A Validatable Droplet Digital Polymerase Chain Reaction Assay for the Detection of Adeno-Associated Viral Vectors in Bioshedding Studies of Tears
Summary
Here, we present a protocol for the development and validation of good laboratory practices in the compliant detection of adeno-associated viral vectors in human tears by droplet digital polymerase chain reaction in support of clinical development of gene therapy vectors.
Abstract
The use of viral vectors to treat genetic diseases has increased substantially in recent years, with over 2,000 studies registered to date. Adeno-associated viral (AAV) vectors have found particular success in the treatment of eye related diseases, as exemplified by the approval of voretigene neparvovec-rzyl. To bring new therapies to market, regulatory agencies typically request qualified or validated bioshedding studies to evaluate release of the vector into the environment. However, no official guidelines for the development of molecular based assays to support such shedding studies have been released by the United States Food and Drug Administration, leaving developers to determine best practices for themselves. The purpose of this protocol is to present a validatable protocol for the detection of AAV vectors in human tears by droplet digital polymerase chain reaction (ddPCR) in support of clinical bioshedding studies. This manuscript discusses current industry approaches to molecular assay validation and demonstrates that the method exceeds the target assay acceptance criteria currently proposed in white papers. Finally, steps critical in the performance of any ddPCR assay, regardless of application, are discussed.
Introduction
Gene therapy definitions vary, but generally induce an intentional and often expected permanent alternation of a specific DNA sequence of the cellular genome to modify or manipulate the expression of a gene or to alter the biological properties of a living cell for a clinical purpose1,2. Viral vectors are increasingly being used as vehicles for gene therapy due to their efficiency of transduction, with one report suggesting that over 70% of current gene therapy clinical trials utilize viral vectors3. Interest in viral vectors for gene therapy has been gaining steadily. The Quarter 4 2022 Quarterly Data Report on the Gene, Cell, and RNA therapy landscape from the American Society of Gene and Cell Therapy reported that in 2022, the gene, cell, and RNA therapy pipeline from preclinical to pre-registration grew by 7%, bringing the total number of therapies in development to 3,726, of which 2,053 (55%) were gene therapies4. The United States Food and Drug Administration (USA FDA) currently has approved 27 cell and gene therapies for clinical use in humans, five of which specifically utilize viral vectors5.
Adeno-associated viruses (AAVs) have gained specific interest as vehicles for gene therapy. A recent meta-analysis revealed that there have been approximately 136 clinical trials investigating the use of AAVs in the past two decades6. Additionally, three of the five USA FDA approved gene therapies are AAV based. This is due to their highly editable nature, broad host range that can be tuned based on the use of specific naturally occurring or artificially engineered vectors, low pathogenicity and toxicity in humans, and generally low immunogenicity7,8. AAVs have also been successfully used to treat ocular diseases in an approved clinical setting. Voretigene neparvovec-rzyl is an AAV2-based therapy that was approved by the USA FDA in 2017 and by the European Medicines Agency (EMA) in 2018 to treat biallelic RPE65 mutation-associated retinal dystrophy9.
With increasing interest in the development of AAV-based therapies comes the need for regulatory guidance on assays. Accurate detection and quantification of any viral vector is an integral part of the discovery, manufacturing, and preclinical/clinical testing phases of product development. The USA FDA has begun to issue some guidance for gene therapies, including on the chemistry, manufacturing, and control for human gene therapy investigational new drug applications10, long-term follow-up after administration of gene therapy11, replication-competent retrovirus testing12, and recommendations for microbial vectors used in gene therapies13. The EMA has also released a series of guidelines concerning the development of gene therapy products that generally align with the FDA recommendations, though some differences do exist14. It is important to note that while these guidance do not establish legally enforceable responsibilities, except where specific regulations are referenced, they provide clarity on the current thinking from regulatory agencies on the topic and their expectations for assays required for drug filings and regulatory approval.
The FDA specifically recommends that studies should be conducted to assess the distribution, persistence, and clearance of a vector from the site of administration to target ocular and non-ocular tissues, intraocular fluids, and blood15. These take the form of biodistribution and shedding studies. Biodistribution studies evaluate exposure by investigating how a product is spread throughout a patient's body from the site of administration. Shedding specifically evaluates the release of the product from the patient into the environment and raises the possibility of transmission of the vector to untreated individuals16. The FDA makes recommendations for the design of biodistribution and shedding studies with respect to the frequency of sample collection, duration of sample collection, types of samples collected, and storage conditions.
Additionally, the FDA recommends the use of quantitative polymerase chain reaction (qPCR, or real-time PCR) for the quantitative detection of vector genomes due to its ease of performance, high-throughput format, rapid turnaround times, and assay sensitivity. However, there is a relative lack of recommendations for the design and performance assessment of molecular methods compared to those which exist for small and large molecules. Many of the guidelines for such studies are difficult to apply to molecular methods due to the unique and complex design of both the products and the assays themselves, raising questions as to the appropriateness of the available platforms for the recommended assessments and appropriate methods for assay validation. To date, the FDA has not required formal validation of PCR-based assays, though the EMA has imposed this requirement17. In light of this void, different groups and workshops have issued white papers and recommendations that manufacturers and contract research organizations have sought to follow18,19,20,21,22,23,24,25. Most of these recommendations are written specifically with qPCR assays in mind, with suggestions or alterations for emerging platforms, such as droplet digital PCR (ddPCR), included only as deemed relevant. More recent recommendations have focused on considerations for ddPCR assays, but have largely focused on their applications to vector genome quantification in a manufacturing setting rather than in the complex biological matrices encountered in bioshedding studies.
Depending on clinical application and goals, ddPCR may be preferred over qPCR in support of biodistribution and shedding studies due to ddPCR's increased sensitivity and ability to handle matrix interference compared to qPCR. Furthermore, due to the partitioning of samples into approximately 20,000 droplets, accurate quantification of the copy number can be achieved without the use of a standard curve using Poisson statistics, simplifying the method development and validation. The goal of this protocol is to describe a standardized approach for the development and validation of a ddPCR-based method for the detection of AAV vectors in tears collected from the ocular surface in support of clinical bioshedding studies.
Protocol
1. Preparation of a synthetic DNA fragment
- Design and order a synthetic DNA fragment containing the target amplification region for use as a quality control.
- Ensure that the sequence contains the entire amplicon sequence from the forward primer to the reverse primer of the target gene of interest, with an extension of four to six base pairs of sequence at the 5' ends of each primer binding sequence.
- Avoid homopolymers of adenine and thymine greater than 12 base pairs or guanine and cytosine base pairs greater than eight base pairs, as long homopolymers may interfere with synthesis of the gene fragment.
NOTE: If amplicons contain such sequences, base substitutions may be made as long as the annealing sites for the primers and probes are maintained. - Alternatively, prepare a linearized plasmid containing the amplicon using typical cloning strategies.
- Centrifuge the tube containing the synthetic DNA fragment in a microcentrifuge for ~10 s to ensure material is collected at the bottom of the tube.
- Resuspend the synthetic DNA fragment using tris-EDTA (TE) buffer to a concentration of 1.0 × 1010 copies/µL, or as appropriate based on the target assay range.
- Vortex briefly, then incubate at 50 °C for 20 ± 5 min. Cool on ice.
- Prepare multiple, ideally single-use aliquots and store at -70 to -90 °C until use.
NOTE: Synthetic DNA fragments prepared in this manner are typically stable for at least 24 months from the date of resuspension. - If desired, determine the exact concentration of the prepared synthetic DNA stock prior to use as a quality control, or estimate the nominal concentration based on the resuspension utilized.
2. Preparation of primers and probe
- Design and order primers and a hydrolysis probe to target the desired amplification region using typical design strategies26,27.
- Utilize a 5' fluorescent reporter dye (e.g., FAM) and a 3' quencher (e.g., Iowa Black dark quencher) compatible with the ddPCR system.
NOTE: Numerous PCR assay design software packages exist, and any may be utilized. For example, Primer-BLAST by the National Center for Biotechnology Information28 is widely used due to the robust options for assay design and the ease at which specificity can be bioinformatically assessed to identify possible off target effects. It should be noted that the preparation of primers and probes may vary from the steps listed here depending on the format in which they are supplied.
- Utilize a 5' fluorescent reporter dye (e.g., FAM) and a 3' quencher (e.g., Iowa Black dark quencher) compatible with the ddPCR system.
- Centrifuge the tubes containing the forward primer, reverse primer, and probe in a microcentrifuge for ~10 s to pellet material to bottom of the tube.
- Resuspend the primers to 20 µM using TE buffer. Vortex briefly.
- Resuspend the probe to 10 µM using TE buffer. Vortex briefly.
- Prepare multiple, ideally single-use aliquots and store at a minimum of -20 °C until use.
NOTE: Primers and probes prepared in this manner are typically stable for at least 24 months from the date of resuspension.
3. Preparation of sample dilution buffer
- Thaw PCR buffer and sheared salmon sperm DNA at room temperature. Vortex thoroughly to mix.
- Prepare a sample dilution buffer, as per Table 1.
- Vortex thoroughly. Store at 2-8 °C for up to 1 month following preparation.
Table 1: Preparation of sample dilution buffer. Please click here to download this Table.
4. Preparation of master mix
- Thaw the ddPCR master mix for probes, forward primer, reverse primer, and probe at room temperature and allow to warm for at least 10 min post-thaw prior to use. Store at room temperature until use.
NOTE: These reagents must be fully brought to room temperature to ensure efficient droplet formation. Do not hold reagents on ice during preparation.- Vortex thoroughly and briefly centrifuge in a mini centrifuge prior to use.
NOTE: Restriction enzymes are typically supplied in glycerol and should be removed from storage immediately prior to use. Mix gently. Do not vortex.
- Vortex thoroughly and briefly centrifuge in a mini centrifuge prior to use.
- Prepare a PCR master mix for each amplification target. See Table 2 for a suggested PCR master mix composition and modify the concentrations of primers and probes as required.
- Thoroughly vortex and briefly centrifuge prior to the addition of restriction enzyme. Add the restriction enzyme and invert to mix.
NOTE: In this step, 22 µL of PCR reaction is required to obtain a final volume of 40 µL of PCR reaction after droplet formation (consisting of 15 µL of PCR master mix, 5.0 µL of template, and 20 µL of droplet generation oil).
- Thoroughly vortex and briefly centrifuge prior to the addition of restriction enzyme. Add the restriction enzyme and invert to mix.
- Add 16.5 µL of master mix to each well according to the plate map. See Figure 1 for an example plate map for a validation accuracy and precision run.
- Ensure that a plate contains three independent preparations of the quality control (QC) series, three independently tested endogenous tear aliquots tested, spiked to a high and low level and unspiked, and three independent no template controls (NTCs).
- Vary the layout of these wells across the plate, where Set 1 is loaded in order of decreasing concentration, Set 2 is loaded in order of increasing concentration, and Set 3 is loaded in a random order to evaluate if there are any plate location-specific effects.
- Array the samples to fill as much of a column as possible and fill unused wells within a column with control buffer. Include multiple endogenous control lots (e.g., more pools of tears or tears collected from individuals) in the remaining wells, if desired.
- Seal the plate with clear adhesive film. Hold the plate at room temperature during template preparation. Alternatively, hold the plate for up to 4 h at 2-8 °C, but bring it back to room temperature for at least 10 min prior to template addition.
Table 2: Example PCR master mix preparation. Please click here to download this Table.
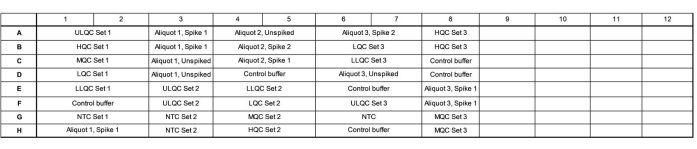
Figure 1: Example plate map for validation accuracy and precision run. Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to view a larger version of this figure.
5. Preparing QCs
- Thaw synthetic DNA fragments or linearized plasmids at room temperature and allow to warm for at least 10 min post-thaw prior to use. Bring the templates to room temperature to ensure efficient droplet formation.
- Store at room temperature until use. Vortex thoroughly and briefly centrifuge in a mini centrifuge prior to use.
- Prepare QC dilutions utilizing the sample dilution buffer as the diluent. An example of the recommended concentrations to prepare for a validation accuracy and precision run is presented in Table 3.
NOTE: Following the successful completion of accuracy and precision runs, only the high quality control (HQC), medium quality control (MQC), and low quality control (LQC) need be run on each plate. For accuracy and precision runs, at least three independent dilutions of the QCs are included for the assessment of intra-assay accuracy and precision. Following accuracy and precision runs, only one dilution series need be included. - Following preparation, store the dilutions at room temperature until added to the plate.
- Store the dilutions on ice or at 2-8 °C if needed. Prior to subsequent use, allow the dilutions to warm to room temperature for at least 10 min prior to use. Discard the QCs at the end of the day.
Table 3: Example quality control (QC) preparation using synthetic double-stranded DNA fragments. Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
6. Preparation of samples
- Thaw tear samples collected from a clinical trial at room temperature until thawed and allow to warm for at least 10 min post-thaw prior to use.
- Store at room temperature until use. Vortex thoroughly and briefly centrifuge in a micro centrifuge prior to use.
- Dilute tear samples 1:10 (or greater) using sample dilution buffer as the diluent into 0.2 mL PCR tubes or 8-well PCR strips. Seal the tubes.
NOTE: Depending on the expected concentration of target in tears, it may be necessary to further dilute the samples or to test multiple dilutions of each sample. - Heat the samples in a thermal cycler at 95 °C for 10 min, followed by holding at 4 °C for at least 5 min to cool. Use a ramp rate of 3 °C/s.
NOTE: Samples may stay in the thermal cycler at 4 °C until use on the same day or may be frozen at -70 to -90 °C for longer storage. This step serves to denature the vector capsid, releasing the genome. As QC synthetic DNA fragments or linearized plasmids are double stranded, they should not undergo this heating step. - Return the samples following cooling to room temperature (or if frozen, thaw at room temperature) and allow to warm for at least 10 min.
NOTE: The samples must be fully brought to room temperature to ensure efficient droplet formation.
7. Template addition
- Retrieve the ddPCR plate containing the master mix. Vortex each sample or QC dilution tube thoroughly and briefly centrifuge to recollect the material.
- Remove adhesive film and add 5.5 µL of QCs or samples to appropriate wells of the 96-well plate, as per the plate map.
NOTE: Refer to step 4.2.1 for explanation of the required volumes - Add 5.5 µL of sample dilution buffer to the NTC wells.
- Droplet generation requires that all wells of a column have a reaction or buffer control. If any wells of a column do not contain sample reactions, dilute 2x ddPCR buffer control 1:2 using nuclease free water. Add 22 µL of 1x ddPCR buffer control to any empty wells of a column.
NOTE: If an entire column is not used, it is not necessary to add buffer control to these wells. - Add a pierceable foil seal to the plate. Place the plate in the plate sealer and seal for 5 s at 180 °C.
- Alternatively, seal the plate in accordance with the ddPCR system manufacturer's recommendations.
- Vortex the plate at maximum speed for at least 30 s (using the continuous vortexing setting; do not use touch vortexing) and centrifuge briefly in a plate spinner.
NOTE: Thorough and complete mixing of the plate at this step is critical for proper partitioning of the PCR reaction into droplets. Ensure that there are no bubbles visible in the wells. If necessary, the plate can be held at 2-8 °C prior to droplet generation for a maximum of 4 h. If held, allow the plate to come to room temperature for a minimum of 10 min prior to droplet generation.
8. Automated droplet generation, thermal cycling, and droplet reading
- Generate droplets in the automated droplet generator as follows.
- On the touch screen, select the columns on the plate map containing samples. The deck of the instrument will light up to indicate which consumables (DG32 cartridges, tips, waste container, droplet generation oil) are required. Yellow lights indicate that it is necessary to add a consumable, while green lights indicate sufficient consumables are available.
- Load the droplet generator from back to front.
- For hydrolysis probes, ensure that the droplet generation oil for probes is installed and sufficient oil for the number of wells remains. If alternative PCR chemistries are utilized, ensure that a compatible droplet generation oil is installed.
- Place a cold block in the droplet plate holder. Ensure that the block is fully blue colored and no pink is visible. Place a new 96-well ddPCR plate in the cold block.
- Place the prepared PCR plate in the sample plate holder. Close the machine lid. Press start for droplet generation.
- Following droplet formation, a total of 40 µL per reaction is transferred automatically to the new PCR plate.
- Within 30 min following the completion of droplet generation, remove the plate containing the droplets from the cold block. Work gently as the droplets are most fragile at this stage.
- Add a pierceable foil seal to the plate. Place the plate in the plate sealer and seal for 5 s at 180 °C.
- Alternatively, seal the plate in accordance with the ddPCR system manufacturer's recommendations.
- Place the plate in a compatible thermal cycler. Enter the cycling conditions (see Table 4).
- Following the end of thermal cycling, hold the plate in the thermal cycler, transferred to 2-8 °C, or read it immediately.
NOTE: Holding the plate for 12 h at 4-12 °C may improve droplet counts, but this is not required. Sufficient droplets should be obtained without the hold. - Load the plate into the droplet reader, ensuring sufficient reader oil remains and the waste container has sufficient space. Read the droplets. Perform droplet reading within 24 h of thermal cycling initiation.
Table 4: Typical thermal cycling conditions. Please click here to download this Table.
9. Data analysis
NOTE: A minimum of 10,000 droplets per well is necessary for the proper calculation of concentration using Poisson statistics. Do not attempt analysis on any wells with fewer than 10,000 droplets.
- A threshold is required to define the droplets as positive or negative. The ddPCR analysis software automatically applies a threshold that may vary across wells. However, manually set a threshold for all wells of the plate at slightly above the fluorescent intensity of the NTC wells for more consistent, accurate, and precise results.
NOTE: Proper placement of the threshold may require optimization depending on the separation of the positive and negative droplets and how much droplet rain exists (see Figure 2). In this example, the droplet amplitude graph shows example wells at each QC level and the NTC. The purple line indicates a threshold of 1,000, set slightly above the negative droplet population. - Poisson statistical modeling requires at least three positive droplets to calculate the concentration with 95% confidence. Consider all wells containing zero, one, or two positive droplets to be negative and set to a concentration of zero27.
- Back-calculate the copy number in each tear sample.
- The concentration, in copies/µL, is provided in the data report. Use this value to determine the concentration in copies/µL of the original sample (i.e., in the tear sample).
- To calculate the ddPCR reaction dilution, divide the initial PCR reaction volume prior to droplet formation by the volume of template added. When the volumes presented in this method are utilized, this yields a value of 4.
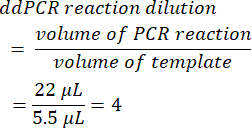
- Determine the serial dilution factor from the original sample (step 6.2).
- To determine the copies/µL in the sample, multiply the copies/µL by the ddPCR reaction dilution, then by the serial dilution factor. For example, the concentration in copies/µL generated in the data report was 966; 5.5 µL of template was added per 22 µL of reaction. A 1:50,000 serial dilution of the sample was utilized.
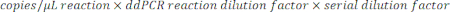
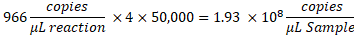
- If multiple dilutions of the same sample were tested, analyze all valid, in-range dilutions and calculate the mean.
- For each QC, calculate the expected copies/µL PCR reaction by dividing the concentration of the given QC dilution (in copies/µL) by the ddPCR reaction volume (20 µL). This allows for direct comparison of this nominal value to the copies/µL value provided in the data report without further calculations.
NOTE: This approach was also used for analysis of the spiked tear samples utilized in the representative results. - Determine the mean value, standard deviation, coefficient of variation (%CV), and percent relative error to the nominal concentration (%RE) of the sample or QC value using the replicate wells (include multiple dilutions if applicable).
- For the assessment of inter-well precision, determine this for each of the well duplicates, if included.
- For the assessment of intra-assay accuracy and precision, determine this for each dilution series or aliquot utilized within a batch.
- For the assessment of inter-assay accuracy and precision, determine this using the intra-assay means of each of the included batches.
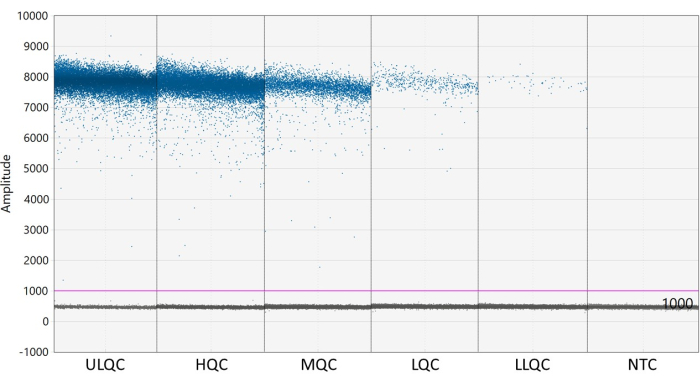
Figure 2: Example of setting threshold. Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to view a larger version of this figure.
10. Assay acceptance criteria
- Utilize the following specifications to the calculated data for each batch to determine if the batch is acceptable. If these conditions are not met, invalidate and repeat the batch.
NOTE: These criteria were determined as a consensus from published white papers on PCR-based assay validation18,19,20,21,22,23,24,25. It may be necessary to modify the target criteria as appropriate for clinical application. - No template control (NTC)
- Ensure that each NTC well has at least 10,000 droplets.
- Ensure that each NTC well has less than 3 positive droplets.
- QCs and assay range
- Ensure that each QC well has at least 10,000 droplets.
- Make sure that the precision of replicate wells of a QC concentration is ≤25.0% CV, except at the upper and lower limits of quantification, where ≤30.0% is acceptable. Assess this independently for each QC set and concentration level.
- Ensure that the relative error of the back-calculated concentration at each mean QC level is within ±25.0% RE of the nominal concentration (copies/PCR reaction), except at the upper and lower limits of quantification, where ±30.0% RE is acceptable. Assess this independently for each QC set and concentration level.
- Make sure that at least 2/3 of the QC samples (e.g., four out of six results) and 50% of the QC samples at each level (low, medium, high) meet these guidelines.
- Samples
- Ensure that the sample wells to be analyzed have at least 10,000 droplets.
- Make sure that the precision of replicate wells of a sample dilution to be analyzed is ≤25.0% CV.
- Ensure that at least one included dilution of the given sample is within the defined quantification range of the assay, as defined above based on the upper and lower limit QCs.
- If all dilutions included yield results greater than the defined upper limit of quantification, and if a sufficient sample volume remains, repeat the assay using a higher dilution of the sample.
- If all dilutions included yield a result lower than the lower limit of quantification, and if a sufficient sample volume remains, repeat the assay using a lower dilution of the sample.
NOTE: Samples containing more than three positive droplets, but that have a concentration below the lower limit of quantification, can be described as detectable, but not quantifiable.
Representative Results
For demonstrative purposes, an assay designed to detect a commercially available, enhanced green fluorescent protein (eGFP)-expressing AAV2 vector, with a synthetic double-stranded DNA fragment containing eGFP as a quality control, was developed. Currently, there is ongoing debate as to whether the vector itself or a synthetic DNA fragment or linearized plasmid is most appropriate for use as the QC. Generally, a synthetic DNA fragment or linearized plasmid may be used if equivalency to the vector is demonstrated in method development (data not shown). Primers and probes were designed and optimized to detect the eGFP transgene. Refer to Supplementary Table S1 for the sequences used in this work. The concentration of the QC fragment stock was empirically determined using ddPCR. All assays were performed using the concentrations and PCR conditions given as examples in the protocol section.
For qPCR assays, it is recommended to evaluate the linearity, sensitivity, dynamic range, accuracy, and precision of the standard curve. Since ddPCR does not rely on a standard curve for target quantification, these recommendations must be modified. Instead, QCs consisting of synthetic double-stranded DNA fragments diluted to various concentrations to span the expected quantifiable range of a ddPCR reaction based on the Poisson statistical modeling were utilized29,30,31,32 to define the dynamic range and sensitivity and to evaluate accuracy and precision. The choice of QC concentrations was based primarily on the expected ratio of positive to total droplets within a well at a given concentration. Mathematically, ddPCR is theoretically most accurate when approximately 80% of the partitions positively amplify. As the positive to total droplet ratio increases above 0.8, the accuracy decreases due to saturation of the partitions, with quantification not being possible once 100% of the droplets are positive. On the low end, theoretically, as little as one positive droplet may be detected and quantified, though the accuracy is poorer and the assay is subject to low-level false positives. Typically, at least three droplets must be positive for a result to be calculated with 95% confidence, which is the threshold to calculate a concentration we used here.
A series of five different QC concentrations was prepared, with the target copy number/µL PCR reaction volumes calculated expected to yield positive to total droplet ratios spanning the quantifiable range of ddPCR, as shown in Table 5. These were used to evaluate the accuracy and precision of the assay. In the assessment here, the upper and lower limits of quantification were not pushed to the theoretical maximum possible in ddPCR. Accurate quantification may be possible at higher and lower levels than demonstrated here. The range should be developed in alignment with the downstream applications of this method.
A total of three independently prepared dilution series of these QCs were prepared in sample dilution buffer for each batch to evaluate the intra-assay accuracy and precision. Duplicate wells of each QC dilution were included. To simulate an actual validation protocol, a total of six accuracy and precision batches were performed by multiple analysts over multiple days. The results from these six batches were analyzed to define the intra-assay and inter-assay accuracy and precision of the method and to define the dynamic range of the assay.
Intra-assay performance was assessed for each batch at each QC level. We expected that all QC and NTC wells would have at least 10,000 droplets. This was met in 216 out of 216 wells tested across all six batches, with an average droplet count of 19,748 droplets/well (Table 6). Next, the inter-well %CV of each set of duplicate wells of each QC was expected to be ≤25.0%, except for the upper and lower limit QC, where ≤30.0% was expected. This was met in sets of 90 out of 90 wells tested across all six batches for the QCs, with an average inter-well %CV of 3.9% across all the QC levels (Table 7). All QCs yielded mean positive to total droplet ratios within the expected ranges outlined above (Table 6).
Within each batch, the intra-assay mean and standard deviation were calculated for each of the independently prepared dilution series points, and these were used to calculate an intra-assay mean for each concentration in each assay. This was used to assess the accuracy and precision of the assay (Table 8). Precision refers to the variability in the data from replicates of the same homogenous sample under normal assay conditions and is evaluated by calculating the %CV of the multiple included aliquots. We expected that the three aliquots tested within each batch would yield an intra-assay %CV ≤25.0%, except for the upper and lower limit QC where ≤30.0% was expected. This was met for all five QC levels in each of the 60 batches (30 out of 30 total performances). Generally, greater intra-assay precision than the target criteria could be achieved, with a mean intra-assay %CV of 7.7% across all QC levels. Accuracy refers to the closeness of agreement between the experimentally determined value and the nominal value. This is evaluated by calculating the percent relative error (%RE, or %Bias) between the calculated concentrations of each QC and their theoretically expected nominal concentrations. It was expected that the intra-assay mean of the three aliquots would be ±25.0% RE of the nominal concentration, except for the upper and lower limit QC where ±30.0% was expected. This was met for all five QC levels in each of the 60 batches (30 out of 30 total performances). Generally, greater intra-assay accuracy than our target could be achieved, with a mean absolute intra-assay %RE of 4.2% across all QC levels. In all performances of the NTC (30 total), no positive droplets were detectable.
Inter-assay accuracy and precision were also calculated using the intra-assay mean of each QC level within each batch. The inter-assay precision was expected to be ≤25.0% CV, except for the upper and lower limit QC where ≤30.0% was expected. Likewise, for inter-assay accuracy, ±25.0% RE was expected, except for the upper and lower limit QC where ±30.0% was expected. A significantly greater inter-assay accuracy and precision than these targets were observed (Table 9), with an inter-assay precision ranging from 4.0% to 8.5% and an inter-assay absolute accuracy ranging from 1.0% to 3.2%. Collectively, these results demonstrate that this method can achieve sufficient intra- and inter-assay accuracy and precision well within current industry targets. A dynamic range of this assay of 2,500-2.5 copies per µL of PCR reaction can be defined based on these results, with an overall assay sensitivity of 2.5 copies per µL of PCR reaction. As previously mentioned, it may be possible to validate wider dynamic ranges.
Next, it was necessary to evaluate assay accuracy and precision within the target matrix – in this case, tears. Typically, assays are validated prior to the initiation of clinical studies, meaning that tears collected from vector-treated patients are unlikely to be available for validation purposes. This can be artificially created by spiking the target AAV vector into tears collected from volunteer donors to create matrix-spiked QCs. Pooled human tears were collected by a third party (BioIVT). For proof of principle, an eGFP-expressing AAV2 vector acquired from a commercial source was utilized. The concentration of the AAV2 vector stock was empirically determined using ddPCR, without the use of a DNA isolation step, as described in this protocol. In each run, the AAV2 was independently spiked into the three tear aliquots at a high (expected 1.41 x 103 copies/µL PCR reaction) and low (28.2 copies/µL PCR reaction) level. Unspiked aliquots were included as a control to demonstrate the specificity of the method.
Intra-assay performance was assessed for each batch at each spike level. It was expected that all tear samples would have at least 10,000 droplets. This was met in 108 out of 108 wells tested across all six batches, with a mean total droplet number of 20,208 droplets/well (Table 10). Next, the inter-well %CV of each set of duplicate wells of each QC was expected to be ≤25.0% for the high and low spike levels. This was met in 36 out of 36 sets of wells tested across all six batches for the QCs, with a mean inter-well %CV of 3.2% (Table 11).
Within each batch, the intra-assay mean and standard deviation were calculated for each of the independently prepared tear spikes, and these were used to calculate an intra-assay mean for each concentration in each assay. This was used to assess the accuracy and precision of the assay in matrix (Table 12). We expected the intra-assay %CV to be ≤25.0% and high and low spike levels. This was met in six out of six batches for each level. Generally, greater intra-assay precision in matrix than the target could be achieved, with a mean intra-assay %CV of 3.7% at the high level and 12.2% at the low level (overall 8.0%). It was also expected the intra-assay %RE would be ±25.0% at both spike levels. This was met in six out of six batches for each level. Likewise, it was generally found that greater intra-assay accuracy in matrix than the target could be achieved, with a mean intra-assay absolute %RE of 8.1% at the low level and 11.3% at the high level (overall 9.7%). For the unspiked control, no eGFP signal was detectable in any of the aliquots (Table 12), demonstrating the specificity of the method in the human tear matrix.
Inter-assay accuracy and precision in tear matrix were also calculated using the intra-assay mean of each spike level within each batch. It was expected that the inter-assay precision would be ≤25.0% CV, and for inter-assay accuracy, we expected ±25.0% RE. A significantly greater inter-assay accuracy and precision than these targets were observed (Table 13), with an inter-assay precision of 5.5% at the high level and 7.1% at the low level, and with absolute inter-assay accuracy of 11.3% at the high level and 8.1% at the low level. Collectively, these results demonstrate the accuracy, precision, and specificity of the method in tear matrix.
Table 5: Quality controls used to define the dynamic range of the assay. Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
Table 6: Total droplet counts and positive to total droplet ratios of synthetic double-stranded DNA quality control and NTC. Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
Table 7: QC inter-well statistics (copy targets/µL PCR reaction). Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
Table 8: Intra-assay accuracy and precision of QCs (copy targets/µL PCR reaction). Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
Table 9: Inter-assay accuracy and precision of QCs (copy targets/µL PCR reaction). Abbreviations: ULQC = upper limit quality control; HQC = high quality control; MQC = medium quality control; LQC = low quality control; LLQC = lower limit quality control; NTC = no template control. Please click here to download this Table.
Table 10: Total droplet counts of tear samples. Please click here to download this Table.
Table 11: Tear sample inter-well statistics (copy targets/µL PCR reaction). Please click here to download this Table.
Table 12: Intra-assay accuracy and precision of tear samples (copy targets/µL PCR reaction). Please click here to download this Table.
Table 13: Inter-assay accuracy and precision of tear samples (copy targets/µL PCR reaction). Please click here to download this Table.
Supplementary Table S1: Sequences of primer, probes, and synthetic double-stranded DNA quality control utilized in this study. Please click here to download this Table.
Discussion
There are several steps of the ddPCR protocol that are critical to proper performance of the assay. The first critical step is the design and optimization of the primers and probe. In general, the use of hydrolysis probe-based chemistry over dye-based chemistry (e.g., SYBR Green) in a preclinical or clinical setting is recommended due to their superior specificity. Additionally, the choice of amplification target is a critical one. Typically, the transgene of interest of the vector is targeted. However, in earlier preclinical stages or in vectors where it may not be possible to distinguish vector transgene versus genomic DNA, it may be appropriate to use standardized vector targets. For example, one could target the inverted terminal repeat region, promoter, poly-A tail, or the inter-segment junctions between these vector components. The choice of target will vary based on vector design. Traditional qPCR primer and probe design strategies and software are typically appropriate for ddPCR. Design parameters that are expected to yield a consistent annealing temperature (for example, 60°C) should be selected to reduce the amount of optimization required. It has also been recommended to design, order, and evaluate at least three different sets for each target. One should then select the set that shows the greatest specificity (no amplification in the negative control well or in a matrix of related target DNA) and sensitivity (i.e., limit of detection)20.
If it is advantageous to be able to transition the assay between qPCR and ddPCR, it is recommended to optimize the assay conditions using qPCR first, and to identify conditions for the selected set that result in amplification efficiencies of 90%-110% with an R2 ≥ 0.98. However, ddPCR as an endpoint method is typically less sensitive than qPCR due to variances in amplification efficiencies. At a minimum, it is recommended to run a thermal temperature gradient in the annealing/extension step to cover temperatures above and below the expected annealing temperatures and to evaluate the rain and fluorescent amplitude separation between the negative and positive droplet clusters as a function of temperature. If the workspace allows, it is recommended to have individual dedicated workstations for master mix preparation, template addition, and amplification. Where possible, these should physically be segregated by a unidirectional workflow with built-in engineering controls, such as controlled access and differential air pressures, to reduce the risk for cross contamination and false positives. If this is not possible, extreme caution must be taken to prevent cross contamination.
There are two steps in this protocol that may appear unusual to those who are more accustomed to qPCR assay development. The first is the inclusion of a restriction enzyme in the PCR master mix. During ddPCR amplification, each droplet is thermocycled to endpoint. In a properly optimized assay, this results in two populations of droplets, one set displaying a consistently high level of fluorescent signals-the positives-and another consistently displaying a low level of fluorescent signal-the negatives. If PCR interference occurs, it may desynchronize the initiation of PCR amplification, resulting in the droplet not reaching an amplification plateau, and thus inconsistent fluorescent endpoints. In this case, the droplets will be distributed between the negatives and positives, resulting in a phenomenon called ddPCR rain. This can result in inaccurate quantification of the target and inconsistently and subjectively applied thresholds. Our recommendation to set the threshold slightly above the signal of the NTC, which should minimize the effects of rain in the final quantification as all droplets are still considered positive, even if not fully cycled to endpoint. AAVs have a highly complex secondary structure that, depending on the amplification target, may reduce accessibility to the primers and probes, resulting in PCR interference and thus rain. The inclusion of the restriction enzyme in the master mix cleaves this secondary structure to increase access by the primers and probes, reducing the rain, which may thereby improve the accuracy of the assay. The effects of the inclusion of a restriction enzyme in the ddPCR reaction have been described previously25,32. Any restriction enzyme can be used, so long as it is confirmed to not cut within the target amplification region. No predigestion steps or alternative buffer compositions are required.
The second unusual step is the preparation of the tear sample containing AAV. In this protocol, a 1:10 (or greater) ratio of tears was utilized and subsequently the sample was heated. Typically, when tears are collected via a capillary tube, which is a widely utilized collection method, on average approximately 10.0 µL can be collected33. The dilution helps to address the limited sample volume and provide enough material for duplicate well testing. While this does reduce the theoretical limit of detection, the robust sensitivity of ddPCR should still result in the detection of all but an extremely few number of vector particles. This approach additionally creates a "backup" well if one were to unexpectedly fail. In this case, or in cases of insufficient sample volume to run two wells, the Poisson error could be used to assess precision. Furthermore, in cases where the concentration is below the limit of detection, it creates an opportunity to merge well data to determine a concentration. It is necessary to liberate the AAV vectors from the viral capsids for ddPCR detection. Some methods for the quantification of AAV have included a proteinase K digestion step to remove the viral capsid34,35,36. All naturally occurring AAV serotypes have melting temperatures at or below approximately 90 °C, with most falling below 80 °C; therefore, this appears to be an unnecessary inclusion37. Heating alone appears to be sufficient to release vector DNA.
Furthermore, ddPCR is generally less susceptible to PCR inhibitors that may be present in a sample that may affect a qPCR assay. If a specific DNA isolation step is included, this would also require specific validation, which is avoided in this protocol. Samples are diluted prior to heating due to the kinetics of diffusion of the vector genomes in a liquid. During the heating and subsequent cooling process, the positive and negative sense strands of the single-stranded DNA genome can anneal together to produce a double-stranded intermediate if the concentrations are sufficiently high. Dilution prior to heating reduces the concentrations and makes it mathematically unlikely that enough double-stranded intermediates form to have an adverse effect on the accuracy of quantification. It should be noted that the synthetic DNA fragments or linearized plasmids used as quality controls must not undergo this heating step. As these are double-stranded, heating would result in conversion to single-stranded intermediates. Following independent partitioning of these single-stranded QCs into droplets, this would be expected to result in a twofold increase in QC concentration relative to the nominal concentration. Alternatively, if QCs are to be heated to standardize the method, this must be factored into the reconstitution and assignment of a nominal concentration.
Finally, with regards to sample preparation, many protocols also recommend the inclusion of a DNase treatment step to remove any unencapsidated vector DNA. This step is critical in cases where it is not desired to quantify free DNA associated with the vector preparation (such as during quantification for dosage purposes). However, in the context of biodistribution and bioshedding studies, one typically desires to know where any vector DNA has traveled, regardless of if it is encapsidated or not. Therefore, it is suggested to typically not perform a DNase treatment step during such studies. If it is determined to be necessary to include a DNase step, this step should be prior to dilutions and heating.
In this paper, data representative of the approach to assessment of the dynamic range, sensitivity, accuracy, and precision of the method within the context of a fit-for-purpose, good laboratory practice compliant validation are presented. The current lack of guidance on this topic leaves validating laboratories to determine target assay criteria for themselves, in line with current industry thinking. Different groups have posed both higher and lower target criteria than used in this study19,20,21,22,23,24,25. The target assay criteria, until more rigidly defined, should be selected prior to validation based on the intended clinical applications of the method. Depending on the downstream decisions to be made on the basis of the data, higher levels of accuracy and precision may be needed. Conversely, a simple positive versus negative result may be sufficient.
The approach also addressed recommendations for the assessment of specificity and a matrix effect. A pool of tears collected from untreated individuals failed to produce a positive result in this assay, whereas the target could be detected when the vector was spiked into the tears at a high and low concentration within the recommended recovery rates. Ideally, matrix containing endogenous vector (e.g., collected following treatment with viral vector) would also be included in these assessments. However, it is unlikely that such samples will be available for use in a validation. To increase the robustness of the validation, multiple pools of tears, or tears collected from a variety of individuals, could be assessed to determine if a patient-specific matrix effect occurs. Finally, it is recommended to evaluate the stability. In workflows where DNA extraction occurs out of the biological matrix, it may be necessary to evaluate the stability of both the sample and the extracted DNA. In this workflow, the sample is tested directly in the assay without the need for DNA extraction. Therefore, in consideration of the evaluation of the stability for this method, one must evaluate the stability of the tear samples. Typically, benchtop, refrigerator, freeze/thaw, and long-term stability assessments are recommended. These were not performed as part of this study, but the methods developed here can be used in this assessment, following manipulations to the input samples.
Overall, this method has been demonstrated to be a robust, repeatable, and validatable assay to detect AAV-based vectors in tear samples. It may serve as a platform to be adapted to specific vectors to support clinical trials and provides a basis for validation of an assay consistent with good laboratory practices.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We would like to thank Nick Russell and Brandon McKethan of Bio-Rad for their helpful discussions during the development of this method.
Materials
| AAV-eGFP Vector | Charles River Laboratories | RS-AAV2-FL | Lot AAV2-0720-FL, used as a proof of principle vector |
| AutoDG Droplet Digital PCR system | Bio-Rad | QX200 | Alternative ddPCR system may be used following manufacturer’s protocol. |
| AutoDG Oil for Probes | Bio-Rad | 1864110 | Or use material compatible with ddPCR system. |
| ddPCR Buffer Control for Probes | Bio-Rad | 1863052 | Or use material compatible with ddPCR system and PCR chemistry. |
| ddPCR Droplet Reader Oil | Bio-Rad | 1863004 | Or use material compatible with ddPCR system. |
| ddPCR Piercable Foil Seals | Bio-Rad | 1814040 | Or use material compatible with ddPCR system. |
| ddPCR Plates 96-Well, Semi-Skirted | Bio-Rad | 12001925 | Or use material compatible with ddPCR system. |
| ddPCR Supermix for Probes (no dUTP) | Bio-Rad | 1863023, 1863024, or 1863025 | Use master mix compatible with primers/probes and ddPCR system. |
| DG32 AutoDG Cartidges | Bio-Rad | 1864108 | Or use material compatible with ddPCR system. |
| Droplet Reader | Bio-Rad | QX200 | Alternative ddPCR system may be used following manufacturer’s protocol. |
| GeneAmp PCR Buffer | Applied Biosystems | N8080129 | N/A |
| Nuclease-Free Water | Ambion | AM9906 | N/A |
| PCR Plate Sealer | Bio-Rad | PX1 | Or use material compatible with ddPCR system. |
| Pipet Tips for AutoDG | Bio-Rad | 1864120 | Or use material compatible with ddPCR system. |
| Pluronic F-68 Non-ionic Surfactant | Gibco | 24040 | N/A |
| Primer and Hydrolysis Probes | Various | Various | Design based on target sequence using general approaches for primer/probe design. Select fluorphores and quenchers compatible with ddPCR system. |
| Restriction Enzyme | Various | Various | Varies with target amplification sequence. Use restriction enzyme that does not cut in the amplified sequence |
| Sheared salmon sperm DNA | ThermoFisher | AM9680 | N/A |
| Synthetic DNA gene fragment or linearized plasmid | Various | Various | Design a synthetic DNA fragment containing the target amplification region for use as a quality control |
| TE Buffer | Teknova | T0224 | Ensure prepared or purchases nuclease free. 10 mM Tris-HCl, 1.0 mM EDTA, pH=8.0 |
| Touch Thermal Cycler | Bio-Rad | C1000 | Or use material compatible with ddPCR system. |
Riferimenti
- Sherkow, J. S., Zettler, P. J., Greeley, H. T. Is it ‘gene therapy. Journal of Law and the Biosciences. 5 (3), 786-793 (2018).
- Ginn, S. L., Amaya, A. K., Alexander, I. E., Edelstein, M., Abedi, M. R. Gene therapy clinical trials worldwide to 2017: an update. The Journal of Gene Medicine. 20 (5), 3015 (2018).
- Ghosh, S., Brown, A. M., Jenkins, C., Campbell, K. Viral vector systems for gene therapy: a comprehensive literature review of progress and biosafety challenges. Applied Biosafety. 25 (1), 7-18 (2020).
- Gene, Cell, & RNA therapy landscape, Q4 2022 quarterly data report. American Society for Gene & Cell Therapy Available from: https://asgct.org/global/documents/asgct_citeline-q4-2022-report_final.aspx (2022)
- Approved Cellular and Gene Therapy Products. United States Food and Drug Administration Available from: https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products (2022)
- Au, H. K. E., Isalan, M., Meilcarek, M. Gene therapy advances: a meta-analysis of AAV usage in clinical settings. Frontiers in Medicine. 8, 809118 (2022).
- Lundstrom, K. Viral vectors in gene therapy. Diseases. 6 (2), 42 (2018).
- Naso, M. F., Tomkowicz, B., Perry, W. L., Strohl, W. R. Adeno-associated virus (AAV) as a vector for gene therapy. BioDrugs. 31, 317-334 (2017).
- Russell, S., et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomized, controlled, open-label, phase 3 trial. Lancet. 390 (10097), 849-860 (2017).
- Chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drugs (INDs). United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/chemistry-manufacturing-and-control-cmc-information-human-gene-therapy-investigationsal-new-drug (2020)
- Long term follow-up after administration of human gene therapy products. United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/long-term-follow-after-adminstration-human-gene-therapy-products (2020)
- Testing of retroviral vector-based human gene therapy products for replication competent retrovirus during product manufacture and patient follow-up. United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/testing-retroviral-vector-based-human-gene-therapy-products-replication-competent-retrovirus-during (2020)
- Recommendations for microbial vectors used in gene therapy. United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/recommendations-microbial-vectors-used-gene-therapy (2016)
- Multidisciplinary: gene therapy. European Medicines Agency Available from: https://www.europa.eu/en/human-regulatory-development/scientific-guidelines/multidisciplinary/multidisciplinary-gene-therapy (2023)
- Human gene therapy for retinal disorders. United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/human-gene-therapy-retinal-disorders (2020)
- Design and analysis of shedding studies for virus or bacterial-based gene therapy and oncolytic products. United States Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/design-and-analysis-shedding-studies-virus-or-bacteria-based-gene-therapy-and-oncolytic-products (2015)
- Guideline on the quality, non-clinical and clinical aspects of gene therapy medicinal products. European Medicines Agency Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-non-clinical-clinical-aspects-gene-therapy-medicinal-products_en.pdf (2018)
- Kaur, S. white paper on recent issues in bioanalysis: mass spec of proteins, extracellular vesicles, CRISPR, chiral assays, oligos; nanomedicines bioanalysis; ICH M10 section 7.1; non-liquid & rare matrices; regulatory inputs (part 1A – recommendations on endogenous compounds, small molecules, complex methods, regulated mass spec of large molecules, small molecule, PoC & part 1B – regulatory agencies’ inputs on bioanalysis, biomarkers, immunogenicity, gene & cell therapy and vaccine). Bioanalysis. 14 (9), 505-580 (2022).
- Hays, A., Islam, R., Matys, K., Williams, D. Best practices in qPCR and qPCR validation in regulated bio analytical laboratories. The AAPS Journal. 24 (2), 36 (2022).
- Ma, H., Bell, K. N., Loker, R. N. qPCR and qRT-PCR analysis: Regulatory points to consider when conducting biodistribution and vector shedding studies. Molecular Therapy. Methods & Clinical Development. 17 (20), 152-168 (2020).
- Wissel, M. Recommendations on qPCR/ddPCR assay validation by GCC. Bioanalysis. 14 (12), 853-863 (2022).
- Expectations for biodistribution (BD) assessments for gene therapy (GT) products. International Pharmaceutical Regulators Programme Available from: https://admin.iprp.global/sites/default/files/2018-09/IPRP_GTWG_ReflectionPaper_BD_Final_2018_0713.pdf (2018)
- Pinheiro, L., Emslie, K. R. Basic concepts and validation of digital PCR measurements. Methods in Molecular Biology. 1768, 11-24 (2018).
- Tzonev, S. Fundamentals of counting statistics in digital PCR: I measured two target copies-what does it mean. Methods in Molecular Biology. 1768, 25-43 (2018).
- Prantner, A., Marr, D. Genome concentration, characterization, and integrity analysis of recombinant adeno-associated viral vectors using droplet digital PCR. PLoS One. 18, 0280242 (2023).
- Primer-BLAST. National Library of Medicine, National Center for Biotechnology Information Available from: https://www.ncbi.nim.nih.gov/tools/primer-blast/ (2023)
- Koressaar, T., Remm, M. Enhancements and modifications of primer design program Primer 3. Bioinformatics. 23 (10), 1289-1291 (2007).
- Ye, J. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. 13, 134 (2012).
- Droplet Digital PCR Application Guide. Bio-Rad Available from: https://www.bio-rad.com/webroot/we/pdf/lsr/literature/Bulletin_6407.pdf (2023)
- Qian, P. L., Sauzade, M., Brouzes, E. dPCR: A technology review. Sensors. 18 (4), 1271 (2018).
- Basu, A. Digital assays part I: portioning statistics and digital PCR. SLAS Technology. 22 (4), 369-386 (2017).
- Sanmiguel, J., Gao, G., Vandeberghe, L. H. Quantitative and digital droplet-based AAV genome titration. Methods in Molecular Biology. 1950, 51-83 (2019).
- Bachhuber, F., Huss, A., Senel, M., Tumani, H. Diagnostic biomarkers in tear fluid: from sampling to preanalytical processing. Scientific Reports. 11, 10064 (2021).
- Martinez-Fernandez de la Camara, C., McClements, M. E., MacLaren, R. E. Accurate quantification of AAV vector genomes by quantitative PCR. Genes. 12 (4), 601 (2021).
- Ai, J., Ibraheim, R., Tai, P. W. L., Gao, G. A scalable and accurate method for quantifying vector genomes of recombinant adeno-associated viruses in crude lysate. Human Gene TherapyMethods. 28 (3), 139-147 (2017).
- Dobnik, D., et al. Accurate quantification and characterization of adeno-associated viral vectors. Frontiers in Microbiology. 10, 1570 (2019).
- Bennett, A., et al. Thermal stability as a determinant of AAV serotype identity. Molecular Therapy Methods and Clinical Development. 6, 171-182 (2017).

