: Modern treadmills may collect a lot of other data, so it is essential to familiarize yourself with the treadmill manual to utilize the equipment's full potential. - Note possible changes in animal behavior, such as limping.
NOTE: If needed, contact a veterinarian and make sure that the animal receives necessary analgesia. Remove the animal from future exercises until it fully recovers.
6. Post-procedural care
- Ensure the animal has access to the water point.
- Reward the animal, for example, with treats or toys.
- Monitor the animal for 30 min after the run for possible adverse effects.
Representative Results
One must have experience working with large animals to succeed with this protocol. Researchers need to be able to evaluate whether an animal stops running due to fatigue or lack of motivation. Recording the speed and distance may help to evaluate this, as usually, animals lacking motivation stop running totally, whereas fatigued animals keep running after slowing down the speed (Figure 3). If necessary, the protocol can be repeated the next day if the results seem unreliable.
A representative timeline for adeno-associated virus (AAV)-treated animals is shown in Figure 4. The timeline may vary depending on the study setting, especially regarding the sacrification timepoint. Note the acclimation period when planning the experiments.
The results can be compared to other organ structure and function measurements, such as cardiac echo, to see how exercise tolerance relates to these other measurements. For example, the change in running distance correlates with the change in ejection fraction. With a low ejection fraction, an animal cannot run at full speed throughout the exercise test (Figure 5). Analyzed variables may differ depending on the study settings. This protocol enables the comparison of total running distance, speed variation, METs, heart rate variation, and arrhythmias.
The ECG is recorded during the exercise test (Figure 6). ST segment analysis is difficult due to artifacts. Changes in heart rate intervals can be measured from the ECG throughout the exercise test.
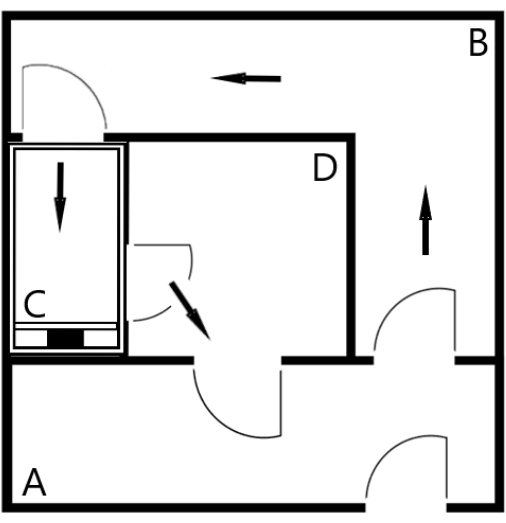
Figure 1: Floor plan of the running track. The place for animals not running is marked with (A). One animal at a time is guided to the treadmill [zone (C)] through a corridor (B). The gate between zones (A) and (B) is closed to ensure that only one animal at a time goes to the running track and the other animals stay in zone (A). It is essential that other animals stay in zone (A), as the animal on the treadmill can see the other animals in zone (A), which motivates them to run. The gate between the treadmill and zone (B) is closed to ensure that the animal cannot back off from the treadmill. The treadmill is operated from zone (D), and the animal is returned to zone (A) through zone (D) after the run. In the present case, a water point where animals can drink and cool off after the run is installed in zone (A). Symbols: black arrows indicate the direction of rotation, and quarter circles indicate gates. Please click here to view a larger version of this figure.

Figure 2: Representative images of the running track. (A) The importance of only one available route for the animals. (B) The treadmill, which should have an adjustable width to prevent animals from turning during the run. (C) An enclosed space meant for another animal in addition to the running animal. Animals are more motivated to run when they see a member of their species. (D) An example of a water point for the animals where the animals can cool off and drink after the stress test. Please click here to view a larger version of this figure.
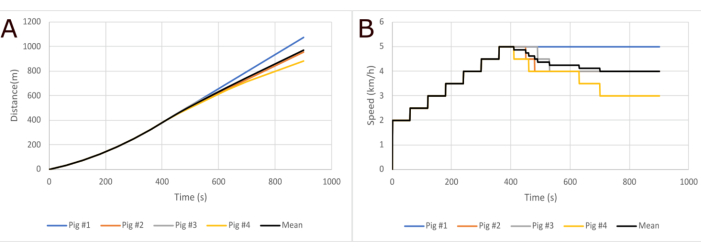
Figure 3: Running distance and speed. (A) Representative data from the total running distances of four healthy pigs. The mean total running distance for the test animals was 970 m, and the standard deviation of the total distances was 80 m. (B) Data on speed variation between pigs. The speed is slowed down by 0.5 km/h intervals until the pig can handle the speed. Please click here to view a larger version of this figure.
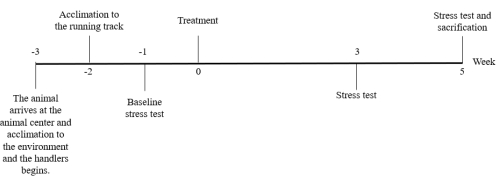
Figure 4: Representative timeline. Note that time points may vary between the studies. However, it is notable that animals should arrive at the laboratory animal center 3 weeks before starting the experiment due to the acclimation period. Please click here to view a larger version of this figure.
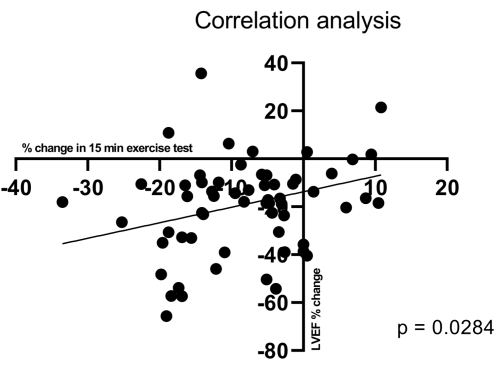
Figure 5: Correlation of the running distance with the change in ejection fraction. Correlation of the change in running distance with the change in ejection fraction. The ejection fraction at rest was measured by the Biplane Simpon method. The change in ejection fraction from the baseline correlates with the change in the running distance of pigs with pacemaker-induced heart failure, with r = 0.2831, p = 0.0284, and R2 = 0.0801. Despite the low r and R2, the change in left ventricular ejection fraction percentage (LVEF%) tends to affect running distances. It is important to note that several factors influence the measured variables, affecting the results. Please click here to view a larger version of this figure.
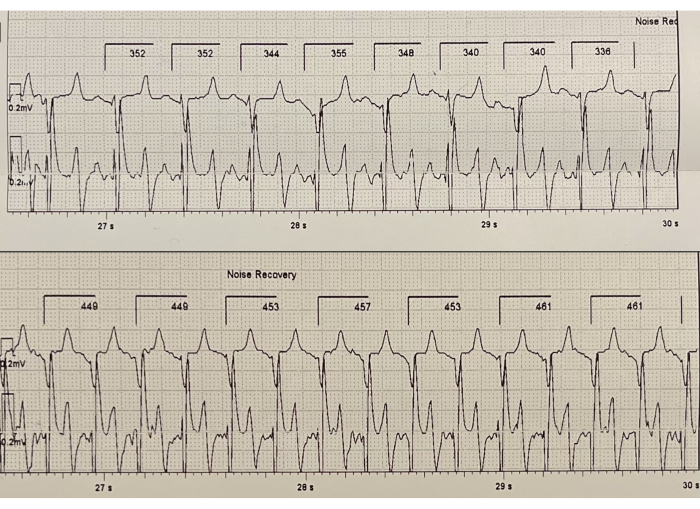
Figure 6: Representative ECG tapes of a healthy pig. The top ECG panel shows an ECG 3 min after the beginning of the exercise test. The bottom ECG strip shows an ECG after running for 10 min. The ECG can be used to evaluate the differences in the heart rate of the test animals. The heart rate of the top ECG panel is 176 beats per minute, and on the bottom ECG strip is 250 beats per minute. Please click here to view a larger version of this figure.
Discussion
This large animal exercise test mimics the test used in clinics, reducing the gap in endpoints between the preclinical studies and clinical trials. It can be applied to evaluate the efficacy of new treatments for severe cardiovascular diseases, such as arteriosclerosis obliterans, heart failure, and ischemic heart diseases. The time points applied in this protocol may vary depending on the tested treatment. This protocol has been standardized based on a long experience of working with large animals and can be used to evaluate the safety and efficacy of cardiovascular gene therapy and other novel therapeutic approaches.
The pig heart and cardiovascular system are similar to human physiology, anatomy, and function. Therefore, pigs have often been used to model cardiovascular disease mechanisms and therapeutic procedures17. The follow-up time in our pig studies has been up to 12 months18; however, handling the animals becomes increasingly challenging as they grow during the long follow-up periods.
This method consists of various critical steps, which are essential for the success of the test and impossible to correct afterward. First, pigs have individual differences in their running motivation. Getting animals motivated to run and maintaining sufficient motivation throughout the test is essential. This assures that all time points are comparable. Maintaining pigs' running motivation requires specific knowledge of their individual behavioral characteristics. Animals must be acclimated to the treadmill and testing environment before the exercise test. Pigs are taught to go on the treadmill, and their successful performances are rewarded. Another way to increase their running motivation is to keep other test animals in the field of vision of the runner.
Avoiding typical anatomical flaws, such as different leg problems, is important. The most common anatomical flaws are hoof diseases, congenital limb deformities, and problems caused by accidents, such as lacerations, scapular injuries, fractures, and wounds. These are mostly due to habitats, accidents, hereditary factors, and aberrations in feeding19. A weakness in the legs leads to uncoordinated walking, making attending exercise tests impossible. Also, if leg weakness appears during the study, the animal must be excluded from the test. Leg problems can be avoided by choosing pigs with intact leg structures. During the research, leg injuries can be prevented by having good conditions in the piggery. Hard, corrosive surfaces must be avoided, and general hygiene must be maintained. Pigs must be fed moderately so that they do not gain weight too rapidly, since this strains their legs. In addition, pigs must be placed in their pens carefully to avoid accidents, and they should have enough stimuli, such as toys, so that their chewing is not directed at other pigs.
During the exercise test, the ECG was recorded with a 3-lead ECG or implantable loop recorder. It is not as accurate as a 12-lead ECG, but can still assess multiple variables, such as arrhythmias and heart rate. Several types of errors and disturbances can falsify ECGs. For example, incorrectly connected electrodes, poor contact between the skin and electrodes, and skeletal muscle contractions can cause errors. The electrodes must remain firmly in place throughout the test. This is challenging as the skin heats up and sweats during running. Contact between the skin and electrodes can be improved by shaving hair, disinfecting, and removing dead skin cells. Also, the movement of muscles causes artifacts that affect ECGs13. This can challenge the interpretation of ST segments. In addition, ECG leads can interfere with running. However, these issues can be reduced by tapping the ECG leads firmly on the skin. ECGs can also be registered with an implantable loop recorder or pacemaker. Using an implantable loop recorder solves many problems that 3-lead ECG usage has. However, installing the implantable loop recorder is an invasive operation with risks, such as infections.
Researchers must observe animal behavior throughout the test to ensure the procedure's overall safety. For example, exhaustion, severe fatigue, nausea, loss of consciousness, severe dyspnea, or cyanotic skin are reasons for terminating the exercise test. Also, researchers must observe changes in the ECG, such as arrhythmias. However, with well-trained personnel and enough experience working with large animals, the current exercise protocol can be routinely utilized in preclinical studies to produce clinically relevant data that should make the clinical transition of new therapeutic approaches more successful regarding clinical benefits to the patients.
Poole et al.20 have published guidelines for animal exercise and training protocols for cardiovascular studies. In these protocols, pigs exercise on a treadmill for approximately 30 min after warming up. During this 30 min, the target heart rate zone for the test animals is 65%-75% of the maximum heart rate. The heart rate is modified either by changing the speed or the incline of the treadmill. The protocol of Poole et al. and the 15 min exercise test presented in this manuscript have multiple similarities, such as the acclimation period, treadmill requirements, the weight of selected test animals, and positive reinforcement by rewarding the animal after the exercise. In both protocols, the test animals can outgrow the treadmill's capacity, limiting the follow-up time.
The main difference between the protocol described by Poole et al. and the exercise test presented in this manuscript is the purpose of the testing. The protocol described by Poole et al. is designed to elicit classical training adaptations noted in humans. Therefore, it focuses on moderate-intensity exercise, whereas the 15 min exercise test method aims to make a near-maximal effort to better evaluate cardiorespiratory fitness. This is achieved when the subjective level of exertion is approximately 90% of the maximum heart rate13. A 15 min exercise test mimics the test used in clinics by gradually increasing the level of exertion until it is near maximal. Due to the difference in the aims of the protocols, the exercise frequency of the test animals differs. Poole et al. describe that pigs can run up to four times a week to achieve better cardiovascular adaptations caused by exercising. The 15 min exercise test evaluates the functional efficacy of gene therapy and other novel therapies, which is why the required frequency is significantly lower and depends on the treatment's requirements. An example of these requirements has been described in Figure 4.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The author would like to thank Minna Törrönen, Riikka Venäläinen, Heikki Karhunen, and Inkeri Niemi from the National Laboratory Animal Center for their assistance in animal work. This study is supported by Finnish Academy, ERC, and CardioReGenix EU Horizon grant.
Materials
| Defibrillator | Zoll M series | TO9K116790 | All portable defribrillators will work |
| Defibrillator pads | Philips | M3713A | All pads work, as long as the pads are compatible with the defibrillator |
| ECG electrodes | Several providers | Prefer ECG electrodes designed for exercise tests | |
| Loop recorder | Abbott Oy | DM3500 | Optional for rhythm monitoring |
| Patient monitor | Schiller Argus LCM Plus | 7,80,05,935 | All portable ecg monitors will work |
| Pigs | Emolandia Oy | ||
| Treadmill | NordicTrack | All treadmills with adjustable incline and speed are suitable for the exercise test. The treadmill should be as long and wide as possible. | |
| Ultrasound system | Philips EPIQ 7 ultrasound | ||
| Various building materials | Several providers | For building fences, ramps and gates according to the Figure 1 and Figure 2 | |
| Various treats for the animals |
Riferimenti
- Virani, S., et al. Heart disease and stroke statistics-2020 update: A report from the American Heart Association. Circulation. 141 (9), e139 (2020).
- Townsend, N., et al. Epidemiology of cardiovascular disease in Europe. Nature Reviews Cardiology. 19 (2), 133-143 (2022).
- Knuuti, J., et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). European Heart Journal. 41 (3), 407-477 (2020).
- Ylä-Herttuala, S., Baker, A. H. Cardiovascular gene therapy: past, present, and future. Molecular Therapy. 25 (5), 1096-1106 (2017).
- Hedman, M., et al. Eight-year safety follow-up of coronary artery disease patients after local intracoronary VEGF gene transfer. Gene Therapy. 16 (5), 629-634 (2009).
- Rosengart, T. K., et al. Long-term follow-up of a phase 1 trial of angiogenic gene therapy using direct intramyocardial administration of an adenoviral vector expression the VEGF121 cDNA for the treatment of diffuse coronary artery disease. Human Gene Therapy. 24 (2), 203-208 (2013).
- Muona, K., Mäkinen, K., Hedman, M., Manninen, H., Ylä-Herttuala, S. 10-year safety follow-up in patients with local VEGF gene transfer to ischemic lower limb. Gene Therapy. 19 (4), 392-395 (2012).
- Leikas, A. J., et al. Long-term safety and efficacy of intramyocardial adenovirus-mediated VEGF-DΔNΔC gene therapy eight-year follow-up of phase I KAT301 study. Gene Therapy. 29 (5), 289-293 (2022).
- Telukuntla, K. S., Suncion, V. Y., Schulman, U. H., Hare, J. M. The advancing field of cell-based therapy: insights and lessons from clinical trials. Journal of the American Heart Association. 2 (5), e000338 (2013).
- Ylä-Herttuala, S., Bridges, C., Katz, M. G., Korpisalo, P. Angiogenic gene therapy in cardiovascular diseases: dream or vision. European Heart Journal. 38 (18), 1365-1371 (2017).
- Lähteenvuo, J., Ylä-Herttuala, S. Advances and challenges in cardiovascular gene therapy. Human Gene Therapy. 28 (11), 1024-1032 (2017).
- Ross, R., et al. Importance of assessing cardiorespiratory fitness in clinical practice: a case for fitness as a clinical vital sign: a scientific statement from the American Heart Association. Circulation. 134 (24), e653-e699 (2016).
- Sietsema, K. E., Stringer, W. W., Sue, D. Y., Ward, S. . Wasserman & Whipp’s Principles of Exercise Testing and Interpretation. 6th. , (2021).
- Darmadi, M. A., et al. Exercise-induced sustained ventricular tachycardia without structural heart disease: a case report. The American Journal of Case Reports. 21, e928242 (2020).
- Casella, G., Pavesi, P. C., Sangiorgio, P., Rubboli, A., Bracchetti, D. Exercise-induced ventricular arrhythmias in patients with healed myocardial infarction. International Journal of Cardiology. 40 (3), 229-235 (1993).
- Gimeno, J. R., et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. European Heart Journal. 30 (21), 2599-2605 (2009).
- Lelovas, P. P., Kostomitsopoulos, N. G., Xanthos, T. T. A comparative anatomic and physiologic overview of the porcine heart. Journal of the American Association for Laboratory Animal Science. 53 (5), 432-438 (2014).
- Korpela, H., et al. AAV2-VEGF-B gene therapy failed to induce angiogenesis in ischemic porcine myocardium due to inflammatory responses. Gene Therapy. 29 (10-11), 643-652 (2022).
- Swindle, M. M. . Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. 2nd edition. , (2007).
- Poole, D. C., et al. Guidelines for animal exercise and training protocols for cardiovascular studies. American Journal of Physiology. Heart and Circulatory Physiology. 318 (5), H1100-H1138 (2020).

