An Automated Culture System for Maintaining and Differentiating Human-Induced Pluripotent Stem Cells
Summary
Here, we present a protocol for an automated cell culture system. This automated culture system reduces labor and benefits the users, including researchers unfamiliar with handling induced pluripotent stem (iPS) cells, from the maintenance of iPS cells to differentiation into various types of cells.
Abstract
Human induced pluripotent stem cells (hiPSCs) with infinite self-proliferating ability have been expected to have applications in numerous fields, including the elucidation of rare disease pathologies, the development of new medicines, and regenerative medicine aiming to restore damaged organs. Despite this, the social implementation of hiPSCs is still limited. This is partly because of the difficulty of reproducing differentiation in culture, even with advanced knowledge and sophisticated technical skills, due to the high sensitivity of iPSCs to minute environmental changes. The application of an automated culture system can solve this issue. Experiments with high reproducibility independent of a researcher’s skill can be expected according to a shared procedure across various institutes. Although several automated culture systems that can maintain iPSC cultures and induce differentiation have been developed previously, these systems are heavy, large, and costly because they make use of humanized, multi-articulated robotic arms. To improve on the above issues, we developed a new system using a simple x-y-z axis slide rail system, allowing it to be more compact, lighter, and cheaper. Furthermore, the user can easily modify parameters in the new system to develop new handling tasks. Once a task is established, all the user needs to do is prepare the iPSC, supply the reagents and consumables needed for the desired task in advance, select the task number, and specify the time. We confirmed that the system could maintain iPSCs in an undifferentiated state through several passages without feeder cells and differentiate into various cell types, including cardiomyocytes, hepatocytes, neural progenitors, and keratinocytes. The system will enable highly reproducible experiments across institutions without the need for skilled researchers and will facilitate the social implementation of hiPSCs in a wider range of research fields by diminishing the obstacles for new entries.
Introduction
This article aims to provide actual and detailed handling procedures for an automated culture system for human induced pluripotent stem cells (iPSC), which we produced by collaborating with a company, and to show representative results.
Since the publication of the article in 2007, iPSC has been attracting attention all over the world1. Due to its greatest feature of being able to differentiate into any type of somatic cell, it is expected to be applied in various fields such as regenerative medicine, elucidating the causes of intractable diseases, and developing new therapeutic drugs2,3. In addition, using human iPSC-derived somatic cells could reduce animal experiments, which are subject to significant ethical restrictions. Although numerous homogeneous iPSCs are constantly required to research new methods with iPSCs, it is too laborious to manage them. Moreover, handling iPSC is difficult because of its high sensitivity, even to subtle cultural and environmental changes.
To solve this problem, automated culture systems are expected to perform tasks instead of humans. Some groups have developed a few automated human pluripotent stem cell culture systems for cell maintenance and differentiation and published their achievements4,5,6. These systems equip multiarticulated robotic arm(s). Robotic arms have not only merit in that they highly mimic human arm movements but also demerit in that they require higher cost(s) for the arm(s), larger and heavier system packaging, and time-consuming education efforts by the engineers to obtain the aimed movements7,8. In order to make it easier to introduce the apparatus to more research facilities at the points of economic, space, and human resource consumption, we have developed a novel automated culture system for the maintenance and differentiation of iPSC into various cell types9.
Our rationale for the new system was to adopt an X-Y-Z axis rail system instead of multiarticulated robotic arms9. To replace the complex hand-like functions of robotic arms, we applied a new idea to this system, which can automatically change three types of specific functional arm tips. Here, we also indicate how users can easily make task schedules with simple orders on software because of the lack of requirements for engineers' contributions throughout the process.
One of the robotic culture systems has demonstrated the making of embryoid bodies using 96-well plates as 3D cell aggregates for differentiation4. The system reported here cannot handle 96-well plates. One achieved the current good manufacturing practice (cGMP) grade using a cell line, although it was not a human pluripotent stem cell5. The automated culture system detailed here has now been developed with the specific aim of helping laboratory experiments (Figure 1). However, it has enough systems to keep clean levels equivalent to a level IV safety cabinet.
Protocol
The Ethics Committee of the Kansai Medical University approved the generation and use of the healthy volunteer-derived iPSCs named KMUR001 (approval No. 2020197). The donor, who was openly recruited, provided formal informed consent and agreed with the scientific usage of the cells.
NOTE: The current interface (the special software named "ccssHMI" running under the Windows XP operating system) is the fundamental operation screen. Under the aforementioned interface, a series of tabs are arranged, allowing users to initiate various operations.
1. Loading operations
- Click the Loading button on the software's top screen. Click the Loading Preparation Start button.
- Place the dish(es) or plate(s) to be entered into the apparatus in position in the apparatus.
NOTE: Necessary information for dish identification should be written on each lid. - Manually close the front sliding window and push the mechanical safety-confirmation button.
- Select the type and quantity of dish(es) or plate(s) in the software.
- Click the Loading Preparation Completed button. Click the Loading Start button.
- After a dish is uploaded into the system, select the information on the dish, such as with iPS cells or without iPS cells, on the software. Register notes about each dish in the software.
- Click the Registration button at the end to complete the loading operation.
2. Unloading operation
- Click the Unload button on the software's top screen. Select the dish(es) to be removed in the software.
- After selecting the dish(es), click the Unloading Preparation Start button. Click the Unloading Start button.
- After the dish(es) have been transferred from the incubator to the workbench in the system, press the Dish Removal button.
- Manually open the front sliding window and take out the dish(es). Manually close the front sliding window and push the mechanical safety-confirmation button.
3. Supplement of consumables: pipettes, tubes, and medium
- To replenish consumables such as pipettes, tubes, and medium, click the Consumables button on the software's top screen, then select the item to be replenished.
- Pipettes
- Click the Pipette button. Select the Replenish button.
- Select a rack that the user wants to replenish on the software. Click the Replenish Start button.
- After confirming that the lid of the pipette storage area on the workbench is opened, manually open the front sliding window and replenish the pipettes as needed.
- Manually close the front sliding window and push the mechanical safety-confirmation button. Click the Replenish Completed button.
- Click the Replenishment Setup button and enter the information for the replenishment, then click the Registration button.
- Click the Replenish Completed button.
- Tubes
- Click the Tube button. Select the Replenish button.
- Select a rack that the user wants to replenish on the software. Click the Replenish Start button.
- After confirming that the rack has moved to the top, click the Replenish Tube button.
- Manually open the front sliding window and replenish the tubes as needed.
- Manually close the front sliding window and push the mechanical safety-confirmation button.
- Click the Replenish Completed button.
- Click the Replenishment Setup button and enter the information for the replenishment, then click the Registration button. Click the Close button.
- Medium
- Click the Medium button. Select the Replenish button in the software.
- Select one rack from three that users want to replenish. Click the Replenish Start button.
- After confirming that the lid of the medium storage area has been opened, manually open the front sliding window and replenish the medium.
- Manually close the front sliding window and push the mechanical safety-confirmation button.
- Click the Replenish Completed button.
- Click the Replenish button and enter the information for the medium, including the name and the amount of the medium. Enter additional comments if necessary.
- Click the Registration button.
- Click the Close button.
- Pipettes
4. Task selection
- Click the Task button on the software's top screen.
- Select the Task Setting button. Select the desired task from the task list and click the Next Step button.
- Specify the date and time to perform the task, and then click the Registration button. Select a dish or plate to perform the task, and then click the Registration button.
- After reconfirming the selected task, click the Registration button. Confirm that the task has been registered on the next screen.
- If necessary, set the next task in the same way.
- Click the Start button at the end. Then, the task will start automatically at the specified date and time.
NOTE: Immediately after finishing every task, UV lights (located on two sides of the hood) automatically turn on, and after 5-30 min, in accordance with the auxiliary setting, they are turned off to keep the hood in aseptic condition. To stop, users can click the Start button. - If users want to cancel a scheduled task in advance, click the Stop button.
- After selecting the task to be aborted, click the Edit Task button.
- Click the Task Cancel button. Confirm that the task has been deleted on the next screen.
5. Check cell pictures
- Every task includes microscopic observations(taking photos) before and after the task work. Observe the progress of cell culture photographically by incorporating a microscopic photography task before or after each task.
- Select pre-set task programs, including selecting multiple specific locations of the dish or the well plate in advance for fixed-point observation to monitor the same location over time.
6. Passaging and differentiation
- Follow the steps in sections 1-5 and set the automated cell culture system to perform passaging and differentiation. The instrument settings for passaging, cardiomyocyte differentiation, hepatocyte differentiation, neural precursor cell differentiation, and keratinocyte differentiation are shown in Table 1, Table 2, Table 3, Table 4, and Table 5, respectively.
Representative Results
Maintenance of human-induced pluripotent stem cells
We used three hPSC lines (RIKEN-2F, 253G1, and KMUR001). We have optimized the maintenance protocol through daily manually performed experiments and further optimized the detailed programs through the seven preliminary experiments performed by the system. For example, shear stresses caused by the liquid speeds of the spit flow from different pipets handled by humans and the system are quite different; therefore, we optimized the time length of the enzymatic digestion and the number of pipetting for cell dispersion by the system.
Human induced pluripotent stem cells were maintained on a 10 cm dish coated with 0.5 µg/cm2 cell culture matrix with hiPSC culture medium (e.g., Neutristem or StemFit AK02N). For passage, the system was programmed to wash hiPSC three times with 5 mL of phosphate buffer saline without calcium (PBS[-]), then treat with 3 mL of TrypLE express enzyme supplemented with 10 µM of the Rho kinase inhibitor (Y-27632) for 15 min at 37 °C. After that, the system added 9 mL of PBS(-) with 0.15% bovine serum albumin fraction V (BSA) to the plate and performed two sets of motion that aspirated 10 mL of cell-containing liquid and dispensed the liquid from the pipette tip in a zig-zag motion across the top of the plate, which was tilted 20° closer to the plate surface, and repeated the above sequence of operations with the plate tilted at 20° on the opposite side. Then, the system collected the cell-containing medium in a 15-mL tube and capped it, after which it was centrifuged at 115 x g for 5 min to deposit the cells. Thereafter, the system aspirated the supernatant and dispersed the cell pellet into single cells using 10 mL of culture medium with 10 µM Y-27632 by pipetting 10 times. The system transferred 1 mL of trypan blue solution into a new 15 mL tube, then added 1 mL of the cell suspension and mixed them by pipetting five times. After that, 100 µL of the Trypan blue-stained cell solution was aspirated and dispensed into the inlet window of the disposable hemocytometer in the holder. The system moved the hemocytometer holder to the microscopic observation area, and captured a photo, which was immediately analyzed by software, and the cell concentration obtained was applied to the next step. For iPSC maintenance, the system seeded the cells at a cell density of 15,000 cells/cm2 in new 10-cm diameter plastic plates coated with 0.5 µg/cm2 cell culture matrix. For differentiation experiments, the system seeded the cells at the required cell density in the medium prepared for each somatic cell type in 6-well plates coated with a cell culture matrix diluted to 1/100. Finally, the system performed cross-locking five times before transferring the plates to the incubator. Three new 10-cm plates containing iPSCs and three cell culture matrix-pre-coated plates were loaded into the system, and other necessary items were also prepared according to the respective procedures mentioned above. One cycle of maintenance culture consists of a passaging task on day 1 followed by a culture medium change once a day for 3 days. All through the experiment, this cycle was set up for five cycles. In addition, consumables were replenished in turn.
According to the ordered schedule, five cycles of maintenance culture were successfully performed as planned by the system. From the cell photographs taken automatically during each task, it was confirmed that the cells proliferated smoothly over time. The cell expansion (n = 3) was calculated and shown in Figure 2. To confirm whether the undifferentiated state of iPSCs remains unchanged even after maintenance culture in the automated culture system, immunocytometoric analysis (Oct3/4 and SSEA4) was performed using the remaining samples at each passaging (Figure 3A). Furthermore, after five cycles, the three dishes were unloaded from the system, and immunohistochemical analysis (Oct3/4, SSEA4, and Tra1-81) was performed as well (Figure 3B). Both analyses using fluorescent immunostaining showed that the undifferentiated state of iPS cells was preserved. Karyotyping of iPSCs was also performed before and after maintenance culture in an automated culture system. Although an abnormality was observed in an allele of chromosome 17 before the start of the maintenance culture, no particular change was observed by the system after 5 maintenance cultures, including the abnormality (Figure 3C). The settings used for maintenance of iPSCs are summarized in Table 1.
Differentiation
Cardiomyocytes
The differentiation protocol from a previous publication was followed here10. The system seeded undifferentiated hiPSCs (KMUR001) at a density of 30,000 cells/cm2 on cell culture matrix-coated 6-well plates in hiPSC culture medium supplemented with 10 µM of Y-27632. The system changed the medium to human pluripotent stem cell medium with 6 µM of the GSK-3ß inhibitor CHIR-99021, 20 ng/mL Activin A, and 10 ng/mL BMP4 after 3 days (differentiation day 1). On differentiation day 3, the system changed to CDM3 medium: RPMI 1640, 500 µg/mL recombinant human albumin, and 213 µg/mL L-ascorbic acid 2-phosphate with 2 µM Wnt-C59. On differentiation day 5, the system changed to fresh CDM3 medium; thereafter, it repeated changing to fresh CDM3 medium every other day. The settings used for cardiomyocyte differentiation of iPSCs are summarized in Table 2.
Hepatocytes
The differentiation protocol from a previous publication was followed here11. The system seeded undifferentiated hiPSCs (RIKEN2F) at a density of 25,000 cells/cm2 on cell culture matrix-coated 6-well plates in hiPSC culture medium supplemented with 10 µM of Y-27632. After 2 days (differentiation day 1), the system changed the medium to RPMI-1640 plus 2% B27 Minus Insulin (RPMI-B27) containing 100 ng/mL Activin A, 6 µM CHIR-99021, and 1% glutamine supplement (e.g., GlutaMAX) for 24 h. On differentiation day 2, we changed the medium to RPMI-B27 with 50 ng/mL Activin A. On differentiation day 5, the system changed the medium to RPMI-B27, containing 1% glutamine supplement and 10 ng/mL BMP-4. On differentiation day 9, the system changed the medium to hepatocyte maturation medium: Leibovitz's L-15 medium containing 8.3% tryptose phosphate broth, 10 µM hydrocortisone 21-hemisuccinate, 50 µg/mL sodium L-ascorbate, 100 nM dexamethasone, 0.58% insulin-transferrin-selenium, 2 mM glutamine supplement, 8.3% fetal bovine serum, and 100 nM rac-1,2-dihexadecylglycerol. Thereafter, the system repeated changing the medium to a fresh medium every other day. The settings used for hepatocyte differentiation of iPSCs are summarized in Table 3.
Neuronal precursor cells
The differentiation protocol from a previous publication was followed here12. The system seeded undifferentiated hiPSCs (RIKEN2F) at a density of 25,000 cells/cm2 on basement membrane matrix-coated 6-well plates in Dulbecco's modified Eagle medium (DMEM)/F12 and neurobasal medium (1:1) supplemented with 10% Knockout serum replacement, 0.1 mM non-essential amino acids, 1 mM glutamine supplement with 10 µM TGF-beta receptor inhibitor (SB431542), 10 µM BMP signal inhibitor (dorsomorphin), and 10 µM Y-27632 (differentiation day 1). On differentiation day 5, the system changed the medium to DMEM/F12 and Neurobasal medium 1:1 supplemented with 0.1mM non-essential amino acids, 1 mM glutamine supplement, 1% N-2 supplement, 1% B-27 supplement, 50 µg/mL ascorbic acid 2-phosphate, 10 µM SB431542, and 10 µM dorsomorphin. On differentiation day 9, the system changed the medium to DMEM/F12 and neurobasal medium 1:1 supplemented with 0.1 mM non-essential amino acids, 1 mM glutamine supplement, 1% N-2 supplement, 1% B-27 supplement, 50 µg/mL ascorbic acid 2-phosphate, and 1 µM all-trans retinoic acid. On differentiation day 13-16, the system changed the medium every day with DMEM/F12 and neurobasal medium 1:1 supplemented with 0.1 mM non-essential amino acids, 1 mM glutamine supplement, 1% N-2 supplement, 1% B-27 supplement, 50 µg/mL ascorbic acid 2-phosphate, and 10 ng/mL bFGF and 10 ng/mL EGF. The settings used for neural precursor cell differentiation of iPSCs are summarized in Table 4.
Keratinocytes
The differentiation protocol from a previous publication was followed here13. The system seeded undifferentiated hiPSCs (253G1) at a density of 15,000 cells/cm2 in cell culture matrix-coated 6-well plates in hiPSC culture medium with 10 µM Y-27632. After 2 days later (differentiation day 1), the system changed the medium to Dulbecco's modified Eagle medium (DMEM)/F12 and Neurobasal medium (1:1) with 0.1 mM non-essential amino acids, 1 mM glutamine, 55 µM 2-mercaptoethanol, 1% N-2 supplement, 2% B-27 supplement, 50 µg/mL ascorbic acid 2-phosphate, 0.05% bovine serum albumin, and 100 ng/mL FGF-basic. On differentiation day 3, and thereafter, the medium was changed to human keratinocyte medium (without supplement for day 3 and with a supplement on/after day 5) with the addition of 0.5 µg/mL hydrocortisone, 1 µM all-trans retinoic acid, 25 ng/mL hBMP-4, 2.4 µg/mL adenine, 1.37 ng/mL triiodothyronine, 0.3 mM ascorbic acid 2-phosphate, and 2 µM forskolin, every 2 days by the system. The settings used for keratinocyte differentiation of iPSCs are summarized in Table 5.
As mentioned above, the entire process was performed using only automated culture equipment, from seeding iPS cells to the subsequent series of differentiation protocols. The expression of characteristic markers in each cell was evaluated (Figure 4). It was shown that differentiation could be induced using only an automated culture system.
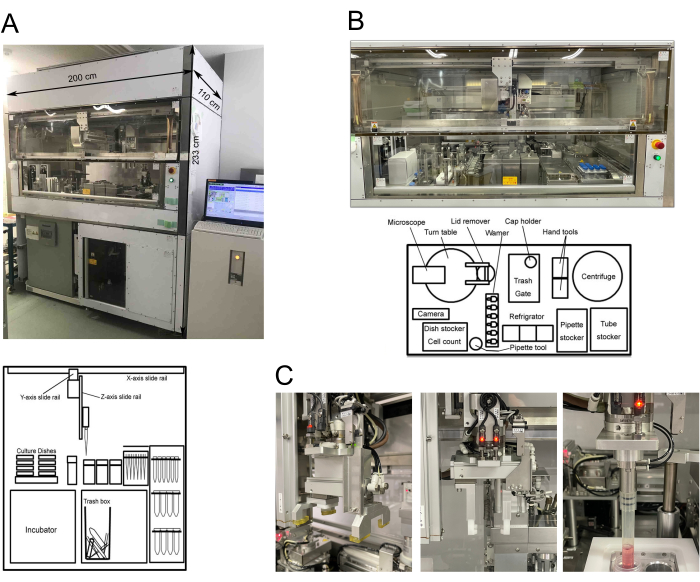
Figure 1: Summary of the automated culture system. (A) Picture of the system showing size and layout sketch 1. (B) Picture of the workbench and layout sketch 2. (C) Hand tools: dish, tube, and pipette. This figure has been modified with permission from Bando et al.9. Please click here to view a larger version of this figure.
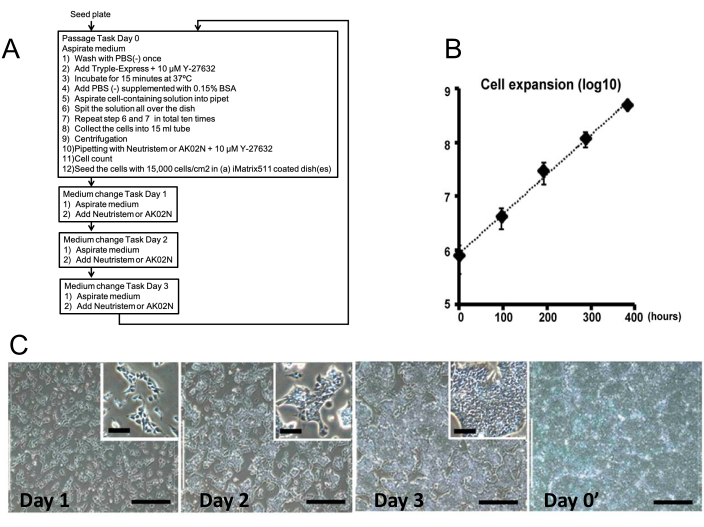
Figure 2: Passaging task and cell growth. (A) The square illustrates each process. (B) iPSC expansion calculated using every automated cell count in the three independent experiments. (C) Representative images captured automatically from passage to passage. Scale bars = 400 µm and 100 µm (inset). This figure has been modified with permission from Bando et al.9. Please click here to view a larger version of this figure.
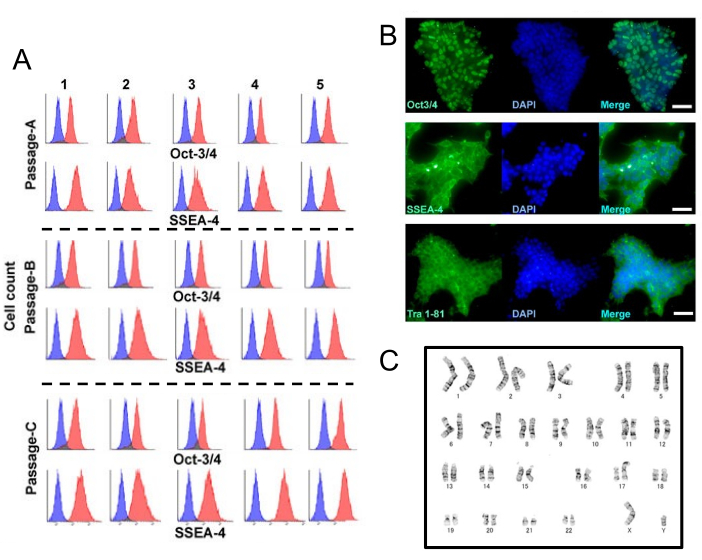
Figure 3: Automated long-term maintenance of iPSC. (A) All immunocytometoric analyses of the three independent experiments for Oct-3/4 and SSEA-4. Each blue histogram indicates no primary antibody control. Each red histogram represents the indicated antigen-specific signal. (B) Representative images of immunohistochemically stained cells for Oct-3/4, SSEA-4, and Tra 1-81. Scale bars = 50 µm. (C) Karyotype of the RIKEN2F-iPSC after the fifth passage. This figure has been modified with permission from Bando et al.9. Please click here to view a larger version of this figure.
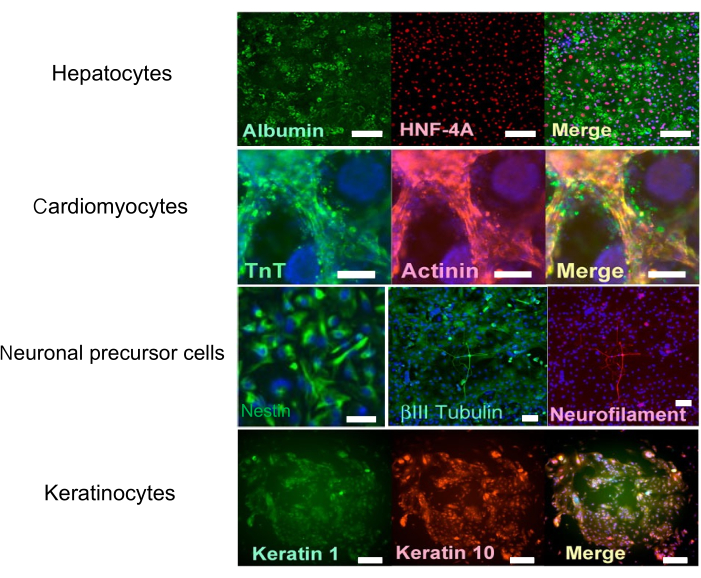
Figure 4: Immunohistochemical staining. Immunohistochemical pictures of hepatocytes (Scale bars = 100 µm), cardiomyocytes (Scale bars = 100 µm), neuronal progenitor cells (Scale bars = 100 µm), and keratinocytes (Scale bars = 200 µm). This figure has been modified with permission from Bando et al.9. Please click here to view a larger version of this figure.
Table 1: Passage preparations and system settings. Please click here to download this Table.
Table 2: Cardiomyocyte differentiation preparations and system settings. Please click here to download this Table.
Table 3: Hepatocyte differentiation preparations and system settings. Please click here to download this Table.
Table 4: Neural precursor cell differentiation preparations and system settings. Please click here to download this Table.
Table 5: Keratinocyte differentiation preparations and system settings. Please click here to download this Table.
Discussion
A critical step in the protocol is that if a user finds any faults, click the cancel, stop, or reset button anytime and start over from the first step. The software can avoid human mistakes, including double booking, opening doors while the system tasks are active, and a lack of replenishment. Another critical point for successful and efficient differentiation to the desired somatic cell is the proper selection of pluripotent stem cell lines because each pluripotent stem cell has an uncontrollable bias in its differentiation properties14,15.
This automated culture system for induced pluripotent stem cells can maintain and differentiate them into various somatic cell types. The size of this system is 200 cm (width), 110 cm (depth), and 233 cm (height), with a total weight of 1.2 tons. The incubator built into the device can simultaneously hold thirty-six 10-cm dishes and nine multiwell plates.
Improvements over previous models are that first, as a handling arm, the X-Y-Z-axis rail system was newly converted from a 6-axis articulated arm, which works by changing the tip tool of the arm to three different parts for dishes, tubes, and pipettes. The arm is suspended from the ceiling of the system and moves along the X-Y-Z axis. Second, a cell-counting system was introduced for feeder-free maintenance cultures and differentiation induction protocols that are performed continuously from the passage. After mixing the single-cell suspension solution with the trypan blue solution, it was transferred to a disposable hemocytometer for microscopic observation and imaging. The software automatically analyzes the obtained image to count the number of live cells and calculate the volume required to seed the required number of cells. The accuracy of cell counts obtained by this image analysis was consistent with that obtained manually.
The X-Y-Z-axes rail system was applied to handle each task quicker than the previous version (multi-axes articulated arm). With this automated culture system, five continuous passages were performed for 16 days, and iPSCs were expanded 625 ± 93 fold. Although the proof of endurance for the time of cell maintenance without feeder cells was shorter than our previous demonstration with feeder cells, the cell expansion was more efficient compared with the previous demonstration6. The shortened task time seemed to contribute to preventing cell damage from drying during the tasks.
Furthermore, differentiation tasks from maintenance culture to differentiation into cardiomyocytes, hepatocytes, neuronal precursor cells, and keratinocytes were performed without human intervention. After a series of these courses, it was confirmed by fluorescence immunostaining that the cells obtained in the automated culture system alone had differentiated into the desired cells. Therefore, by sharing a task program and using this system, researchers who are not familiar with iPS cell management can obtain iPSC-derived somatic cells for their own research. Moreover, differences in reproducibility between researchers or facilities could be eliminated as much as possible, and it could assure that cells of homogeneous quality are obtained every time.
By adopting a new rail system, the equipment could be downsized and reduced in weight to 1.2 tons, ~200 kg lighter than before. Furthermore, parts and program setting costs could be reduced by approximately $10,000 US dollars. The maintenance and programming costs were estimated to decline by 13%. In order to motivate more researchers to enter iPS cell-related research easily, it is also important to keep the equipment costs low6. We hope that current and next-generation automatic culture systems will be one of the contributions to helping provide iPSCs and iPSC-derived differentiated cells of more uniform quality.
This system’s major limitations are the refrigerator’s limited capacity for tentative storage of cultures and its weakness in handling small amounts (<100 µL) of liquid. Furthermore, the lack of sophisticated artificial intelligence disables automatic optimization according to the on-time cell condition. The minor limitation is the requirement of special pipette tips provided by the system manufacturers4,5. We are developing the next generation of culture systems that will be able to handle small amounts of liquid for medium preparations and include a feedback optimization system based on high-performance artificial intelligence (system learning). Our collaborating manufacturer9 has enough know-how to build custom-made systems according to the users’ special needs. In addition to this, next-generation systems equipped with more general and wider functions will be on the market soon. We are also considering a subscription-based rental system to supply up-to-date systems to all users.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study was supported by a grant from the New Business Promotion Center, Panasonic Production Engineering Co., Ltd., Osaka, Japan.
Materials
| 0.15% bovine serum albumin fraction V | Fuji Film Wako Chemical Inc., Miyazaki, Japan | 9048-46-8 | |
| 1% GlutaMAX | Thermo Fisher Scientific | 35050061 | |
| 10 cm plastic plates | Corning Inc., NY, United States | 430167 | |
| 253G1 | RKEN Bioresource Research Center | HPS0002 | |
| 2-mercaptoethanol | Thermo Fisher Scientific | 21985023 | |
| Actinin mouse | Abcam | ab9465 | |
| Activin A | Nacali Tesque | 18585-81 | |
| Adenine | Thermo Fisher Scientific | A14906.30 | |
| Albumin rabbit | Dako | A0001 | |
| All-trans retinoic acid | Fuji Film Wako Chemical Inc. | 186-01114 | |
| Automated culture system | Panasonic | ||
| B-27 supplement | Thermo Fisher Scientific | 17504044 | |
| bFGF | Fuji Film Wako Chemical Inc. | 062-06661 | |
| BMP4 | Thermo Fisher Scientific | PHC9531 | |
| Bovine serum albumin | Merck | 810037 | |
| CHIR-99021 | MCE, NJ, United States #HY-10182 | 252917-06-9 | |
| Defined Keratinocyte-SFM | Thermo Fisher Scientific | 10744019 | Human keratinocyte medium |
| Dexamethasone | Merck | 266785 | |
| Dihexa | TRC, Ontario, Canada | 13071-60-8 | rac-1,2-Dihexadecylglycerol |
| Disposable hemocytometer | CountessTM Cell Counting Chamber Slides, Thermo Fisher Scientific | C10228 | |
| Dorsomorphin | Thermo Fisher Scientific | 1219168-18-9 | |
| Dulbecco’s modified Eagle medium/F12 | Fuji Film Wako Chemical Inc. | 12634010 | |
| EGF | Fuji Film Wako Chemical Inc. | 053-07751 | |
| Essential 8 | Thermo Fisher Scientific | A1517001 | Human pluripotent stem cell medium |
| Fetal bovine serum | Biowest, FL, United States | S140T | |
| FGF-basic | Nacalai Tesque Inc. | 19155-07 | |
| Forskolin | Thermo Fisher Scientific | J63292.MF | |
| Glutamine | Thermo Fisher Scientific | 25030081 | Glutamine supplement |
| Goat IgG(H+L) AlexaFluo546 | Thermo Scientific | A11056 | |
| HNF-4A goat | Santacruz | 6556 | |
| Hydrocortisone | Thermo Fisher Scientific | A16292.06 | |
| Hydrocortisone 21-hemisuccinate | Merck | H2882 | |
| iMatrix511 Silk | Nippi Inc., Tokyo, Japan | 892 021 | Cell culture matrix |
| Insulin-transferrin-selenium | Thermo Fisher Scientific | 41400045 | |
| Keratin 1 mouse | Santacruz | 376224 | |
| Keratin 10 rabbit | BioLegend | 19054 | |
| KMUR001 | Kansai Medical University | Patient-derived iPSCs | |
| Knockout serum replacement | Thermo Fisher Scientific | 10828010 | |
| L-ascorbic acid 2-phosphate | A8960, Merck | A8960 | |
| Leibovitz’s L-15 medium | Fuji Film Wako Chemical Inc. | 128-06075 | |
| Matrigel | Corning Inc. | 354277 | |
| Mouse IgG(H+L) AlexaFluo488 | Thermo Scientific | A21202 | |
| N-2 supplement | Thermo Fisher Scientific | 17502048 | |
| Nestin mouse | Santacruz | 23927 | |
| Neurobasal medium | Thermo Fisher Scientific | 21103049 | |
| Neurofilament rabbit | Chemicon | AB1987 | |
| Neutristem | Sartrius AG, Göttingen, Germany | 05-100-1A | cell culture medium |
| Oct 3/4 mouse | BD | 611202 | |
| PBS(-) | Nacalai Tesque Inc., Kyoto, Japan | 14249-24 | |
| Rabbit IgG(H+L) AlexaFluo488 | Thermo Scientific | A21206 | |
| Rabbit IgG(H+L) AlexaFluo546 | Thermo Scientific | A10040 | |
| Recombinant human albumin | A0237, Merck, Darmstadt, Germany | A9731 | |
| Rho kinase inhibitor, Y-27632 | Sellec Inc., Tokyo, Japan | 129830-38-2 | |
| RIKEN 2F | RKEN Bioresource Research Center | HPS0014 | undifferentiated hiPSCs |
| RPMI 1640 | Thermo Fisher Scientific #11875 | 12633020 | |
| SB431542 | Thermo Fisher Scientific | 301836-41-9 | |
| Sodium L-ascorbate | Merck | A4034-100G | |
| SSEA-4 mouse | Millipore | MAB4304 | |
| StemFit AK02N | Ajinomoto, Tokyo, Japan | AK02 | cell culture medium |
| TnT rabbit | Abcam | ab92546 | |
| TRA 1-81 mouse | Millipore | MAB4381 | |
| Triiodothyronine | Thermo Fisher Scientific | H34068.06 | |
| TripLETM express enzyme | Thermo Fisher Scientific, Waltham, MA, United States | 12604013 | |
| Trypan blue solution | Nacalai Tesque, Kyoto, Japan | 20577-34 | |
| Tryptose phosphate broth | Merck | T8782-500G | |
| Wnt-C59 | Bio-techne, NB, United Kingdom | 5148 | |
β  Tublin mouse Tublin mouse |
Promega | G712A |
Riferimenti
- Okita, K., et al. A more efficient method to generate integration-free human iPS cells. Nature Methods. 8 (5), 409-412 (2011).
- Tanaka, T., et al. In vitro pharmacologic testing using human induced pluripotent stem cell-derived cardiomyocytes. Biochemical and Biophysical Research Communications. 385 (4), 497-502 (2009).
- Egashira, T., et al. Disease characterization using LQTS-specific induced pluripotent stem cells. Cardiovascular Research. 95 (4), 419-429 (2012).
- Sasamata, M., et al. Establishment of a robust platform for induced pluripotent stem cell research using Maholo LabDroid. SLAS technology. 26 (5), 441-453 (2021).
- Tristan, C. A., et al. Robotic high-throughput biomanufacturing and functional differentiation of human pluripotent stem cells. Stem Cell Reports. 16 (12), 3076-3092 (2021).
- Konagaya, S., Ando, T., Yamauchi, T., Suemori, H., Iwata, H. Long-term maintenance of human induced pluripotent stem cells by automated cell culture system. Scientific Reports. 5, 16647 (2015).
- McClymont, D. W., Freemont, P. S. With all due respect to Maholo, lab automation isn’t anthropomorphic. Nature Biotechnology. 35 (4), 312-314 (2017).
- Gonzalez, F., Zalewski, J. Teaching joint-level robot programming with a new robotics software tool. Robotics. 6 (4), 41 (2017).
- Bando, K., Yamashita, H., Tsumori, M., Minoura, H., Okumura, K., Hattori, F. Compact automated culture system for human induced pluripotent stem cell maintenance and differentiation. Frontiers in Bioengineering and Biotechnology. 10, 1074990 (2022).
- Tohyama, S., et al. Glutamine oxidation is indispensable for survival of human pluripotent stem cells. Cell Metabolism. 23 (4), 663-674 (2016).
- Yamashita, H., Fukuda, K., Hattori, F. Hepatocyte-like cells derived from human pluripotent stem cells can be enriched by a combination of mitochondrial content and activated leukocyte cell adhesion molecule. JMA journal. 2 (2), 174-183 (2019).
- Shimojo, D., et al. Rapid, efficient and simple motor neuron differentiation from human pluripotent stem cells. Molecular Brain. 8 (1), 79 (2015).
- Nishimoto, R., Kodama, C., Yamashita, H., Hattori, F. Human induced pluripotent stem cell-derived keratinocyte-like cells for research on Protease-Activated Receptor 2 in nonhistaminergic cascades of atopic dermatitis. The Journal of Pharmacology and Experimental Therapeutics. 384 (2), 248-253 (2023).
- International Stem Cell Initiative. Screening ethnically diverse human embryonic stem cells identifies a chromosome 20 minimal amplicon conferring growth advantage. Nature Biotechnology. 29 (12), 1132-1144 (2011).
- Keller, A., et al. Genetic and epigenetic factors which modulate differentiation propensity in human pluripotent stem cells. Human Reproduction Update. 24 (2), 162-175 (2018).

