Isolation, In Vitro Expansion, and Characterization of Mesenchymal Stem Cells from Mouse Epididymal Adipose Tissue
Summary
The adipose tissue is an excellent source of mesenchymal stem cells. Here, we bring the step-by-step extraction, cultivation, and characterization of adipose tissue-derived stem cells (ADSCs) from Swiss mice epididymal adipose tissue.
Abstract
Mesenchymal stem cells (MSCs) have been extensively studied as a new therapeutic approach, mainly to stop exacerbated inflammation due to their potential to modulate the immune response. The MSCs are immune-privileged cells capable of surviving in immunologically incompatible allogeneic transplant recipients based on low expression of class I major histocompatibility complex (MHC) molecules and in the use of cell-based therapy for allogeneic transplant. These cells can be isolated from several tissues, the most commonly used being the bone marrow and adipose tissues. We provide an easy protocol to isolate, culture, and characterize MSCs from epididymal adipose tissue of mice. The epididymal adipose tissue is surgically excised, physically fragmented, and digested with 0.15% collagenase type II solution. Then, primary adipose tissue-derived stem (ADSCs) cells are cultured and expanded in vitro, and the phenotypic characterization is performed by flow cytometry. We also provide the steps to differentiate the ADSCs into osteogenic, adipogenic, and chondrogenic cells, followed by functional characterization of each cell lineage. The protocol provided here can be used for in vivo and ex vivo experiments, and as an alternative, the adipose-derived stem cells can be used to generate MSCs-like immortalized cells.
Introduction
Mesenchymal stem cells (MSCs) are adult multipotential cells differentiating into cells such as osteoblast, chondroblast, and adipocyte1,2. These cells reside in several organs, and because of that, they can be extracted from adult tissues such as bone marrow, muscle, fat, hair follicle, tooth root, placenta, dermis, perichondrium, umbilical cord, lung, liver, and spleen3,4.
The effects of MSCs on physiology and the immune system have been reported5,6. These cells have been promising for treating several diseases, both in human and veterinary medicine. The MSCs can control inflammation and promote angiogenesis and tissue homeostasis through different mechanisms, such as cell-cell contact, soluble factors, and small extracellular vesicles7,8,9,10. Furthermore, the MSCs are immune-privileged cells capable of surviving in immunologically incompatible allogeneic transplant recipients because these cells show low expression of class I major histocompatibility complex (MHC) molecules and are used in cell-based therapy for allogeneic transplant11,12. The low immunogenicity combined with the regenerative potential makes MSCs ideal candidates for cell therapy, such as graft-versus-host disease (GvHD)13, systemic lupus erythematosus (SLE)14, and multiple sclerosis15, among others16,17.
Despite the fact that MSCs reside in several adult tissues, the adipose tissue offers advantages over other sources, such as accessibility for harvesting, with minimal surgical intervention; large number of available cells with high expansion rate; and easy in vitro expansion using an easy-to-perform protocol without the need for specific equipment and low-cost materials18,19,20. Once extracted, the adipose tissue-derived stem cells (ADSCs) must be characterized as established by the International Society for Cellular Therapy (ISCT)21. Thus, MSCs must show morphology fibroblast-like, adherence to plastic culture, expressing a high percentage (≥95%) of mesenchymal markers such as endoglin (CD105), ecto-5'-nucleotidase (CD73) and Thy-1 (CD90), and low percentage (≤2%) of hematopoietic markers such as leukocyte common antigen (CD45), transmembrane phosphoglycoprotein (CD34), glycolipid-anchored membrane glycoprotein (CD14), integrin alpha M (CD11b), B-cell antigen receptor complex-associated protein alpha chain (CD79α) or B-lymphocyte surface antigen B4 (CD19) and class II human leukocyte antigen (HLA-II). Furthermore, a functional characterization is required, and the cells should be able to differentiate into osteoblast, chondroblast, or adipoblast cells21.
Here, we show how to obtain the MSCs from epididymal adipose tissue using mechanical dissociation and enzymatic digestion for in vitro studies and the morphological characterization preconized by ISCT.
Protocol
All animal experiments were conducted according to international guidelines for animal ethics and were approved by institutional committees of care and use from the State University of Santa Cruz under protocol number 021/22. Swiss male mice (6-8 weeks) were acquired from the Animal Breeding, Maintenance and Experimentation Laboratory – State University of Santa Cruz (LaBIO-UESC) Animal Research Facility, maintained in specific pathogen-free conditions, receiving water and food ad libitum with 12 h light/dark cycles.
NOTE: Other mice lineage can be used; we recommend the use of adult mice (6-8 weeks) since they have more developed adipose tissue and cells with high proliferative activity.
1. Preparation
NOTE: Before proceeding, prepare the following reagents described below. See the Table of Materials for reagent and material supplier information. Personal protective equipment such as masks, caps, lab coats, and gloves must be worn in all practical procedures.
- Epididymal adipose tissue extraction
- Reserve the surgery materials: three sets of sterile tweezers and scissors, five needles, and a beaker containing 200 mL of 70% ethanol.
- Prepare a terminal anesthetic, mixing ketamine (100 mg/kg) and xylazine (10 mg/kg).
- ADSCs culture
- Basal medium: Prepare Dulbecco's Modified Eagle Medium (DMEM) high glucose media supplemented with 10% fetal bovine serum (FBS), 50 µg/mL gentamicin, 0.25 µg/mL amphotericin B, 100 U/mL penicillin, and 100 mg/mL streptomycin.
- Digest solution: Prepare 0.15% of collagenase type II in PBS sterilized in a 0.25 µm filter.
NOTE: It is not necessary to use the four antibiotics described in the basal medium. It will depend on the cell culture conditions of the laboratory where this is performed.
- ADSCs phenotypic characterization
- Prepare 1% bovine serum albumin (BSA) in PBS.
- Dilute the anti-mouse antibodies as mentioned in Table 1.
- Prepare 2% formaldehyde in PBS.
- ADSCs osteogenic differentiation
- Osteogenic differentiation medium: Prepare DMEM supplemented with 5.67 M ascorbic acid, 10 nM dexamethasone, 0.02 M β-glycerophosphate, 10% FBS, and 1% penicillin/streptomycin.
- For Von Kossa staining, prepare the following solutions: 70% ethanol in water, 5% silver nitrate in water, distilled water, 5% sodium thiosulfate in water, and 1% eosin B in water.
- Adipogenic differentiation:
- Adipogenic differentiation medium: Prepare DMEM supplemented with 0.5 mM 3-Isobutyl-1-methylxanthine, 200 µM indomethacin, 1 µM dexamethasone, 10 µM insulin, 10% FBS, and 1 % penicillin/streptomycin.
- For Oil-red staining, prepare the following solutions: 10% formalin in deionized water, 60% isopropanol in deionized water, lysochrome (fat-soluble dye) diazo dye (Oil-Red O solution) in 60% isopropanol, and 1% hematoxylin in deionized water.
- Chondrogenic differentiation:
- Chondrogenic differentiation medium: Prepare chondrogenic medium supplemented with 10% FBS and 1% penicillin/streptomycin.
- Prepare 10% formaldehyde in PBS.
- For proteoglycans and glycosaminoglycans staining, prepare the following solutions: Heteroglycan stain (Alcian Blue 1%) in acetic acid, pH 2.5, paraffin, hematoxylin, ethanol series dilution in water (100%, 96%, 80%, and 70%).
| Antibody/Fluorophore | Dilution | Clone | Final concentration (µg/mL) | Function and cell in which it is expressed | ||
| CD34- PE | 1:100 | RAM34 | 2 | Cell adhesion factor. Hematopoietic stem cells. | ||
| CD45- APC | 1:100 | 30-F11 | 2 | Assistence in activation of leukocytes. Expressed in leukocytes. | ||
| CD71- FITC | 1:100 | C2 | 5 | Controls iron uptake during cell proliferation. Proliferating cells, reticulocytes, and precursor cells. | ||
| CD29- FITC | 1:100 | Ha2/5 | 5 | Adhesion and activation, embryogenesis, Leukocytes, dendritic cells, platelets, mast cells, fibroblasts, and endothelial cells. | ||
| CD90- PerCP | 1:100 | OX-7 | 2 | Signaling, adhesion. T lymphocyte, NK, monocyte, Hemtopoietic Stem Cells, neuron, and fibroblast. | ||
Table 1: Antibodies used for phenotypic characterization of ADSCs by flow cytometer. List of antibodies with their respective fluorochromes, dilutions, clone, and final concentration as well as their function in the cell which is expressed.
2. Methods
NOTE: The adipose tissue is distributed throughout the body in subcutaneous or intra-abdominal locations. In mice, the most common adipose tissues used for experiments include the subcutaneous epididymal, mesenteric, and retroperitoneal22. Here, the steps for obtaining epididymal adipose tissue are shown (Figure 1).
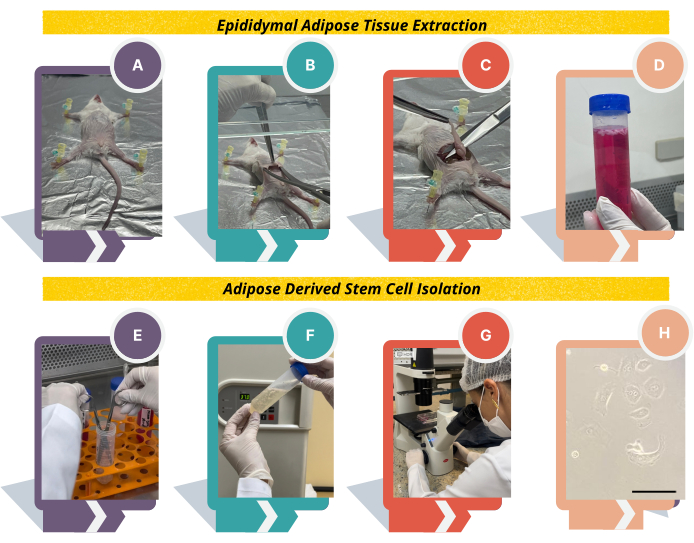
Figure 1: Experimental design to obtain adipose tissue-derived stem cells from Swiss mice. (A) Using needles, place the animal on the cork or styrofoam board; (B) Lift the skin using tweezers, make a cut in the center of the abdominal region, and detach the skin from the peritoneum; (C) identify the white adipose tissue above the epididymis; (D) transfer the tissue to DMEM medium supplemented with 10% FBS and 1% antibiotics, wash the epididymal adipose tissue thoroughly with PBS, and transfer the tissue to digest solution; (E), fragment the tissue and (F) incubate at 37 °C for 1 h vigorously shaking every 10 min; (G) after counting the cells, plate in 6-well plates and observe the cell morphology under an inverted microscope. (F) Cell morphology will change from round to fibroblast-like. Scale bar = 22.22 µm. Please click here to view a larger version of this figure.
- Extraction of epididymal adipose tissue
NOTE: Be aware that all procedures must be carried out under sterile conditions in a laminar flow cabinet in order to ensure pure and contamination-free cell culture.- Euthanize the animals using ketamine and xylazine solution (100 and 10 mg/kg, as described in step 1.1.2) by intraperitoneal route.
NOTE: Other euthanizing methods can be used23. Make sure the animal is dead by confirming the absence of a heartbeat. - Place the animal with the ventral side facing up on a cork or styrofoam board covered with aluminum foil and fix it using sterile needles (Figure 1A).
NOTE: To avoid contamination, spray 70% alcohol in the abdominal region. Alternatively, shave the hair with a scalpel. - Lift the skin using tweezers in the center of the abdominal region and make a cut with sterile scissors.
NOTE: Two sets of sterile tweezers and scissors are necessary to avoid contamination. The first one is used to open the animal, cutting the skin and peritoneal layer; the second one is used to pick up the epididymal adipose tissue. Also, reserve 150 mL of 70% alcohol in a beaker to clean scissors and tweezers between steps. - Introduce the scissors under the animal's skin and open it to detach it from the peritoneum (Figure 1B). Lift the peritoneal layer using tweezers and cut with sterile scissors.
- Use another set of sterile tweezers and scissors and identify the epididymis above the testes and the white adipose tissue above the epididymis. Pull the entire epididymal adipose tissue using sterile tweezers, approximately 2 cm in size, and cut very close to the epididymis (Figure 1C).
- Put the fat into a 50 mL sterile conical tube containing basal medium and place it on ice (Figure 1D). In the hood, proceed to the next steps as described below.
- Euthanize the animals using ketamine and xylazine solution (100 and 10 mg/kg, as described in step 1.1.2) by intraperitoneal route.
- Adipose tissue-derived stem cells (ADSCs) isolation
NOTE: Process the samples in a biological class II laminar flow hood, and personnel should wear a lab coat, gloves, and surgical mask.- Wash the epididymal adipose tissue thoroughly with PBS.
NOTE: This step is essential to remove the blood and other particles from the sample. The blood can inhibit collagenase type II enzyme and compromise the experiment. - Using a sterile tweezer, transfer the epididymal adipose tissue into a 50 mL tube containing 15-20 mL of digest solution, prepared as step 1.2.2. Fragment the tissue using sterile scissors and incubate the tissue at 37 °C for 1 h (Figure 1E, F).
NOTE: A water bath or incubator at 37 °C can be used in this step. Every 10 min, the suspension must be vigorously shaken to facilitate digestion. Do not extend the time of 1h incubation. - Inactivate the collagenase by diluting the sample 1:1 in basal medium and centrifuge the suspension at 250 x g for 10 min at 4 °C to obtain the vascular fraction of the stroma. Discard the supernatant and resuspend the pellet in 1 mL of basal medium.
- Check the viability and count the cells through the trypan blue dye exclusion method in a Neubauer chamber using a dilution of 1:10 or 1:100.
NOTE: The expected yield is around 0.5-1 million cells/g of processed adipose tissue and viability of 80%. - Plate the isolated cells (0.3-0.5 million) in 3 mL of basal medium in one well of a 6-well plate. Incubate the cells overnight at 37 °C with 5 % CO2.
- Next day: Remove the medium from the well and wash the cells with PBS. Add 3 mL of basal medium. Repeat this process every 2 days until the cells become 90% confluent.
NOTE: Always observe the morphology of the cells under the inverted microscope, changing from round to fibroblast-like (Figure 1H).
- Wash the epididymal adipose tissue thoroughly with PBS.
- Adipose tissue-derived stem cells (ADSCs) expansion
NOTE: Once the cells grow ~90% confluent, proceed to subculture as described below.- Remove the medium from the well and wash the cells with PBS. Add 0.5 mL/well of trypsin/EDTA (0.05 % trypsin; 200 mg/L EDTA) and incubate the plate for 3-5 min at 37 °C to detach the cells.
NOTE: Do not exceed 5 min in trypsin/EDTA. The complete detachment of the cells should be verified under the inverted microscope. The process can be accelerated by pipetting up and down carefully or tapping the side of the plate. - Inactivate the enzyme by diluting the sample 1:1 in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics.
- Split the cell suspension into 2 wells and add 3 mL of basal medium (DMEM supplemented with 10 % FBS and 1 % antibiotics). Incubate overnight at 37 °C with 5% CO2.
- Change medium the next day, then every 2 days until 90% confluence.
- Repeat the process until the third passage and proceed to phenotype and functional characterization.
NOTE: Always observe the morphology of the cells under the inverted microscope, changing from round to fibroblast-like.
- Remove the medium from the well and wash the cells with PBS. Add 0.5 mL/well of trypsin/EDTA (0.05 % trypsin; 200 mg/L EDTA) and incubate the plate for 3-5 min at 37 °C to detach the cells.
- Adipose tissue-derived stem cells (ADSCs) characterization
- Phenotypic characterization by flow cytometry
- Detach the cells using Trypsin/EDTA, as described in steps 2.3.1 to 2.3.2.
- Collect the cells in a conical tube (50 mL) and centrifuge at 250 x g for 10 min at 4 °C. Discard the supernatant and resuspend the pellet in 1 mL of PBS.
- Count the cells as described in step 2.2.4 and seed 0.5-1 x 106 cells in 100 µL of PBS in a 96 well-plate.
NOTE: The number of wells will depend on the color of the fluorochrome conjugate and the filters/lasers of the cytometer. - Add antibodies (Table 1) diluted in 1% BSA in PBS.
NOTE: It is not necessary to use Fc block to avoid nonspecific binding because we already use BSA to dilute antibodies. Reserve a sample of cells without receiving antibodies that will be used as a control of staining. The antibodies can be combined in the same well according to the fluorochrome dye and cytometer available in the lab. All the antibodies described in Table 1 can be added in the same well, except the CD71 FITC and CD29 FITC, which must be in different wells because of the same fluorochrome. - Incubate the plate for 30 min at 4 °C in the dark.
- Wash cells, then complete the volume to 200 µL using PBS, centrifuge the plate at 252 x g for 10 min at 4 °C, and discard the supernatant. Repeat this step one more time.
- Fix the cells by adding 200 µL per well of 2% formaldehyde in PBS. Store the cells in the dark at 4 °C until analysis in the flow cytometer.
NOTE: For best results, acquire the cells on the flow cytometer as soon as possible. The brightness of fluorescence of markers decays significantly after 48 h for most fluorochromes. So, do not exceed 48 h to read the plate. Acquisition of 30,000 events is recommended. - Use the gating strategy presented in Figure 2B to select the ASC population.
- Functional characterization: Osteogenic differentiation
- Detach the cells using Trypsin/EDTA (as described in steps 2.3.1 to 2.3.2), collect and centrifuge the cells (as described in steps 2.4.1.2 and 2.4.1.2), and count them (as described in steps 2.2.4).
- Plate, in triplicate, 2 x 105 cells per well in 6-well plates in basal medial (DMEM supplemented with 10 % FBS and 1 % antibiotics); incubate at 37 °C in 5% CO2.
NOTE: Two 6-well plates will be needed, one for each differentiation time (14 and 21 days). - Next day, change the medium to osteogenic medium (step 1.4.1).
NOTE: reserve 3 wells being cultured only with the basal medium, which will be the control for differentiation. - Culture cells for 14 and 21 days in osteogenic media and the control cells, changing media every 3 days. Proceed to Von Kossa staining (step 1.4.2) to assess the mineralized nodules at the end of each culture period.
- Aspirate the medium from each well and wash the cells twice with PBS. Fix the cells with 2 mL of 70% ethanol overnight at room temperature (RT). Wash the cells carefully in running water.
- Add 2 mL of 5% silver nitrate solution and incubate the plate in ultraviolet light for 1 h. Wash the cells with distilled water. Add 2 mL of 5% sodium thiosulfate, and incubate for 5 min. Wash with distilled water.
- Counterstain with 2 mL of eosin for 40 s. Wash with distilled water until the staining solution is removed, and visualize the staining using an optical microscope.
- Functional characterization: Adipogenic differentiation
- Detach the cells using Trypsin/EDTA (as described in steps 2.3.1 to 2.3.2), collect and centrifuge the cells (as described in steps 2.4.1.2 and 2.4.1.2), and count them (as described in steps 2.2.4).
- Plate 2 x 105 cells per well in 6-well plates in basal medial (DMEM supplemented with 10 % FBS and 1 % antibiotics); incubate at 37 °C in 5% CO2.
NOTE: Two 6-well plates will be needed, one for each differentiation time (14 and 21 days). - The next day, change the medium to adipogenic medium (step 2.5.1).
NOTE: Reserve 3 wells being cultured only with the basal medium as control. - Culture cells for 14 and 21 days in adipogenic medium, changing media every 3 days. At the end of each culture period, proceed to Oil-Red O staining (steps 2.4.3.5-2.4.3.7), an indicator of intracellular lipid accumulation.
- Aspirate the media from each well. Wash the cells twice with PBS. Fix cells in 2 mL of 10% formalin for 1 h. Wash once with PBS, and then one time with water.
- Stain with 2 mL of Oil-Red O solution in 60% isopropanol for 5 min. Wash once with distilled water.
- Counterstain with 2 mL of 1% hematoxylin diluted 1:2 in distilled water for 1 min. Wash with distilled water until the staining solution is removed, and visualize the stained samples using an optical microscope.
- Functional characterization: Chondrogenic differentiation
- Detach the cells using Trypsin/EDTA (as described in steps 2.3.1 to 2.3.2), collect and centrifuge the cells (as described in steps 2.4.1.2 and 2.4.1.2), and count them (as described in steps 2.2.4).
- Place 5 x 105 ADSCs in four polypropylene conical tubes and centrifuge at 450 x g for 5 min to form a pellet. Discard the supernatant.
- Culture the pellets in a conical tube in a chondrogenic medium (step 1.6.1) or only basal medium (control of differentiation) for 14 and 21 days at 37 °C in 5% CO2. Change medium every 3 days and avoid removing pellets.
NOTE: Each cell pellet refers to each culture time, as well as their respective controls grown only with basal medium. - At the end of each culture time point, collect pellets using fine-tip tweezers and place them in a 24-well plate. Proceed for histological sectioning and staining of proteoglycans and glycosaminoglycans (steps 2.4.4.5.-2.4.4.9).
NOTE: Each pellet is processed in a separate well. - Fix pellets in 2 mL of 10% buffered formaldehyde for 30 min. Discard the formaldehyde solution, and add 2 mL of 70% ethanol. Remove the pellet from the conical tube using a tip and embed the pellets in paraffin.
- Using a rotating paraffin microtome, make 5 µm histological sections. Incubate the sections for 15 min at 60 °C, and immerse them twice in xylene (for 5 and 15 min).
- Rehydrate the samples by immersing the histological sections in alcohol baths at decreasing concentrations (100%, 96%, 80%, and 70%) for 2 min each, then rinse with deionized water for 5 min.
- Proceed to stain the samples with Alcian blue 1% in acetic acid, pH 2.5, for 30 min.
- Counterstain with hematoxylin for 1 min, and wash with distilled water. Mount with DPX (mountant for histology) or other mount solution and visualize samples using an optical microscope.
- Phenotypic characterization by flow cytometry
Representative Results
Cells extracted from adipose tissue according to the protocol presented here showed morphology matching the minimal criteria for MSCs proposed by ISCT. An overview of the protocol is shown in Figure 1. Phenotypically, ADSCs showed adherence to plastic and fibroblast-like morphology in the first days of cell culture (Figure 2A). In addition, they grew homogeneously and formed colonies. Furthermore, ADSCs showed low expression of CD34 (2.12%) and CD45 (1.81%), both hematopoietic markers, and high expression of CD71 (42.6%), CD29 (74.2%), and CD90 (55.1%), all mesenchymal cell markers (Figure 2B).
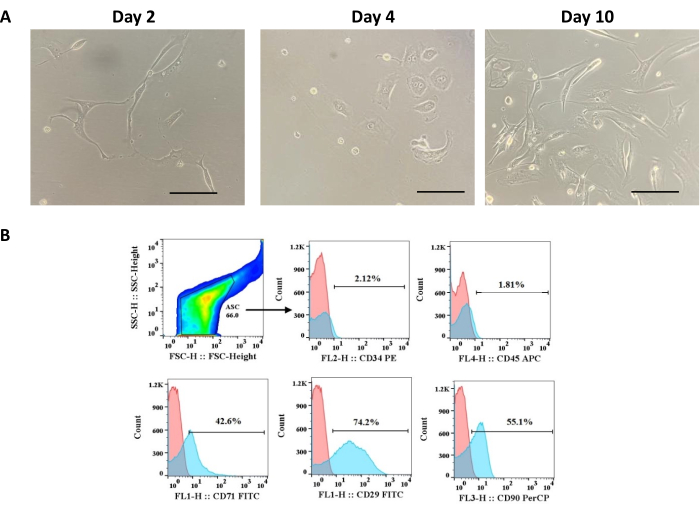
Figure 2: Phenotypic characterization of adipose tissue-derived cells. (A) ADSCs morphology changing from round to fibroblast-like on days 2, 4, and 8 of culture; scale bar = 22.22 µm. (B) Histograms for markers expressed (CD71, CD29, and CD90) or not (CD34 and CD45) by ASC. This figure has been reproduced with permission from Miranda et al.24. Please click here to view a larger version of this figure.
Functionally, ADSCs showed multipotency to differentiate into osteoblast, adipoblast, and chondroblast when cultured in conditioned media for each lineage for 14 or 21 days (Figure 3). The Von Kossa staining revealed mineralized nodules in the extracellular matrix, characteristic of the osteogenesis process (Figure 3). The Oil-red O staining evidenced lipid vacuoles in the cytoplasm of ADSCs, and the Alcian Blue staining confirmed the presence of glycosaminoglycan in the extracellular matrix (Figure 3). Together, phenotypic and functional characteristics have confirmed the population of cells extracted from epididymal adipose tissue as MSCs.
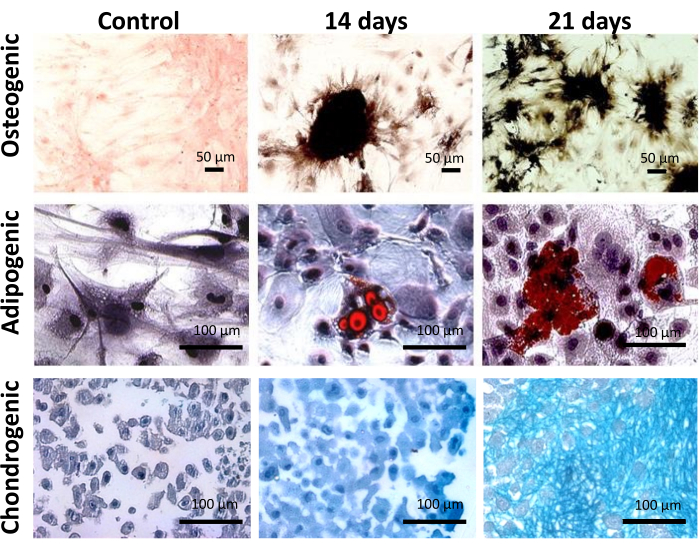
Figure 3: Functional characterization of adipose tissue-derived cells. Osteogenic, adipogenic, and chondrogenic multilineage potential of ADSC after 14 and 21 days on culture. Scale bar = 50 µm (top panel), 100 µm (middle and bottom panels). This figure has been reproduced with permission from Miranda et al.24. Please click here to view a larger version of this figure.
Discussion
The MSCs can be extracted from different tissues. Despite bone marrow representing a common source of MSCs in both murine and humans25,26, we have chosen to work with adipose tissue in this study because of its richness in MSCs, distribution in the body, and ease of accessing it. As an alternative, adipose-derived stem cells can be used to generate MSCs-like immortalized cells27.
Some points of the extraction deserve special attention for the success of the protocols shared here. First, we highlight the care to collect the tissue that must be done in aseptic conditions. So, ensure the mice are properly disinfected with 70% alcohol before starting the surgery. Another important point is not to use the same set of tweezers and scissors to pick up the epididymal adipose tissue, thus avoiding any contamination source.
A second concern is the processing of tissue. The fragments in the conical tube must be properly and vigorously shaken with 10 min of incubation at 37 °C. It is very important to change the medium the next day and then every 2-3 days. It is recommended to observe the culture daily, checking the medium color, cell shape, and any interference in the environment of the culture.
A third concern is regarding the best time/passage for MSC characterization. Here, we recommend doing it at the third passage of cells, but this is not mandatory. It depends on the objective of the study. In the third passage, we have around 80% of the cells characterized as MSCs, which is enough for the objectives of this study for example. The phenotypic characterization is performed by analyzing the expression of hematopoietic markers (CD34 and CD45, for example) and mesenchymal markers (CD29, CD73, CD90, and CD105, for example). Furthermore, the International Society for Cell and Gene Therapy (ISCT)21 highlights that the ADSCs should have fibroblast-like morphology and adhere to the plastic of the culture platform. The morphology can be observed under a microscope during the growing culture. The functional characterization is performed by analyzing the potential of ADSCs to differentiate into osteoblast, adipoblast, and chondroblast cells. In the phenotypic characterization, the percentage of mesenchymal and hematopoietic markers may vary according to the animal species and cell source, not fully meeting the numbers recommended by the ISCT. For example, the ISCT recommends for human MSCs ≥95% of CD105, CD73, CD90, and ≤2% of CD45, CD34, CD14 or CD11b, CD79a or CD19, Human leukocyte antigen – DR Isotype (HLA-DR)21. Furthermore, it is important to do the functional characterization and confirm the multipotency of cells differentiating into osteoblasts, chondrocytes, and adipocyte lineages. At least two of these lineages are recommended for proving multipotency.
In addition to these highlighted points, some assays suggested here can be substituted, for example, Alizarin Red S instead of Von Kossa staining, Periodic Acid Shiff instead of Alcian Blue, and polymerase chain reaction (PCR) instead of flow cytometry.
Among the three types of adipose tissue described in humans (white, brown, and beige), white adipose tissue has the highest source of MSCs28. This tissue can be obtained from subcutaneous, abdominal, and inguinal regions, such as the fat above the epididymis reported here. The physical fragmentation and enzymatic digestion by collagenase used in the protocol shared here derive the stromal vascular fraction (SVF) composed of many cell types, except for the adipocytes, which are depleted during the processing.
In conclusion, the main advantages of using adipose tissue as a source of MSCs include their abundance and ease of methods for cell isolation, associated with the MSCs’ therapeutic potential as anti-inflammatory and regenerative properties.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Research supported by a grant from the Conselho Nacional de Desenvolvimento Científico e Tecnologico (480807/2011-6) and Fundação de Amparo à Pesquisa de Minas Gerais (APQ-01237-11). This study was financed in part by the PROPP UESC (073.6764.2019.0021079-85). MGAG and URS thanks to the scholarship granted by Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), respectively.
Materials
| 140 °C High Heat Sterilization CO2 Incubator | RADOBIO SCIENTIFIC CO. LTD, China | C180 | |
| 3-Isobutyl-1-methylxanthine | Sigma-Aldrich, San Luis, Missouri, USA | I7018 | |
| Acetic acid glacial | Sigma-Aldrich, San Luis, Missouri, USA | PHR1748 | |
| Alcian Blue 8GX | Sigma-Aldrich, San Luis, Missouri, USA | A9186 | BioReagent, suitable for detection of glycoproteins. 1% in acetic acid, pH 2.5 |
| Alcohol 70% | Sigma-Aldrich, San Luis, Missouri, USA | 65350-M | 70% in water |
| Amphotericin B | Sigma-Aldrich, San Luis, Missouri, USA | PHR1662 | |
| Antibodies anti-mouse anti-CD29 FITC (Clone Ha2/5) | BD Biosciences, San Diego, CA, USA | 555005 | Functions in the cell: Adhesion and activation, embryogenesis, Leukocytes, DC, platelets, mast cells, fibroblasts and endothelial cells |
| Antibodies anti-mouse anti-CD34 PE (Clone RAM34) | BD Biosciences, San Diego, CA, USA | 551387 | Functions in the cell: Cell adhesion factor. Hematopoietic stem cells |
| Antibodies anti-mouse anti-CD45 APC (Clone 30-F11) | BD Biosciences, San Diego, CA, USA | 559864 | Functions in the cell: Assists in the activation of leukocytes |
| Antibodies anti-mouse anti-CD71 FITC (Clone C2) | BD Biosciences, San Diego, CA, USA | 553266 | Functions in the cell: Controls iron uptake during cell proliferation. Proliferating cells, reticulocytes and precursors |
| Antibodies anti-mouse anti-CD90 PerCP (Clone OX-7) | BD Biosciences, San Diego, CA, USA | 557266 | Functions in the cell: Signaling, adhesion. T lymphocyte, NK, monocyte, HSC, neuron, fibroblast |
| Ascorbic acid | Sigma-Aldrich, San Luis, Missouri, USA | PHR1008 | |
| Automatic pipettes | Thermo Fisher Scientific, Waltham, Massachusetts, USA | 4700850N | Finnpipette F1 Good Laboratory Pipetting (GLP) Kits |
| Beaker | Not applicable | 1 unit | |
| Bovine serum albumin | Sigma-Aldrich, San Luis, Missouri, USA | A7906 | |
| Cell culture plates (6-well) | Merck, Darmstadt, Germany | Z707759 | 07 units sterile. TPP tissue culture plates |
| Cell culture plates (96-well. Round or V bottom) | Merck, Darmstadt, Germany | CLS353077 | 01 unit sterile. Wells, 96, Tissue Culture (TC)-treated surface, round bottom clear wells, sterile |
| Chondrogenic medium | Stem Pro Chondrogenesis Differentiation–Life Technologies | A1007101 | TGF-β2, TGF-β3, dexamethasone, insulin, transferrin, ITS, sodium-l – ascorbate, sodium pyruvate, ascorbate-2-phosphate |
| Collagenase type II | Life Technologies, California, USA | 17101015 | |
| cork or styrofoam board covered with aluminum | Not applicable | 1 unit | |
| cotton | Not applicable | 50 g | |
| Dexamethasone | Sigma-Aldrich, San Luis, Missouri, USA | D4902 | |
| Dissecting scissor | Not applicable | 03 units sterile | |
| DPX Mountant for histology | Sigma-Aldrich, San Luis, Missouri, USA | 6522 | |
| Dulbecco’s modified Eagle’s medium (DMEM) | Sigma-Aldrich, San Luis, Missouri, USA | D5523 | With 1000 mg/L glucose and L-glutamine, without sodium bicarbonate, powder, suitable for cell culture |
| Eosin B | Sigma-Aldrich, San Luis, Missouri, USA | 861006 | |
| Fetal bovine serum (FBS) | Sigma-Aldrich, San Luis, Missouri, USA | F4135 | |
| Formaldehyde | Sigma-Aldrich, San Luis, Missouri, USA | 47608 | |
| Formalin | Sigma-Aldrich, San Luis, Missouri, USA | HT501128 | |
| Gentamicin | Sigma-Aldrich, San Luis, Missouri, USA | G1397 | |
| Hematoxylin | Sigma-Aldrich, San Luis, Missouri, USA | H3136 | |
| Hypodermic Needle (0.3mm x 13mm) | Not applicable | 5 units | |
| Indomethacin | Sigma-Aldrich, San Luis, Missouri, USA | I0200000 | |
| Insulin | Sigma-Aldrich, San Luis, Missouri, USA | I3536 | |
| Isopropanol | Sigma-Aldrich, San Luis, Missouri, USA | 563935 | 70% in H2O |
| Ketamine-D4 hydrochloride solution | Sigma-Aldrich, San Luis, Missouri, USA | K-006 | 1.0 mg/mL in methanol (as free base), certified reference material, Cerilliant® |
| Neubauer chamber | Sigma-Aldrich, San Luis, Missouri, USA | BR718620 | BRAND counting chamber BLAUBRAND Neubauer pattern. With clips, double ruled |
| Nichiryo pipette tips (0.1–10 μL) | Merck, Darmstadt, Germany | Z645540 | Volume range 0.1–10 μL, elongated, bulk pack. Sterile |
| Nichiryo pipette tips (1–10 mL) | Merck, Darmstadt, Germany | Z717401 | Volume range 1–10 mL, universal, bulk pack. Sterile |
| Nichiryo pipette tips (200 μL) | Merck, Darmstadt, Germany | Z645516 | Maximum volume 200 μL, graduated, ministack. Sterile |
| Oil-Red O solution | Sigma-Aldrich, San Luis, Missouri, USA | O1391 | 0.5% in isopropanol |
| Paraffin | Sigma-Aldrich, San Luis, Missouri, USA | 107.151 | 46–48, in block form |
| Penicillin/Streptomycin | Sigma-Aldrich, San Luis, Missouri, USA | P4333 | Solution stabilized, with 10,000 units penicillin and 10 mg streptomycin/mL, 0.1 μm filtered, BioReagent, suitable for cell culture |
| Phosphate-buffered saline solution 1x (PBS). | Sigma-Aldrich, San Luis, Missouri, USA | P3813 | Powder, pH 7.4, for preparing 1 L solutions. Balanced and sterile |
| Polypropylene conical tubes (15 mL) | Falcon, Fisher Scientific | 14-959-53A | Sterile |
| Polypropylene conical tubes (50 mL) | Falcon, Fisher Scientific | 14-432-22 | 2 units sterile |
| scalpel (optional) | Not applicable | 1 unit | |
| Silver nitrate | Sigma-Aldrich, San Luis, Missouri, USA | 85228 | |
| Sodium thiosulfate | Sigma-Aldrich, San Luis, Missouri, USA | 72049 | |
| Surgical tweezer (15 cm) | Not applicable | 3 units sterile | |
| Swiss male mice (6–8 weeks) | Bioterium, Santa Cruz State University | 021/22 | |
| syringe (1 mL) | Not applicable | 1 unit | |
| Trypan Blue Dye | Sigma-Aldrich, San Luis, Missouri, USA | T8154 | 0.4%, liquid, sterile-filtered, suitable for cell culture |
| Trypsin/EDTA (ethylenediaminetetraacetic acid) | Sigma-Aldrich, San Luis, Missouri, USA | T3924 | |
| Xylazine | Sigma-Aldrich, San Luis, Missouri, USA | PHR3263 | |
| β-glycerophosphate disodium salt hydrate | Sigma-Aldrich, San Luis, Missouri, USA | G9422 | BioUltra, suitable for cell culture, suitable for plant cell culture, ≥99% (titration) |
Riferimenti
- Caplan, A. I. Mesenchymal stem cells. J Orthop Res. 9 (5), 641-650 (1991).
- Pittenger, M., et al. Mesenchymal stem cell perspective: cell biology to clinical progress. npj Regen Med. 4, 22 (2019).
- Lin, W., et al. Mesenchymal stem cells and cancer: Clinical challenges and opportunities. BioMed Res Int. 2820853, 1-12 (2019).
- Mazini, L., et al. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int J Mol Sci. 21 (4), 1306 (2020).
- Shi, Y., et al. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat Rev Nephrol. 14 (8), 493-507 (2018).
- Uccelli, A., et al. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 8 (9), 726-736 (2008).
- Dong, J., et al. Human adipose tissue-derived small extracellular vesicles promote soft tissue repair through modulating M1-to-M2 polarization of macrophages. Stem Cell Res Ther. 14 (1), 67 (2023).
- Maffioli, E., et al. Proteomic analysis of the secretome of human bone marrow-derived mesenchymal stem cells primed by pro-inflammatory cytokines. J. Proteomics. 166, 115-126 (2017).
- Akiyama, K., et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 10, 544-555 (2012).
- Wang, Y., et al. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 15, 1009-1016 (2014).
- Li, C., et al. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 11, 187 (2021).
- Zhang, J., et al. The challenges and promises of allogeneic mesenchymal stem cells for use as a cell-based therapy. Stem Cell Res Ther. 6, 234 (2015).
- Jurado, M., et al. Adipose tissue-derived mesenchymal stromal cells as part of therapy for chronic graft-versus-host disease: A phase I/II study. Cytotherapy. 19 (8), 927-936 (2017).
- Zhang, M., et al. Mesenchymal stem cell-derived exosome-educated macrophages alleviate systemic lupus erythematosus by promoting efferocytosis and recruitment of IL-17+ regulatory T cell. Stem Cell Res Ther. 13 (1), 484 (2022).
- Fernández, O., et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One. 13 (5), 0195891 (2018).
- Abdolmohammadi, K., et al. Mesenchymal stem cell-based therapy as a new therapeutic approach for acute inflammation. Life Sci. 312, 121206 (2023).
- Hoang, D. M., et al. Stem cell-based therapy for human diseases. Signal Transduct Target Ther. 7 (1), 272 (2022).
- Lee, R. H., et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 14 (4-6), 311-324 (2004).
- Strem, B. M., et al. Multipotential differentiation of adipose tissue derived stem cells. Keio J Med. 54 (3), 132-141 (2005).
- Kern, S., et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 24 (5), 1294-1301 (2006).
- Dominici, M. D., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 8 (4), 315-317 (2006).
- Berryman, D. E., et al. Growth hormone’s effect on adipose tissue: Quality versus quantity. International Journal of Molecular Sciences. 18 (8), 1621 (2017).
- Shomer, N. H., et al. Review of rodent euthanasia methods. J Am Assoc Lab Anim Sci. 59 (3), 242-253 (2020).
- Miranda, V. H. S., et al. Liver damage in schistosomiasis is reduced by adipose tissue-derived stem cell therapy after praziquantel treatment. PLoS Negl Trop Dis. 14 (8), e0008635 (2020).
- Li, H., et al. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann N Y Acad Sci. 1370 (1), 109-118 (2016).
- Boregowda, S. V., et al. Isolation of mouse bone marrow mesenchymal stem cells. Methods Mol Biol. 1416, 205-223 (2016).
- Sreejit, P., et al. Generation of mesenchymal stem cell lines from murine bone marrow. Cell Tissue Res. 350 (1), 55-68 (2012).
- Bunnell, B. A. Adipose tissue-derived mesenchymal stem cells. Cells. 10 (12), 3433 (2021).

