Enhancing the Development and Growth of Infant Cerebral Palsy Rats Using Selective Spinal Manipulations
Summary
This study illustrates the effect of selective spinal manipulation on the growth and development of infant rats with cerebral palsy, emphasizing the specific procedure and standardized protocol. Body weight measurement, Rotarod test, Foot-fault score, other behavioral tests, and growth hormone detection were performed to evaluate the protocol.
Abstract
Cerebral palsy (CP) is a refractory pediatric disease with a high prevalence, high disability rate, and difficult treatment. A variety of treatments are currently used for CP. The treatment involves drug and non-drug therapy. Traditional Chinese medicine external therapy is a very distinctive treatment method in non-drug therapy. As one of the external therapies of traditional Chinese medicine, massage is used in treating cerebral palsy and has good efficacy, small side effects, and strong operability. As a part of TCM external therapy, selective spinal manipulation can effectively promote the growth and development of infant rats with cerebral palsy.The operation was mainly divided into four steps: first, the rubbing method was applied to the spine and both sides of the spine for 1 min. The pressing and kneading method was applied to the spine for 5 min, and the muscles on both sides of the spine for 5 min. Second, pressing and kneading the sensitive local acupoints in the spine for 2 min were performed. Thirdly, the affected limb was treated by twisting method for 1 min. Fourth, the rubbing method was applied to a midline from the forehead to the back of the brain for 1 min. This study aimed to use selective spinal manipulation to treat infant rats with cerebral palsy. The weight, Rotarod test, Foot-fault score, and growth hormone of infant rats with cerebral palsy were detected to understand the effect of selective spinal manipulation on the growth and development of infant rats with cerebral palsy. The results showed that it can promote weight gain, improve balance ability and motor function, promote growth and development of infant cerebral palsy rats, promote growth hormone secretion, and increase the temperature of sensitive parts of the back.
Introduction
Cerebral Palsy (CP), caused by non-progressive damage to the brain in the fetus or infancy, is a group of disorders characterized by abnormal motor and postural development1, developmental disorders including slow weight gain2,3,4, and motor dysfunction. The incidence of cerebral palsy in China is 2.48%, and the prevalence is 2.46‰ (1-6 years old)5, respectively. Foreign studies reported a prevalence of 2.4%-3.6%6. Cerebral palsy is a major cause of physical injury and disability in children, among which balance and movement disorders significantly impact daily living activities1. Cerebral palsy can be caused by premature birth, infection, genetics, neonatal ischemia and hypoxia, neonatal jaundice, and other complex and diverse pathogenic factors7. The goal of treatment for cerebral palsy is to improve physical function and quality of life 8. Currently, the treatment methods for cerebral palsy include deep brain stimulation9, robot-assisted gait training10, wrist and ankle acupuncture11, and meridian acupuncture combined with massage12.
Traditional Chinese medicine (TCM) has been increasingly used as an effective treatment for CP13,14. Massage, as a part of it, also plays a certain role. For example, selective spinal manipulation can regulate the state of DNA hydroxymethylation to regulate neural development and improve learning and memory function15. In the treatment of infant rat with cerebral palsy, selective spinal manipulation may improve the inflammatory homeostasis of the cortex and hippocampus by regulating the methylation level of inflammatory cytokines TNF-α and IL-10 gene promoter region16 and play a positive therapeutic role in the balance ability of infant rat with cerebral palsy16. Selective spinal manipulation can improve the cognitive function of infant rats with cerebral palsy17. Selective spinal manipulation was used in the treatment of infant rats with cerebral palsy, and it was observed that it also had therapeutic effects on their growth and development18.
The purpose of this study is to illustrate the effect of selective spinal manipulation on the growth and development of infant rats with cerebral palsy by measuring the body weight, Rotarod test19, Foot-fault score20,21, and other behavioral tests and the detection of growth hormone, and to provide research ideas for relevant personnel.
Protocol
This study was approved by the Experimental Animal Ethics Committee of Yunnan University of Traditional Chinese Medicine. All experimental operations on animals followed the 3R principle of experimental animal reduction, optimization, and replacement (No. R-06202018). Healthy Sprague Dawley (SD) rats (14 males and 7 females) of Specific pathogen-free (SPF) grade with an average body weight of 250-300 g were used in this experiment. The rats were raised in the SPF animal room of Yunnan University of Traditional Chinese Medicine, Certificate number: SYXK(Yunnan)K2022-0004. All rats were housed under a 12-h light/dark cycle with a natural light-controlled environment at a room temperature of 22-26 °C and a relative humidity of 40%-50%. After 1 week of adaptive feeding, the male/female ratio was maintained at 1:2. After 1 week, the pregnant rats were housed in a single cage, fed normally, eating and drinking freely. After natural delivery, the healthy infant rats born to the pregnant rats were selected. The infant rats were divided into the Sham, Control, and Treatment groups to observe the effect of selective spinal manipulation on the growth and development of infant rats with cerebral palsy. From each group, 6 male pups were selected for the experiment. The Treatment group was treated with selective spinal manipulation from the 5th day after birth, and the treatment was given for 6 days followed by rest for 1 day.
1. Establishing cerebral palsy model
- Sterilize the microscope, ophthalmic scissors, forceps, suture, surgical plate, scissors, cotton swabs, and gloves required for surgery, and place them in the operating room.
- Separate a pup temporarily from its mother on the third day of life, place it in a single frame, and transport it to the sterile operating room.
- Measure the body weight. Include infant rats with body weight ranging from 7-9 g in the study, and exclude those who did not conform to body weight. Randomly divide the infant rats into Sham, Control, and Treatment groups.
- Induce anesthesia with isoflurane at a concentration of 2% and maintain anesthesia at 1% using a small-animal inhalation gas anesthesia machine.
- Connect the small animal gas anesthetic to the power supply and open the valve connected to the induction box.
- Put the infant rat into the induction box, turn the concentration adjustment knob, and adjust the concentration of isoflurane to 2% to induce anesthesia.
- When the infant rat is unconscious, remove it from the induction box. Place it on the operation plate and close channel 1 of the anesthesia machine.
- Place the infant rat supine. Fix the anesthesia inhalation mask to maintain anesthesia.
- Open the channel connected to the anesthesia inhalation mask. Maintain anesthesia by turning the concentration adjustment knob and adjusting the concentration of isoflurane to 1%.
- Disinfect the neck skin of the infant rat and cut a longitudinal 1 cm incision on the neck skin to expose the subcutaneous tissue.
- Cut the skin of the left neck under a dissecting microscope, and use ophthalmic tweezers to bluntly separate the sternohyoid muscle and the left sternomastoid muscle.
- Find the left common carotid artery and vagus nerve, and separate the left common carotid artery and vagus nerve.
- In the Model group, cut off the left common carotid artery with an electric coagulation pen. Clean and suture the neck incision.
- In the Sham group, separate the left common carotid artery from the vagus nerve.
- After the operation, place the infant rat in the thermostatic water bath in the prone position for resuscitation for 1 h.
NOTE: After natural recovery, the skin color of the limbs and the whole body was ruddy. The infant rat could move its limbs autonomously and crawl independently. - Place the resuscitated rats of the Control and Treatment groups in a closed incubator at 37 °C in the prone position. Introduce a mixture of 5% oxygen and 95% nitrogen gas to induce hypoxia, and remove the rats after 2 h.
- Remove the infant rat that completed the hypoxia process from the hypoxia box and place it in the prone position in a box with normal atmospheric oxygen content. Place them in a 37 °C thermostatic water bath for 1 h. After complete recovery, transfer the infant rat back to the mother's cage.
NOTE: After recovering naturally, the skin color of the limbs and the whole body was red. Infant rat could move their limbs autonomously and could crawl independently. - Place the infant rat of the Sham group in one box with normal atmospheric oxygen content, and place the box in a 37 °C thermostatic water bath for 1 h. After complete recovery, put the infant rat back into the mother's cage for feeding.
NOTE: After recovering naturally, the skin color of the limbs and the whole body was red. Infant rat could move their limbs autonomously and could crawl independently. The whole operation process was completed in the special operation room of the SPF animal room of Yunnan University of Traditional Chinese Medicine. During resuscitation and hypoxia, the operator observes the infant rat at all times to prevent death caused by improper posture.
2. Righting reflex experiment
- On the 4th day after birth (the 2nd day after modeling), perform the righting reflex experiment to verify whether the model was successful (Figure 1 and Table 1).
- Lift the tail of the infant rat, rest its back on a horizontal board, and use the thumb and index finger to secure its belly and neck. Check if the rat can return to its normal position. The inability of the rat to return to the normal position from the abnormal position due to the loss of the righting reflex is recorded as a successful model16,22.
- Record the time each group of pups takes to turn over from the supine to the prone position. Begin to record the time when the thumb and index fingers are released simultaneously in the supine position, allow the infant rat to turn to the prone position, and stop recording when the front and back paws are placed on the floor. If the infant rat could not return to the normal position for more than 20 s, record the righting time as 20 s.
3. Preparation before the operation
- After modeling, separate the infant rat with cerebral palsy from its mother and bring it to an operating room with a constant room temperature of 22-26 °C for 2 min to adapt to the surrounding environment.
- Wear disposable gloves to keep the palm mild and soft and the temperature between 36-37 °C.
- Place the rat gently in the palm of the left hand. Bend the left thumb to cover the eyes of the rat for 2 min to form a dark field of vision so that the rat can adapt to the environment of the operator's palm. Use the right index and middle fingers for manipulation.
NOTE: Covering the eyes of the infant rat can create a dark environment. This will keep the infant rat from becoming agitated because of the unsafe and uncertain environment affecting the experimental results.
4. Dividing the rats into the Control and the Treatment groups
- On the second day after modeling (P5), verify the model by the righting reflex test and randomly divide the infant rats into the Control and Treatment groups. Perform selective spinal manipulation on the infant rats of the Treatment group.
- Place the infant rat in the prone position. Ensure that the spine is always kept at a straight level (Figure 2A).
- Massage the spine and both sides of the spine for 1 min using the rubbing method, and use the pressing and kneading method to the spine and the muscles on both sides of the spine for 5 min.
- Massage the spine and skin on both sides using the rubbing method to relax the infant rat and relieve its tension fully. Ensure that the frequency of rubbing is 100-120 times/min for 1 min after the infant rat is quiet and without agitation.
- Use the right index finger, middle finger, and ring finger as the contact surface, and make a circular rubbing motion on the surface skin of the cervical spine, thoracic spine, and lumbar spine from head to tail without disturbing the subcutaneous tissue.
- With the right index finger, middle finger, and ring finger as contact surfaces, perform circular motion on the skin surface of the trapezius muscle, superficial gluteus muscle, cervical rhomboid muscle, pectoral rhomboid muscle, latissimus dorsi muscle, and external oblique muscle in order from head to tail according to the animal anatomical standard23, without disturbing the subcutaneous tissue.
- Treat the spinal column first with pressing and kneading for 5 min, followed by pressing and kneading the muscles on both sides of the spine for 5 min.
- Apply the kneading method to the spine at a frequency of 120 times/min for 5 min. Use the right index or middle fingers as the contact surface. From head to tail, perform circular kneading on the surface skin of the cervical, thoracic, and lumbar vertebrae, combined with downward pressing (1.77 ± 0.54 N). Ensure that the force reaches the cervical vertebrae, thoracic vertebrae, lumbar vertebrae, and supraspinous ligaments.
- Apply the pressing and kneading method to the muscles on both sides of the spine at a frequency of 120 times/min for 5 min. Use the right index finger or middle finger as the contact surface. According to the animal anatomical standard23, press down the skin surface of the trapezius, superficial gluteus, rhomboid neck, rhomboid pectoralis, latissimus dorsi, and external oblique abdominal muscles in a round kneading motion from head to tail, with a force of 1.77 ± 0.54 N, so that the force reaches the trapezius, superficial gluteus, rhomboid neck, rhomboid pectoralis, latissimus dorsi, and external oblique abdominal muscles (Figure 2B)23.
- Massage the spine and skin on both sides using the rubbing method to relax the infant rat and relieve its tension fully. Ensure that the frequency of rubbing is 100-120 times/min for 1 min after the infant rat is quiet and without agitation.
- Press and knead the sensitive spinal acupoint points (such as Ganshu, Xinshu, Pishu, Shenshu, and Feishu24) at a frequency of 100-120 times/min for 2 min. Using the right index or middle finger as the contact surface, perform circular kneading and downward pressing(1.77 ± 0.54 N) on the surface skin of local acupoints (such as Ganshu, Xinshu, Pishu, Shenshu, and Feishu) in infant rats with cerebral palsy.
NOTE: The acupoints were selected according to the "Animal Acupuncture Point Map" formulated by the Experimental Acupuncture Research Branch of the Chinese Acupuncture Society) (Figure 3)25. - Locally stimulate the affected limb by the twisting method at a frequency of 25 times/min for 1 min.
- With the right thumb and index finger, pinch the affected limb of the infant rat with cerebral palsy, exerting symmetrical force on the two fingers. Ensure that the force is gentle and the action is light and gentle. Rub the right extensor carpi radialis, extensor digitorum common, extensor carpi ulnaris, phalanx, interphalangeal joint, gastrocnemius muscle, semitendinosus muscle, metatarsal bone, and intermetatarsal joint of infant rat back and forth.
- Apply the rubbing method to the midline of the forehead to the back of the brain at a frequency of 100-120 times/min for 1 min. Use the right index finger as the contact surface, and use the rubbing method on an imaginary line from the forehead to the middle of the posterior brain of the infant rat (namely, one line of Baihui, Fengfu, and Dazhui) (Figure 3).
NOTE: Massage is mainly applied to the skin. It is a circular movement on the skin surface of the head of the infant rat with cerebral palsy. It does not need to drive the subcutaneous muscle movement of an infant rat with cerebral palsy. - At the end of the procedure, leave the infant rat alone for 30 min before returning it to the mother rat cage.
NOTE: With age, the hair of the infant rats becomes thick, so it is necessary to remove the back hair of all groups of infant rats during the massage operation to avoid affecting the experimental effect.
5. Detecting the temperature of the local acupoints
- Use an infrared thermal imager to detect the temperature of the local acupoint area before and after the operation.
- Before selective spinal massage, connect the Type-C interface of the mobile phone with the USB interface of the infrared imaging device with a data cable, and turn on the Power button.
- Click Analyze to enter the mobile phone terminal of the software, select Photo to enter the photo mode, and click the Picture di Picture Mode in the lower right corner.
- Focus the camera at the acupoint areas of Dazhui, Xinshu, and Shenshu acupoints (Figure 3) on the back of the infant rat to take temperature shots. Click Photo to complete the image capture and Save it in JPG format.
- Connect the computer with a data cable and upload the saved image to a computer installed with the Fotric software for analysis.
- Open the Fotric software on the PC, open the Local File, and find the location of the image.
- Select the Thermogram workspace, select the image to be analyzed, and enter the analysis interface.
- On the Analysis screen, select the picture in the Thermal image box on the right and open the Lock.
- Select the Zoom Function, adjust the size of the Thermal image and the left box Background image to coincide, and turn off the Lock on the right.
- Find the Set measurement rectangle in the toolbar on top and mark the acupoint to be detected in the figure.
- Click Report on the right to record the temperature value.
6. Detecting the motor balance function of infant rats with cerebral palsy
- Use the Behavioral Rotarod test19 to detect the motor function of rats in the Sham, Control, and Treatment groups on the 61st day after birth.
- Parameter settings: Start the power switch, select Do experiment to enter the next step, select Positive, Rats, Timing 5 min to enter the next step, and select Acc time:10s, Speed:10rpm.
- Then, place the rats on the running track and begin the test by clicking Run after they stand firm. Adjust the running state of each run to ON. Train each rat once for 5 min, and then begin to perform the test thrice.
- After the training, place the infant rats on the rotarod-running track. After it is stable, click Run, and adjust the running state to ON. At the end of 5 min, select Date to record the time and speed of each rat falling from the rotarod-running track for the first time, i.e., the Run time data.
- Use the Foot-fault test20,21 to evaluate the balance motor function of the right forelimb of the Sham, Control, and Treatment groups on the 61st day after birth.
- Place the infant rats at the beginning end of the horizontal ladder and record a video of them crossing it to the other end (100 cm long, and the distance between each ladder is 2 cm). After one training session, perform the formal test thrice for each rat at a 5 min interval.
- Observe the right forelimb when the animal crosses the ladder using low-speed playback to make a score, as shown in the scoring standard (Table 2).
7. Western blotting
- Perform western blotting analysis of the tissue samples as described previously26.
NOTE: The amount of lysate was determined according to the size of the tissue block. Antibodies used were as follows: primary anti-Growth Hormone antibody (0.5 µg [0.5 ng/lane]); anti-Growth Hormone receptor antibody (1/1000); internal reference anti-beta Actin antibody (1/2000) for primary antibodies; Goat anti-RabbitIgG H&L (HRP) (1/10000) for secondary antibody.
8. Detecting the strength of the hand
- Wear the device used to detect the manipulation force on the hand used for massage. Fit the finger used to massage the infant rat with the sensor chip of the device, and turn the Power button on.
- Connect the hard disk interface that stores the manual tester software to the computer, and enter the dedicated IP address of the manual tester in the URL input box.
- Install the SpringVR client on the computer, click to enter the collection interface, and select the Finger Pattern in the lower right corner to test the manipulation force.
- Select the Pressure option and click Start to start recording the manual force of the operator during operation. The software will automatically record the intensity of the manipulation and generate a data table on the local disk where the data in the file name PressureSensor will be found.
9. Data statistics
NOTE: SPSS26.0 software was used for statistical analysis, Graphpad Prism9.0.0 for bar chart production, and Image J for gray value analysis of protein bands.
- Analyze and present all data as mean ± standard deviation (Mean ± SD).
NOTE: The test standard was α = 0.05, and P ≤ 0.05 was considered statistically significant. Fisher's test was used because the data were analyzed by analysis of variance and normality and conformed to the normal distribution characteristics and variance homogeneity.
Representative Results
Selective spinal manipulation can promote body weight gain in infant rats with cerebral palsy.
During the treatment, body weight was measured on postnatal days 3, 14, 28, 42, and 61 (Figure 4, Table 3). On the third day after birth, the body weight of the Sham group was 5.53 ± 0.035 g, and the body weight of the Control group was 3.15 ± 0.43 g. The body weight of the Treatment group was 4.42 ± 0.13 g, and it was not significantly different from that of the Sham and Control groups before modeling.
On postnatal day 14, the body weight of the Sham group was 35.97 ± 1.019 g, the body weight of the Control group was 28.00 ± 0.7403 g, and the body weight of the Treatment group was 33.78 ± 3.705 g. There was no significant difference in body weight between the Treatment and Sham groups. The weight of the Treatment group compared with the Control group was statistically significant (P < 0.01).
On the 28th day after birth, the body weight of the Sham group was 104.9 ± 3.534 g, the body weight of the Control group was 80.35 ± 6.767 g, and the body weight of the Treatment group was 96.73 ± 7.638 g. The Treatment group was compared with the Control group, and it was statistically significant (P < 0.01).
On postnatal day 42, the body weight of the Sham group was 194.1 ± 4.333 g, the body weight of the Control group was 138.0 ± 6.029 g, and the body weight of the Treatment group was 173.9 ± 5.433 g. There was a statistically significant difference in body weight between the Treatment and Control groups (P < 0.0001).
On postnatal day 61, the body weight of the Sham group was 321.5 ± 5.675 g, the body weight of the Control group was 230.3 ± 5.198 g, and the body weight of the Treatment group was 268.9 ± 15.22 g. P < 0.0001 was considered statistically significant. There was a statistically significant difference in body weight between the Treatment and Control groups at P < 0.0001.
Selective spinal manipulation can improve cerebral palsy's effective motor balance function.
On the 61st day after birth, after the end of treatment, we performed the fatigue rotarod test. The first falling time of each group was 296.1 ± 1.65 s in the Sham group, 102.7 ± 7.73 s in the Control group, and 220.1 ± 8.04 s in the Treatment group. There was a significant difference between the Sham and Control groups (P < 0.0001). The difference between the Treatment group and the Control group was statistically significant (P < 0.0001), and the difference between the Treatment group and the Sham group was statistically significant (P < 0.0001) (Figure 5, Table 4)
On the 61st day after birth, after the end of treatment, the Foot-fault scores of the Sham and Control groups were 5.53 ± 0.03 and 3.15 ± 0.43, respectively. The score of the Treatment group, 4.42 ± 0.13, was significantly lower than that of the Sham group (P < 0.0001). The score of the Treatment group was significantly higher than that of the Control group (P < 0.0001). Compared with the Treatment group and the Sham group, the difference was statistically significant (P < 0.0001) (Figure 6, Table 5).
Selective spinal manipulation can regulate the level of growth hormone secretion in cerebral palsy.
Tissue samples were taken on postnatal day 32, and the hypothalamus of rats with cerebral palsy was selected for Western blot, and the corresponding primary and secondary antibodies were selected: Antibodies used were primary anti-Growth Hormone antibody, anti-Growth Hormone receptor antibody, internal reference Anti-beta Actin antibody for primary antibodies and Goat anti-RabbitIgG H&L (HRP) (1/10000) for secondary antibody. Selective spinal manipulation can promote the growth and development of infant rats with cerebral palsy by regulating the expression of growth hormone (GH) and growth hormone receptor (GHR) (Figure 7, Table 6)26.
Promoting the growth and development of infant rats with cerebral palsy can improve the content of serotonin and norepinephrine in the hippocampus, thereby improving the ability to learn and memory27.
Selective spinal manipulation can effectively improve the local acupoint temperature (Figure 8, Table 7).
Infrared thermal imaging was used to detect the temperature of the local acupoint area before and after the treatment in the treatment group. It was found that the temperature was maintained at 32.92 ± 0.55 °C before the treatment, 36.32 ± 0.15 °C immediately after the treatment, and 35.32 ± 0.28 °C 2 min after the treatment. The temperature was 34.61 ± 0.17 °C 5 min after the end of treatment and 34.28 ± 0.60 °C 10 min after the end of treatment. The differences were statistically significant compared with the immediate temperature before the end of treatment and the local acupoint area 2 min, 5 min, and 10 min after treatment (P < 0.05). After selective spinal manipulation, the temperature of the local acupoint area in the treatment group was increased and maintained to a certain extent.
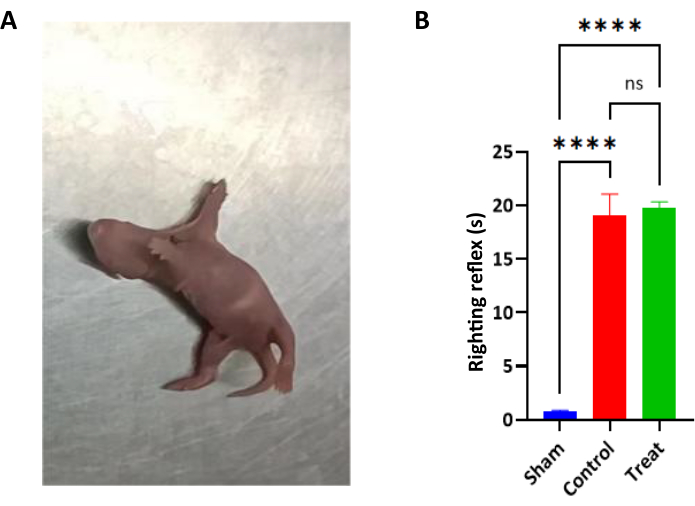
Figure 1: Righting reflex. (A) Schematic representation of the righting reflex experiment. The righting reflex test was performed on the 4th day after birth (the second day after modeling) to verify the success of modeling. (B) Bar graph of righting reflex test in each group (n = 6, Mean ± SD). Note: ns indicates P > 0.05 and no statistical difference. **** indicates a P < 0.0001, indicating a highly significant difference. Please click here to view a larger version of this figure.
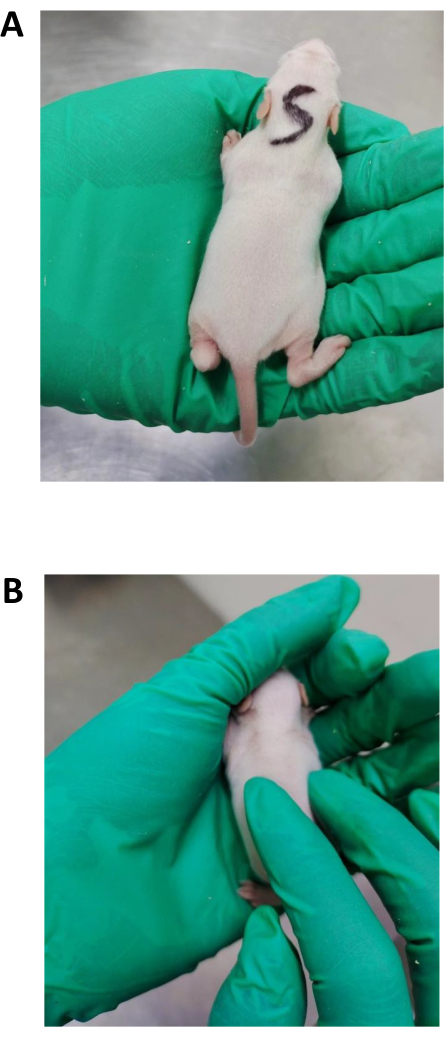
Figure 2: Selective spinal manipulation. (A) Handling of pups before manipulation. The infant rats were placed in the palm of the operator and kept quiet, which was the environment in the camp. (B) Representation of selective spinal manipulation. Press and knead the muscles on both sides of the spine. Please click here to view a larger version of this figure.
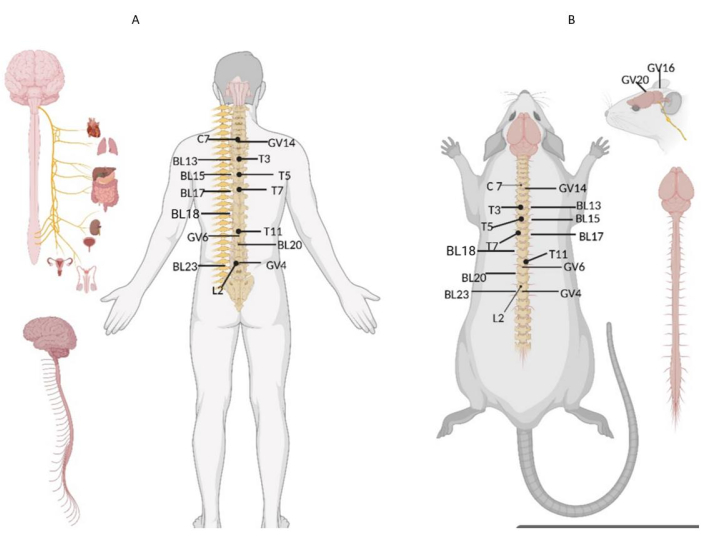
Figure 3: Map of acupoints on the back of human versus SD rat. (A) Human and (B) SD rat. C7: seventh cervical vertebra; GV14: Dazhui (GV14), location: between the seventh cervical vertebra and the first thoracic vertebra, dorsal midline; T3: third thoracic vertebra; BL13: Feishu (BL13), location: between the two ribs below the third thoracic vertebra; T5: fifth thoracic vertebra; BL15: Xinshu point, located between the two sides of the lower rib of the fifth thoracic vertebra; T7: seventh thoracic vertebra; BL17: Geshu point, location: lower and lower ribs of the seventh thoracic vertebra; BL18: Ganshu point, location: lower and lower ribs of the seventh thoracic vertebra; T11: eleventh thoracic vertebra; GV6: Jizhong point, located between the eleventh and twelfth spinous process of thoracic vertebra; BL20: Pishu point, located between the two ribs below the twelfth thoracic vertebra; L2: second lumbar spine; GV4: Mingmen (GV4), location: the dorsal median line, the depression under the second lumbar spinous process; BL23: Shenshu point, located on both sides of the second lumbar spine. GV20: Baihui (GV20), location: median parietal bone; GV16, Fengfu point, location: occipito-atlantoaxial joint dorsal depression behind occipital crest. The location of acupoints in the human body is highly consistent with the location of acupoints in animals from the anatomical position. The abnormal response point was close to the projection point of the innervated body surface. Please click here to view a larger version of this figure.
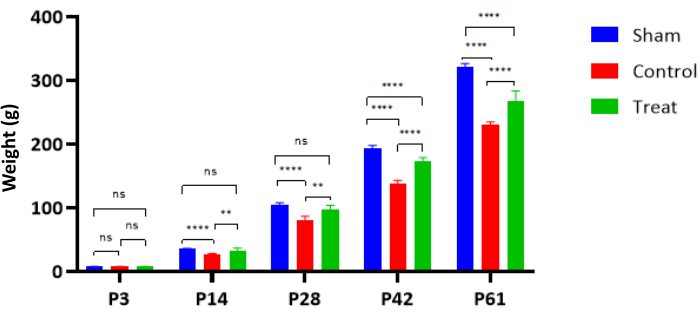
Figure 4: Bar chart of weight measurement in each group (n = 6, Mean ± SD). Note: ns P >0.05, no statistical difference; **P < 0.01, ***P < 0.001,****P < 0.0001, the difference was statistically significant. Please click here to view a larger version of this figure.
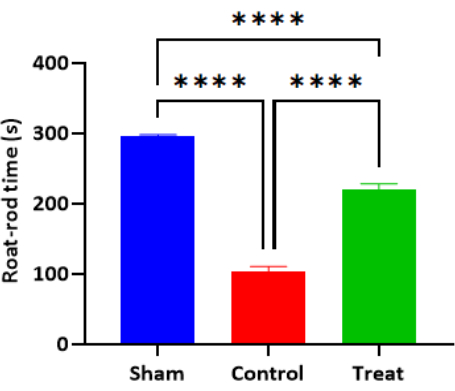
Figure 5: Bar graph of the time of the first drop of the Roat-rod test in each group (n = 6, Mean ± SD). Note: ****P < 0.0001, the difference was statistically significant. Please click here to view a larger version of this figure.
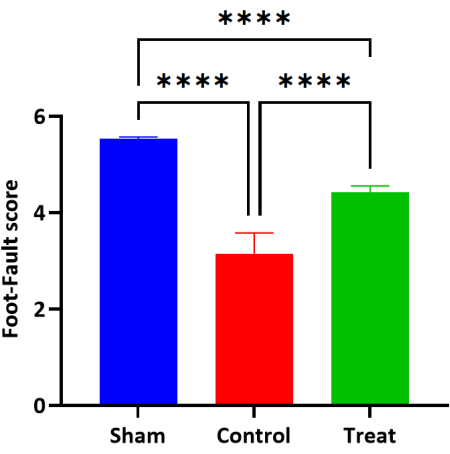
Figure 6: Bar plot of the Foot-fault scores in each group (n = 6, Mean ± SD). Note: ****P < 0.0001, the difference was statistically significant. Please click here to view a larger version of this figure.
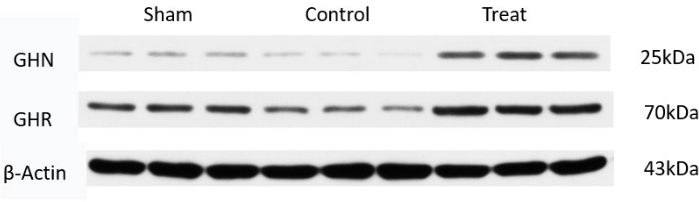
Figure 7: Western blot bands of GH and GH receptor. Western blot was used to detect the secretion of growth hormone at the molecular biological level in each group, and the effect of selective spinal manipulation on growth hormone was observed. Please click here to view a larger version of this figure.
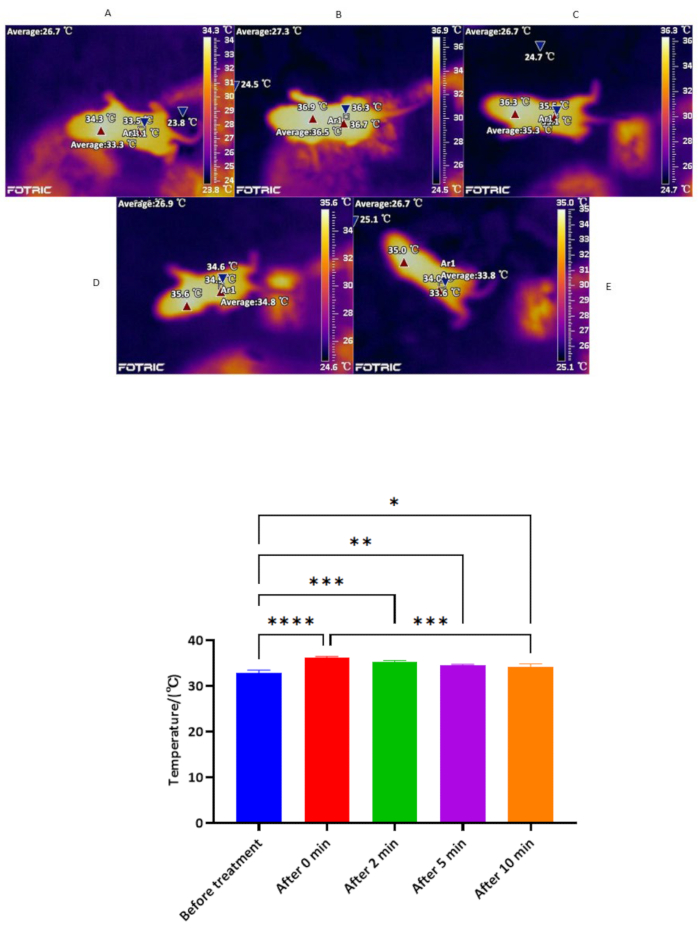
Figure 8: Temperature monitoring before and after treatment. (Top Panel) Thermal images taken by an infrared thermal imager. The temperature (°C) of the local acupoint area was recorded by an infrared thermal imager before and after tuina treatment. (A) Before tuina treatment. (B) Temperature immediately at the end of treatment. (C) Local acupoint temperature 2 min after treatment. (D) Local acupoint temperature 5 min after the end of treatment. (E) local acupoint temperature 10 min after treatment. (Bottom Panel) Bar chart of temperature monitoring before and after treatment (n = 3, Mean ± SD).*P < 0.05, P < 0.01, * * * * *P < 0.001, * * * *P < 0.0001, the difference is statistically significant. Please click here to view a larger version of this figure.
| Group | Number of cases (n) | Righting reflex/s |
| Sham | 6 | 0.77 ± 0.10 |
| Control | 6 | 19.00 ± 2.04* |
| Treatment | 6 | 19.77 ± 0.55# |
Table 1: The time statistics of righting reflex in infant rats with cerebral palsy(Mean ± SD; n = 6). Pups in each group were subjected to the righting reflex test on the 4th day after birth (the 2nd day after modeling) to verify whether the model was successful. * Compared with Sham group, P < 0.0001; # compared with Control group, P < 0.0001.
| Score | Standard for evaluation | ||
| 1 | When the young rat is placed on the horizontal ladder, the foot slips off when bearing weight, affecting the walking | ||
| 2 | When the young rat is placed on the ladder, the foot slips when bearing weight, but does not affect the walking | ||
| 3 | When the young rat is placed on the horizontal ladder, the rat moves the foot quickly to the next step of the ladder bearing weight | ||
| 4 | When the young rat is placed on the horizontal ladder, the rat places the foot partially, and moves across the ladder bearing weight | ||
| 5 | When the young rat is placed on the horizontal ladder, the rat puts its foot on one step and quickly moved to another step | ||
| 6 | When the young rat is placed on the horizontal ladder, the rat places its paw pads correctly and fully bearing the body weight | ||
Table 2: Foot-fault test score standard table
| Time (Day) | Number of cases (n) | Group | ||
| Sham | Control | Treatment | ||
| P3 | 6 | 8.52 ± 0.27 | 8.60 ± 0.08 | 8.45 ± 0.28D |
| P14 | 6 | 35.97 ± 1.01 | 28.00 ± 0.74* | 33.78 ± 3.70Ñ |
| P28 | 6 | 104.9 ± 3.53 | 80.35 ± 6.76* | 96.73 ± 7.63Ñ |
| P42 | 6 | 194.1 ± 4.33 | 138.0 ± 6.02* | 173.9 ± 5.43#D |
| P61 | 6 | 321.5 ± 5.67 | 230.3 ± 5.19* | 268.9 ± 15.22#D |
Table 3: The weight test results of each group (Mean ± SD) (g). Note: Ñ Compared with Control group, P < 0.01; ° Compared with Sham group, P < 0.01; à Compared with Control group, P < 0.001; * Compared with Sham group, P < 0.0001; # Compared with Control group, P < 0.0001; D Compared with Sham group, P < 0.0001.
| Group | Number of cases (n) | Roat-rod/time/s |
| Sham | 6 | 296.1 ± 1.65 |
| Control | 6 | 102.7 ± 7.73* |
| Treatment | 6 | 220.1 ± 8.04#D |
Table 4: The Rotarod test of infant rats in each group (Mean ± SD) (s). Note: * Compared with the Sham group, P < 0.0001; # Compared with the Control group, P < 0.0001; D Compared with the Sham group, P < 0.0001.
| Group | Number of cases (n) | Foot-Fault score |
| Sham | 6 | 5.53 ± 0.03 |
| Control | 6 | 3.15 ± 0.43* |
| Treatment | 6 | 4.42 ± 0.13#D |
Table 5: The Foot-fault scores of each group (Mean ± SD). Note: * Compared with the Sham group, P < 0.0001; # Compared with the Control group, P < 0.0001; D Compared with the Sham group, P < 0.0001.
| Group | GH | GHR |
| Sham | 2.325 ± 0.830 | 1.469 ± 0.265 |
| Control | 1.563 ± 0.070# | 1.563 ± 0.070Ñ |
| Treatment | 2.471 ± 0.956* | 2.471 ± 0.956& |
Table 6: Growth hormone and growth hormone receptor Western blot band gray value data (n = 10). GH:* The pairwise comparison between the Treatment group and the Control group (P = 0.020 < 0.05) showed statistically significant difference; # Pairwise comparison between Sham group and Control group (P = 0.022 < 0.05) showed significant difference; The pairwise comparison between the Treatment group and the Sham group showed no statistical significance (P = 0.076 > 0.05); GHR: & Pairwise comparison between Treatment group and Control group (P = 0.024 < 0.05) showed significant difference; Ñ Pairwise comparison between Sham group and Model group (P = 0.021 < 0.05) showed statistically significant difference; Pairwise comparison between Treatment group and Sham group showed no statistical significance (P = 0.085 > 0.05).
| Time of therapy | Number of cases (n) | Temperature |
| Before treatment | 3 | 32.92 ± 0.55 |
| Immediately | 3 | 36.32 ± 0.15* |
| After 2 min | 3 | 35.32 ± 0.28# |
| After 5 min | 3 | 34.61 ± 0.17Ñ |
| After 10 min | 3 | 34.28 ± 0.60Dà |
Table 7: Temperature monitoring results before and after treatment (Mean ± SD) (°C). Note: * Compared with those before treatment, P < 0.0001; # Compared with that before treatment, P < 0.001; D Compared with 0 min after treatment, P <0.001; Ñ Compared with before treatment, P < 0.01; à Compared with pre-treatment, P < 0.05.
Discussion
Because ischemia and hypoxia are important pathogenic factors of cerebral palsy, the internationally recognized method of establishing the cerebral palsy model is combined with hypoxia to prepare the cerebral palsy model16,28,29,30. When cerebral palsy develops, it causes global developmental delay, including weight and motor function, which may be related to the level of growth hormone secretion31. Selective spinal manipulation was used to treat infant rats with cerebral palsy. The body weight was monitored during the treatment, and behavioral tests such as the Rotarod test, Foot-fault score, and growth hormone were performed. The temperature of local acupoints was observed before and after the treatment. After the ischemia-hypoxia model was established, the infant rat with cerebral palsy lost weight. Still, with the extension of massage, it was found that the weight gain of the Treatment group was significantly better than that of the Control group. It also had obvious advantages in regulating the secretion of growth hormone. These results indicate that selective spinal manipulation can effectively improve the growth and development of infant rats with cerebral palsy. Moreover, because the left common carotid artery was used to establish the model of ischemia and coagulation combined with hypoxia, there must be damage to the left cortex, leading to impaired motor function. After treatment, we used the Rotarod test and Foot-fault score to detect the behavioral ability of infant rats with cerebral palsy32,33,34. It was found that the motor function of cerebral palsy rats improved after selective spinal manipulation35. Selective spinal manipulation can effectively improve the growth development and motor function of infant rats with cerebral palsy.
Traditional Chinese massage is based on acupoints and the meridian system. This experiment's design is mainly to observe the influence of traditional Chinese massage on the growth and development of infant rats with cerebral palsy. Massage treatment is mainly performed on the bladder meridian of foot-taiyang first and second side lines and the Du meridian, but different from acupuncture, there is a clear location of the stimulation point. Massage is a kind of facial stimulation in which the operator's fingers are used for abdominal operation, and the contact surface has a certain area range, a diameter of about 1.0 cm circular. Meridians also have a certain range; however, the human finger contact surface is wider than the SD rat acupoint area. This means there is an inevitable operational procedure at some non-acupoint locations. Therefore, we did not set a non-meridian massage group separately as a control when assigning the groups for this study.
Selective spinal massage selects the spine and muscles on both sides of the spine for stimulation during the operation, which can relieve local muscle spasms, promote local blood circulation, and improve the function of the corresponding innervation area to achieve the effect of relieving meridian and collaterals and overall regulation and treatment. It fully embodies the integration of the holistic concept of traditional Chinese medicine, the meridian theory of traditional Chinese medicine, and the principle of spinal nerve innervation, and it gives consideration to the whole while carrying out local treatment of cerebral palsy8.
In the procedure, the massage method on the spine and the muscles and ligaments on both sides of the treatment, that is, the Du meridian and bladder meridian of foot-taiyang first and second side lines of massage stimulation, can play a role in connecting the back acupoints, spinal cord, and brain. Therefore, when we perform manipulative operations on the back points of an infant rat, we can stimulate the back points and meridians to run qi and blood, regulate the functions of the body's viscera, and thus affect the functions of the spinal cord and brain to promote the growth and development of infant rat. On the whole, by stimulating the Du meridian and bladder meridian of foot-taiyang first and second side lines, the Yang of the whole body can be mobilized, and the overall disease resistance of the body can be improved, which reflects the overall concept of Traditional Chinese Medicine16,26,35,36,37,38. The Du meridian originates from the lower abdomen of the human body and reaches the top of the head along the spine, regulating the Yang qi of the human body36. The bladder meridian of Foot-Taiyang mainly runs along both sides of the governor vessel, and the back-shu points of five zang organs are located on the first lateral line of the Foot-Taiyang bladder meridian, involved in treating diseases of the zangfu organs39,40. The meridians and collaterals are divided into floating collaterals, minute collaterals, and cutaneous regions. Therefore, when we perform the procedure on the back skin of an infant rat with cerebral palsy, we can operate along the direction of meridians and effectively stimulate floating collaterals, minute collaterals, and cutaneous regions. Therefore, by stimulating the acupoints and meridians to run the qi and blood, the function of the body's viscera is regulated and results in weight gain of the pups8.
We found cord-like reaction points on the urinary bladder meridian of foot-taiyang on the back of children with cerebral palsy, and infrared thermography was used to detect the abnormal temperature of some acupoints. Combined with the principle of spinal nerve innervation, we found that these reaction points were close to the projection points on the surface of nerve innervation, which is also consistent with the principle of selective spinal nerve root resection for treating cerebral palsy (Figure 3)41. Acupoints infuse meridians, qi, and blood and connect the body surface and zang-fu organs42,43. Therefore, in the treatment of infant rats with cerebral palsy, selective spinal manipulation is used to operate on the acupoints on the back, activating the local acupoints, unblocking the meridians, qi, and blood, and the acupoints are in a heat-sensitive state44. After the operation and treatment, a certain temperature can still be maintained.
Using the twirling method on the right limb can relieve the spasm of local muscle tissue, decrease local meridians, and improve local dysfunction. Finally, we choose to perform massage therapy on the head because the head is the head of all Yang, where the essence qi and blood of the five zang-fu organs converge. The treatment of this part can reconcile Yin and Yang and achieve the effect of overall rehabilitation16,18. In conclusion, selective spinal manipulation can effectively improve the body weight, motor function, and growth hormone of infant rats with cerebral palsy to promote growth and development.
There are still some important points to pay attention to during the operation. First, it should be noted that the infant rat should adapt to the palm temperature and the agitation condition is stable before treatment. Operators need to use disposable gloves to avoid the abandonment of female rats due to abnormalities after direct contact with the skin of operators. Before the operation, the operators must be uniformly trained in their manipulations and can operate after passing the assessment. Here, we used a specific manipulation tester to test to ensure the standardization of the operation. Because we observed weight changes during growth in infant rats with cerebral palsy, the manipulation must be checked regularly. When pressing and kneading, the downward pressure should be vertical, and the force should be from light to heavy, stable, and continuous so that the stimulation sensation can fully reach the body's deep tissue. Do not use swift violence. The range of kneading should be small and large, and the force should be light and heavy first. The range of kneading should be small and large, and the force should be light and heavy first. The operation of the kneading method needs to reach the muscle and ligament layer. The manual force was 1.77 ± 0.54 N when monitored by the manual tester. In contrast, only the skin surface is treated when rubbing is performed. In addition, the skin of infant rats with cerebral palsy is delicate, and improper operation can lead to skin damage.
As an external treatment of traditional Chinese medicine, selective spinal manipulation is an effective supplement to the existing treatment methods of cerebral palsy, and it is a diversified treatment method of cerebral palsy. It considers both overall and local treatment, which can give children with cerebral palsy a comprehensive rehabilitation and can bring better economic benefits and social effects to the family and society of children. However, this technique also has some shortcomings because the operators have different understandings and mastery of the technique, the degree of unity of the technique is low, the deviation of the therapeutic effect may occur, and the curative effect is not exact. The next step is to make the manipulation reach a uniform standard. Although we have confirmed that selective spinal manipulation positively affects the growth and development of infant rats with cerebral palsy and can play a therapeutic role by regulating inflammatory homeostasis, its mechanism of action on neuroplasticity and white matter regeneration still needs to be studied.
Because of the certainty of curative effect and scientific theory of selective spinal manipulation, based on the operation theory of selective spinal manipulation, this set of manipulations can be extended to treat other pediatric diseases, such as children's growth retardation, language development retardation, etc. Providing research ideas enriches the treatment system for most medical workers and researchers. Therefore, this article is conducive to popularizing and applying the technique.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work has been supported by the General Program of National Natural Science Foundation of China (82374614), the Major Biomedical Project of the Science and Technology Department of Yunnan Province (202102AA100016), the Joint Major Project of Applied Basic Research of the Department of Science and Technology of Yunnan Province — Yunnan University of Traditional Chinese Medicine (201901AI070004), Supported by the Key Laboratory of Acupuncture, Moxibustion, and Massage for Prevention and Treatment of Encephalopathy in Colleges and Universities of Yunnan Province (2019YGZ04), Department of Science and Technology of Yunnan Province — Youth Project of Basic Research Program of Yunnan Province (202101AU070002), Graduate Program of Science Research Fund of Education Department of Yunnan Province, (2023Y0433); Scientific Research Foundation of Education Department of Yunnan Province, (2023Y0462).
Materials
| 96-well plates | Beijing Lanjieke Biotechnology Co., LTD | 11510 | Determination of protein concentration |
| Anti-beta Actin antibody | Abacm | Ab8227 | Dilution: 1/2000 |
| Anti-Growth Hormone antibody | Abacm | Ab126882 | 0.5 µg (0.5 ng/lane) |
| Anti-Growth Hormone receptor antibody | Abacm | Ab202964 | Dilution: 1/1000 |
| Basic operating microscope | Shanghai YuYAN Scientific Instrument Co. LTD | SM-101 | The common carotid artery was isolated under microscope during modeling |
| BCA developer | Biyuntian Biological Engineering Co., LTD | P0010 | Determination of protein concentration |
| Chemiluminescence imaging system | Shanghai Qinxiang Scientific Instrument Co., LTD | 100240073 | Protein banding imaging |
| Direct-load Color Prestained Marker | Beijing Kangrunchengye Biotechnology Co., LTD (GenStar) | M221 | Western Blot |
| DK-30Automatic snow ice maker | Henan Brothers instrument equipment Co., LTD | SHDX0023 | Ice-making |
| ECL luminescent substrate kit | Beijing Lanjieke Biotechnology Co., LTD | BL520B | Convert latent images in exposed film into visible images |
| Electric-heated thermostatic water bath | TAISITE INSTRUMENT | DK-98-II | The young rats were resuscitated after modeling |
| Electronic scales | Kunshan YoukeWEI ELECTRONIC Technology Co. LTD | CN-LQC10002 | The body weight of the young rats was measured |
| German small white electric coagulation pen hemostat | Haohang | L55×W125×H37 | It was used to coagulate the left common carotid artery |
| Glove | Jiangsu YANGzi LiDE Medical Device Co. LTD | Q/320684 YZYL001-2017 | For massage operation |
| Glycine | Beijing Soleibao Technology Co., Ltd. | Cat#G8200 | Electrophoretic solution, Configure the transfer fluid |
| Goat Anti-RabbitIgG H&L (HRP) | Abacm | Ab6721 | Dilution: 1/10000 |
| Intelligent laboratory ultra-pure water machine | Chongqing huachuag water treatment engineering co.,LTD | N/A | Filtration (15 L) |
| Isoflurane | Shandong Ante Animal Husbandry Technology Co. LTD | 15198 | Anesthesia was maintained by induction in young rats |
| LinkIR | FOTIRC | V1.3.2.134 | Infrared image analysis software |
| Low temperature high speed tissue grinder | Wuhan Servicebio technology CO.,LTD | SKZ3F20200191 | Tissue grinding |
| Methanol | Guangdong Guanghua Sci-Tech Co., Ltd | 20220519 | Configure the transfer fluid |
| Mini-PROTEAN Tetra | Bole Life Medical Products (Shanghai) Co., Ltd | 552BR 233193 | Electrophoresis |
| Multiskan Spectrum Microplate Spectrophotometer | TECAN | Spark | The absorbance and concentration of tissue protein were detected |
| Pressure-sensing smart gloves | Jinan Super Sense Intelligent Technology Co. LTD | Miiglove | It is used to measure the manipulative strength of the operator |
| PVDF membrane | MerckMillipore Corporation | IPVH00010 | Western Blot |
| Refrigerated centrifuge | Hettich Precision Technology (Zhuhai) Co., LTD | MIKRO 220R | Centrifuge |
| Research three-in-one thermal imager | FOTIRC | 226S (384 x 288) | Temperature measurement |
| RIPA lysate | Beijing Solaibao Technology Co., LTD (Solarbio) | R0010 | Lytic tissue |
| SHA-CA digital display water bath thermostatic oscillator | Changzhou Aohua Instrument Co. LTD | SHA-CA | Young rats were used in hypoxia |
| Six-rat fatigue rotarod apparatus | Shanghai Duoyi Industry Co., LTD | DO01104RT703 | CP motor function was detected |
| Skim milk powder | Guangzhou Saiguo Biotechnology Co., LTD (BIOFROXX) | 1172GR500 | Confining liquid |
| Surgical plate | Shanghai YuYAN Scientific Instrument Co. LTD | 51002 | The model operating table was established in young rats |
| TS-200 Orbital shaker | Haimen Qilin Bell Manufacturing Co., Ltd. | TS-8S | Gel fixation |
| Tween 80 | MedChemExpress | HY-Y1819 | Configure TBST |
| ZS-MV Portable anesthesia machine | ZHONGSHI SCIENCE &TECHNOLOGY | ZS-MV-I | Anesthesia was induced and maintained in experimental animals |
Riferimenti
- Paul, S., Nahar, A., Bhagawati, M., Kunwar, A. J. A review on recent advances of cerebral palsy. Oxidative Medicine and Cellular Longevity. , 2622310 (2022).
- Şimşek, T. T., Tuç, G. Examination of the relation between body mass index, functional level and health-related quality of life in children with cerebral palsy. Turk Pediatri Arsivi. 49 (2), 130-137 (2014).
- Dahlseng, M. O., et al. Feeding problems, growth and nutritional status in children with cerebral palsy. Acta Paediatrica. 101 (1), 92-98 (2012).
- Fogarasi, A., et al. The purple n study: Objective and perceived nutritional status in children and adolescents with cerebral palsy. Disability and Rehabilitation. 44 (22), 6668-6675 (2022).
- Xiaojie, L., et al. Epidemiological characteristics of cerebral palsy in twelve province in China. Chinese Journal of Practical Pediatrics Clinical. 33 (5), 378-383 (2018).
- Kakooza-Mwesige, A., et al. Prevalence of cerebral palsy in Uganda: A population-based study. The Lancet. Global Health. 5 (12), e1275-1282 (2017).
- Korzeniewski, S. J., Slaughter, J., Lenski, M., Haak, P., Paneth, N. The complex aetiology of cerebral palsy. Nature Reviews. Neurology. 14 (9), 528-543 (2018).
- Vargus-Adams, J. N., Martin, L. K. Domains of importance for parents, medical professionals and youth with cerebral palsy considering treatment outcomes. Child: Care, Health and Development. 37 (2), 276-281 (2011).
- Koy, A., et al. Quality of life after deep brain stimulation of pediatric patients with dyskinetic cerebral palsy: A prospective, single-arm, multicenter study with a subsequent randomized double-blind crossover (stim-cp). Movement Disorders. 37 (4), 799-811 (2022).
- Pool, D., Valentine, J., Taylor, N. F., Bear, N., Elliott, C. Locomotor and robotic assistive gait training for children with cerebral palsy. Developmental Medicine and Child Neurology. 63 (3), 328-335 (2021).
- Li, J., et al. Evaluating the effects of 5-hz repetitive transcranial magnetic stimulation with and without wrist-ankle acupuncture on improving spasticity and motor function in children with cerebral palsy: A randomized controlled trial. Frontiers In Neuroscience. 15, 771064 (2021).
- Chen, K., Shu, S., Yang, M., Zhong, S., Xu, F. Meridian acupuncture plus massage for children with spastic cerebral palsy. American Journal of Translational Research. 13 (6), 6415-6422 (2021).
- Gao, J., et al. Rehabilitation with a combination of scalp acupuncture and exercise therapy in spastic cerebral palsy. Complementary Therapies in Clinical Practice. 35, 296-300 (2019).
- Chen, Z., et al. Effects of traditional Chinese medicine combined with modern rehabilitation therapies on motor function in children with cerebral palsy: A systematic review and meta-analysis. Frontiers In Neuroscience. 17, 1097477 (2023).
- Zhang, Y., et al. Tuina massage improves cognitive functions of hypoxic-ischemic neonatal rats by regulating genome-wide DNA hydroxymethylation levels. Evidence-Based Complementary and Alternative Medicine: ECAM. 2019, 1282085 (2019).
- Zhang, P., et al. Chinese tuina protects against neonatal hypoxia-ischemia through inhibiting the neuroinflammatory reaction. Neural Plasticity. 2020, 8828826 (2020).
- Niu, F., et al. Spinal tuina improves cognitive impairment in cerebral palsy rats through inhibiting pyroptosis induced by nlrp3 and caspase-1. Evidence-Based Complementary and Alternative Medicine: ECAM. 2021, 1028909 (2021).
- Bowen, Z., Guangyi, X., Qian, Z., Xueping, H., Xiantao, T. Time-dose effect of spinal manipulation on growth and motor function in infant rat with cerebral palsy. Shi Zhen National Medicine and National Medicine. 28 (09), 2274-2277 (2017).
- Yang, L. J., Cui, H. Olig2 knockdown alleviates hypoxic-ischemic brain damage in newborn rats. Histology and Histopathology. 36 (6), 675-684 (2021).
- Martins, L. A., Schiavo, A., Xavier, L. L., Mestriner, R. G. The foot fault scoring system to assess skilled walking in rodents: A reliability study. Frontiers in Behavioral Neuroscience. 16, 892010 (2022).
- Li, S., et al. Plxna2 knockdown promotes m2 microglia polarization through mtor/stat3 signaling to improve functional recovery in rats after cerebral ischemia/reperfusion injury. Experimental Neurology. 346, 113854 (2021).
- Wei, W., et al. Neuroprotective effect of verbascoside on hypoxic-ischemic brain damage in neonatal rat. Neuroscience Letters. 711, 134415 (2019).
- Taiyi, W., Ziyu, H. . Anatomical Atlas of Experimental Animals in Chinese and English. , (2000).
- Na, X. . Study on the sensitization rule of dorsal acupoints in children with spastic cerebral palsy based on infrared thermal imaging technology and the effect of massage intervention. , (2021).
- China Association for Acupuncture and Moxibustion. Names and localization of commonly used acupoints in laboratory animals Part 2: Rats. Acupuncture Research. 46 (04), 351-352 (2021).
- Yinghua, S., Xiantao, T. Effect of spinal manipulation on the expression of growth hormone and its receptor protein in hypothalamus of infant rat with cerebral palsy. Sichuan Traditional Chinese Medicine. 39 (02), 55-59 (2021).
- Qi, H., et al. Effect of spinal manipulation on learning and memory in infant rat with cerebral palsy and its mechanism. Guide to Traditional Chinese Medicine. 24 (11), 36-39 (2018).
- Tai, W. -. C., Burke, K. A., Dominguez, J. F., Gundamraj, L., Turman, J. E. Growth deficits in a postnatal day 3 rat model of hypoxic-ischemic brain injury. Behavioural Brain Research. 202 (1), 40-49 (2009).
- Huang, L., et al. Animal models of hypoxic-ischemic encephalopathy: Optimal choices for the best outcomes. Reviews In the Neurosciences. 28 (1), 31-43 (2017).
- Lyu, H., et al. A new hypoxic-ischemic encephalopathy model in neonatal rats. Heliyon. 7 (12), e08646 (2021).
- Hegazi, M. A., et al. Growth hormone/insulin-like growth factor-1 axis: A possible non-nutritional factor for growth retardation in children with cerebral palsy. Jornal de Pediatria. 88 (3), 267-274 (2012).
- Boboc, I. K. S., et al. A preclinical systematic review and meta-analysis of behavior testing in rat models of ischemic stroke. Life. 13 (2), 567 (2023).
- Lubrich, C., Giesler, P., Kipp, M. Motor behavioral deficits in the cuprizone model: Validity of the rotarod test paradigm. International Journal of Molecular Sciences. 23 (19), 11342 (2022).
- Tan, Y., et al. Vof-16 knockout improves the recovery from hypoxic-ischemic brain damage of neonatal rats. Brain Research. 1748, 147070 (2020).
- Xiantao, T., Pengyue, Z., Xinghe, Z. Construction and application of selective spinal manipulation in the treatment of children with cerebral palsy. China’s Scientific and Technological Achievements. 23 (9), 1-2 (2022).
- Matos, L. C., Machado, J., Greten, H. J., Monteiro, F. J. Changes of skin electrical potential in acupoints from ren mai and du mai conduits during qigong practice: Documentation of a clinical phenomenon. Journal of Bodywork and Movement Therapies. 23 (4), 713-720 (2019).
- Levkovets, I. L., Kiryanova, V. V. Systemic and pathogenetic approach: A new look at traditional Chinese medicine. Voprosy Kurortologii, Fizioterapii, I Lechebnoi Fizicheskoi Kultury. 99 (1), 80-88 (2022).
- Efferth, T., Xu, A. -. L., Lee, D. Y. W. Combining the wisdoms of traditional medicine with cutting-edge science and technology at the forefront of medical sciences. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology. 64, 153078 (2019).
- Zhang, Z., et al. Correlated sensory and sympathetic innervation between the acupoint bl23 and kidney in the rat. Frontiers In Integrative Neuroscience. 14, 616778 (2020).
- Zhu, X., et al. Effects of electroacupuncture at st25 and bl25 in a sennae-induced rat model of diarrhoea-predominant irritable bowel syndrome. Acupuncture In Medicine: Journal of the British Medical Acupuncture Society. 35 (3), 216-223 (2017).
- Gimarc, K., Yandow, S., Browd, S., Leibow, C., Pham, K. Combined selective dorsal rhizotomy and single-event multilevel surgery in a child with spastic diplegic cerebral palsy: A case report. Pediatric Neurosurgery. 56 (6), 578-583 (2021).
- Zhang, W. -. B., Wang, Y. -. P., Li, H. -. Y. Analysis on correlation between meridians and viscera in book the yellow emperor’s internal classic. Acupuncture Research. 43 (7), 424-429 (2018).
- Wang, Y. -. P., Hou, X. -. S. Discussion on the classification of acupoints. Chinese Acupuncture & Moxibustion. 39 (10), 1069-1072 (2019).
- Chen, R. -. X., Kang, M. -. F. Clinical application of acupoint heat-sensitization. Chinese Acupuncture & Moxibustion. 27 (3), 199-202 (2007).

.
