Disposable Dosators Intended for Dry Powder Delivery to Mice
Summary
Pharmaceutical dry powder development necessitates reliable in vivo testing, often using a murine model. Device technology for accurately and reproducibly delivering dry powder aerosols to mice is restricted. This study presents disposable dosators for pulmonary drug delivery at mouse-relevant doses, aiding initial proof-of-concept research.
Abstract
Dry powder inhalers offer numerous advantages for delivering drugs to the lungs, including stable solid-state drug formulations, device portability, bolus metering and dosing, and a propellant-free dispersal mechanism. To develop pharmaceutical dry powder aerosol products, robust in vivo testing is essential. Typically, initial studies involve using a murine model for preliminary evaluation before conducting formal studies in larger animal species. However, a significant limitation in this approach is the lack of suitable device technology to accurately and reproducibly deliver dry powders to small animals, hindering such models' utility. To address these challenges, disposable syringe dosators were developed specifically for intrapulmonary delivery of dry powders in doses appropriate for mice. These dosators load and deliver a predetermined amount of powder obtained from a uniform bulk density powder bed. This discrete control is achieved by inserting a blunt needle to a fixed depth (tamping) into the powder bed, removing a fixed quantity each time. Notably, this dosing pattern has proven effective for a range of spray-dried powders. In experiments involving four different model spray-dried powders, the dosators demonstrated the ability to achieve doses within the range of 30 to 1100 µg. The achieved dose was influenced by factors such as the number of tamps, the size of the dosator needle, and the specific formulation used. One of the key benefits of these dosators is their ease of manufacturing, making them accessible and cost-effective for delivering dry powders to mice during initial proof-of-concept studies. The disposable nature of the dosators facilitates use in animal procedure rooms, where cleaning and refilling reusable systems and weighing materials is inconvenient. Thus, developing disposable syringe dosators has addressed a significant hurdle in murine dry powder delivery for proof-of-concept studies, enabling researchers to conduct more accurate and reproducible preliminary studies in small animal models for pulmonary drug delivery.
Introduction
The use of dry powder inhalers (DPIs) for pulmonary drug delivery has garnered significant interest over the past three decades due to the global phase-out of chlorofluorocarbon propellants1,2. DPIs offer numerous benefits over other pulmonary delivery systems, such as metered dose inhalers and nebulizers, including formulation stability, portability, ease of use, and propellant-free dispersal mechanisms2. However, before moving DPI products toward clinical translation, several preclinical studies must be conducted, many of which are initially completed using a murine model. Nevertheless, technologies available to deliver dry powders accurately and reproducibly to small animals are limited.
Common methods to deliver dry powders to small animals, such as mice, include passive inhalation3,4,5,6,7 and direct administration8,9,10,11,12,13. Passive inhalation typically requires a custom chamber that utilizes large doses of spray-dried powder to prepare a sufficient aerosol cloud. As mice are obligate nose breathers14, delivery by passive inhalation requires the powder to travel through the nose and throat to reach the lungs, necessitating the maintenance of an aerosol cloud with sufficient particle aerodynamic properties7,8. While a useful technique that is more physiologically relevant than direct delivery due to inhalation as a result of normal breathing14, it may not be suitable for initial studies where powder mass is limited.
Alternatively, a number of intratracheal delivery devices for direct dry powder delivery have been reported8,9,10,11,12,13. Intratracheal devices bypass the nose and throat, delivering the powder directly to the lungs and allowing for finer control over the delivered dose14. Additionally, some devices, especially those prepared using a tamping loading procedure9, can be prepared with smaller quantities, which is an important consideration for initial proof-of-concept studies. The lack of universally available intratracheal delivery devices has hindered their potential for use, limiting availability and leading to interlaboratory differences14. In this study, we propose a simple, inexpensive, disposable dosator for intratracheal delivery that can be utilized for proof-of-concept murine studies in the development of dry powder aerosols.
Protocol
All animal experiments were conducted in accordance with the Animal Welfare Act and the Public Health Service Policy on Humane Care and Use of Laboratory Animals. The study protocol was approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center. Healthy female BALB/c mice, ~6-8 weeks old, were administered the dry powder content of one dosator by intrapulmonary aerosol delivery for a pharmacokinetic study using spectinamide 1599 dry powders9. The animals were obtained from a commercial source (see Table of Materials).
1. Preparation of the dosator and the filling components
- Trim the plastic luer portion of a 2.54 cm (1 inch) blunt stainless-steel needle (21-25 G) using either a precision sectioning saw (see Table of Materials), or a belt sander until 2-3 mm of the plastic luer remains (Figure 1A and Figure 2A).
NOTE: If a belt sander is used, the stainless-steel needle may need to be cleaned using a smaller needle or wire to remove the possible obstructions created. - Cut off the tip (1-1.5 cm) of a 0.6 mL conical centrifuge tube. Fill the tip of the tube with 30-35 mg of powder.
NOTE: See Representative Results for the details of the example powders used for the present study. The powder aerosol performance should be evaluated prior to use in this application following standard methodology as described in USP General Chapter <601> (see Table of Materials). - If storing and/or transporting the powder, use the tube cap (cut off) to close the vial. Seal with paraffin film to minimize powder exposure to ambient moisture if storing and/or transporting.
2. Loading and assembling dosators
- Tamp the trimmed stainless-steel needle into the powder bed in the 0.6 mL conical centrifuge tube tip as many times as needed to achieve the desired dose (Figure 2B). Gently wipe the sides of the stainless-steel needle with a low-lint wiper to remove any excess powder (Figure 3).
- Gently insert the loaded stainless-steel needle into a 3.81 cm (1.5 inch) polypropylene or 5.08 cm (2 inch) polytetrafluoroethylene (PTFE) needle (16-20 G) (see Table of Materials) to avoid dislodging any powder (Figure 1B,C and Figure 2C).
3. Actuating dosators
- Draw back a disposable syringe to the desired volume, which may vary based on application.
NOTE: For intrapulmonary administration in mice, 0.15-0.6 mL is typically appropriate8,9. - Attach the syringe to the luer lock on the polypropylene or PTFE needle (Figure 2D).
- Insert the needle end of the dosator into the desired target. For analyzing powder content and reproducibility, insert the needle through a perforated rubber septum or paraffin film into a vial containing a small amount (e.g., 1-5 mL) of water and/or organic solvent (e.g., ethanol), with solvent identity and volume dependent on active pharmaceutical ingredient (API) physical characteristics and the quantification method.
- For delivery to mice, insert the needle up to the first bronchial bifurcation of the trachea of anesthetized mice following established protocols9,15.
- Depress the syringe forcefully, expelling the powder out of the device into the collection vial (Figure 2E).
NOTE: The same technique must be followed for delivering the powder to the murine lungs. - For analyzing content and reproducibility from the collection vial, utilize an appropriate analytical method for the specific API, such as UV-Visible (UV-Vis) spectrophotometry or high-performance liquid chromatography (HPLC).
Representative Results
The aerosol performance of various spray-dried powders was established prior to use in this study. The aerodynamic particle size distribution (APSD) was described by the mass median aerodynamic diameter (MMAD), representing the size that divides the distribution in two at the 50th percentile (d50), and the geometric standard deviation (GSD), reflecting the breadth of the distribution. The GSD is defined by the square root of the aerodynamic diameter at the 80th percentile divided by that at the 16th percentile (d84/d16)1/2, with the percentiles representing one standard deviation on either side of the mean for a log-normal distribution of mass with respect to particle size.
Four representative spray dried powders were considered for delivery using the dosators described herein. The spray dried (SD) powders, including tigecycline (SD-1)3, capreomycin sulfate (SD-2)16, spectinamide 1599 (SD-3)9, and albuterol sulfate (SD-4) APIs with excipients, represent a range of antibacterial and bronchodilator formulations that have been developed for a variety of applications. Prior to use in the dosators, the aerodynamic particle size distribution was determined for the four powders using a low-resistance dry powder inhaler and a high-performance cascade impactor following USP General Chapter <601> (see Table of Materials). The MMADs of SD-1, SD-2, SD-3, and SD-4 were 2.6 ± 0.1 µm (GSD = 2.1 ± 0.1), 1.7 ± 0.1 µm (GSD = 2.4 ± 0.1), 1.7 ± 0.4 µm (GSD = 2.7 ± 0.5), and 2.2 ± 0.2 µm (GSD = 2.1 ± 0.3), respectively. The four powders exhibited fine particle fractions (<4.46 µm), with respect to the emitted dose, of 68% ± 1%, 82% ± 1%, 77% ± 1%, and 68% ± 2% for SD-1, SD-2, SD-3, and SD-4, respectively. The four powders are visualized using scanning electron microscopy in Figure 4.
Each powder was prepared into separate 30-35 mg aliquots, and the stainless-steel needle (21 G) of the dosator was tamped into the powder bed 1 to 4 times. The dosator (21 G stainless-steel inner needle and 16 G polypropylene outer needle) was actuated into a sealed vial containing 5 mL of water. After gentle mixing, the solution was analyzed via UV-Visible spectrophotometry (λ = 351 nm, 268 nm, 271 nm, and 230 nm for SD-1, SD-2, SD-3, and SD-4, respectively) to monitor the dose of powder released from the dosator. The delivered dose as a function of the number of tamps in the powder bed is displayed in Figure 5. Notably, all spray dried powders demonstrated a linear dose-response (R2 > 0.97) from 1 to 4 tamps with these dosators. For SD-1, one tamp led to a powder delivery of 209 ± 99 µg, with each subsequent tamp adding ~130 µg (Figure 5A). The other powders exhibited similar trends, with the first tamp incurring a larger dose of powder than subsequent tamps. For SD-2 (Figure 5B), SD-3 (Figure 5C), and SD-4 (Figure 5D), one tamp led to a delivery of 268 ± 88 µg, 332 ± 95 µg, and 412 ± 72 µg, with each subsequent tamp adding a smaller amount of 170-230 µg. The linear response for each powder allows control in drug loading, with the four powders, SD-1, SD-2, SD-3, and SD-4, demonstrating achievable ranges of 210-570 µg, 270-780 µg, 330-870 µg, and 410-1120 µg, respectively. While all linear and reproducible, the differences seen from one spray dried powder to another highlight the necessity of characterizing the dose released from the dosators for the specific dry powder of interest.
Smaller diameter dosators were also prepared to evaluate their use in smaller/younger mice. The initial design described in the previous paragraph was prepared using a 16 G polypropylene outer needle (outer diameter = 1.7 mm). The 21 G stainless-steel needle used in these dosators is also compatible with a 20 G PTFE outer needle (outer diameter = 1.2 mm) as reported by Stewart et al.9 Figure 6A displays the use of 21 G stainless-steel/20 G PTFE dosators with powder formulation SD-1. A slight decrease in achievable dose is observed as compared to the 21 G stainless-steel/16 G polypropylene dosators, with the initial tamp resulting in a dose of 111 ± 62 µg and each subsequent tamp adding ~96 µg (Figure 6A). The increased length of the PTFE needle (5.08 cm) as compared to the polypropylene needle (3.81 cm) and needle flexibility may lead to powder losses. Smaller diameter polypropylene outer needles were also evaluated but required smaller diameter stainless-steel inner needles. 18 G (outer diameter = 1.3 mm) and 20 G (outer diameter = 1.0 mm) polypropylene needles required 22 G and 25 G stainless-steel inner needles, respectively. As expected, decreasing the inner needle diameter decreased the achievable dose. 22 G stainless-steel/18 G polypropylene dosators, displayed in Figure 6B, demonstrated a SD-1 dose of 82 ± 31 µg with one tamp, with each subsequent tamp increasing the dose by ~41 µg. 25 G stainless-steel/20 G polypropylene dosators, displayed in Figure 6C, demonstrated a smaller SD-1 dose of 29 ± 17 µg, with additional tamps minimally increasing the delivered dose (~4 µg/tamp). Figure 6D displays a comparison of the four dosator systems evaluated herein when using 4 tamps of powder formulation SD-1 and highlights that the dosator system can be customized to meet the dosage needs and age/size of the animal.
Figure 1: Modified needle preparation. (A) Modified stainless-steel needle with plastic luer lock portion trimmed to 2-3 mm. (B–C) Insertion of modified stainless-steel needle into polypropylene needle. Please click here to view a larger version of this figure.
Figure 2: Powder loading and actuation schematic. (A–E) Schematic of powder loading, dosator assembly, and actuation from assembled dosator. Air is forced through the inner stainless-steel needle, dispensing powder out of the dosator. Please click here to view a larger version of this figure.
Figure 3: Removing powder residue from outside of inner needle. (A) Modified stainless-steel needle with powder retained on the outside of the needle after tamping into powder bed. (B) Modified stainless-steel needle with clean surface after gentle wiping with low-lint wiper. (C) Visualization of inside of needle containing powder. Please click here to view a larger version of this figure.
Figure 4: Spray dried powders. Representative scanning electron microscopy images of spray dried powders prepared from four distinct APIs. Dry powders include (A) SD-1, (B) SD-2, (C) SD-3, and (D) SD-4. All imaging was performed at a 10,000x magnification, with the scale bar equal to 5 µm. Please click here to view a larger version of this figure.
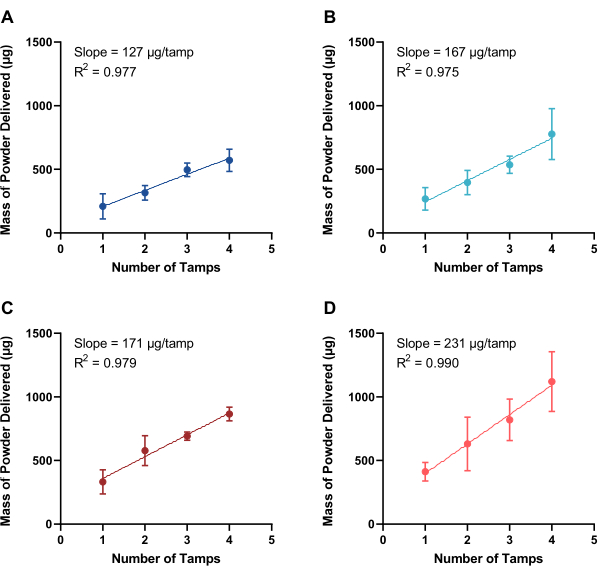
Figure 5: Quantification of powder delivered. Mass of powder delivered from dosators (21 G stainless-steel inner needle and 16 G polypropylene outer needle) as a function of tamps into a powder bed of four spray dried powders, including (A) SD-1, (B) SD-2, (C) SD-3, and (D) SD-4 (n ≥ 3, mean ± standard deviation). The slope, demonstrating the mass of powder dispersed per loading tamp, and the goodness-of-fit (R2) to a linear curve are included. Please click here to view a larger version of this figure.
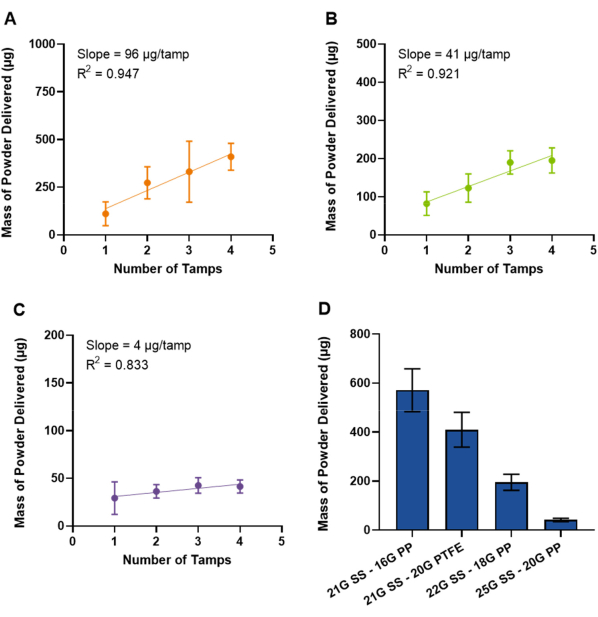
Figure 6: Quantification of powder delivered with smaller diameter needles. Mass of SD-1 powder delivered from dosators prepared using smaller diameter needles, including (A) 21 G stainless-steel inner needle with 20 G PTFE outer needle, (B) 22 G stainless-steel inner needle with 18 G polypropylene outer needle, and (C) 25 G stainless-steel inner needle with 20 G polypropylene outer needle (n ≥ 3, mean ± standard deviation). The slope, demonstrating the mass of powder dispersed per loading tamp, and the goodness-of-fit (R2) to a linear curve are included. The y-axis is scaled to fit the data in each figure. A comparison of dose of SD-1 delivered from all dosator types after 4 tamps into the powder bed is shown in (D). Abbreviations include: SS, stainless-steel; PP, polypropylene; PTFE, polytetrafluoroethylene. Please click here to view a larger version of this figure.
Discussion
As mice are obligate nose breathers, delivery via passive inhalation for initial proof-of-concept studies makes efficiency and dose estimation challenging as the powder must pass the nose and throat in a manner dependent on particle properties and powder dispersion efficiency7,8,14. The use of the dosators developed herein bypasses the nose and throat, with the dosator inserted to the first bronchial bifurcation9, and delivers the full dose directly to the lungs of mice, allowing for more precise dose control for initial studies. These dosators represent a reproducible and customizable delivery method for intratracheal administration to mice and in vitro evaluation of powder performance.
Dosators using 21 G stainless-steel and 16 G polypropylene needles were capable of loading and delivering 200-1100 µg depending on the formulation and the number of tamps, which is typically a suitable dose for mice. Loading beyond 4 tamps was possible for certain formulations, such as SD-1 and SD-2, which retained powder dispersion up to at least 5 tamps, but loading beyond 4 tamps became challenging for formulations such as SD-3 and SD-4. If the powder became too packed in the inner needle after further tamping, a bolus of 0.15-0.6 mL of air was insufficient to dislodge and disperse the powder. While a greater volume of 1-2 mL may be able to disperse these loaded powders, these volumes may cause trauma to mice and should be avoided8,15. In all cases, tamping should be performed gently to minimize this effect. As a result, this effect limits loading above 600-1100 mg, depending on the formulation. While suitable for mice, a larger reservoir-type dosator should be used for animals requiring a greater dose10. Smaller diameter dosators (1.0-1.3 mm outer diameter) were also developed and evaluated with SD-1. The greatest dose for a reduced size dosator was observed when combining a 21 G stainless-steel inner needle with a 20 G PTFE outer needle. Pharmacokinetic studies in mice have been performed previously by Stewart et al. with this dosator system, highlighting its successful use9. Smaller dosators were also possible using polypropylene outer needles but resulted in lower achievable doses, highlighting a limitation in the system. Dose is strongly influenced by needle diameter, and the greater doses reported for the 21 G stainless-steel/16 G polypropylene dosators may not be possible for use in mice that are too small/young.
The dosator systems are confirmed to work across the four spray dried powders discussed herein. However, all particle systems in this study are low-density engineered particles that are of uniform bulk density. Efficacy in other particle systems where uniformity of the powder bed cannot be guaranteed has not yet been evaluated and may not result in reproducible delivery. Additional evaluation will be necessary on a case-by-case basis prior to use of the dosator system.
We describe the use of a precision sectioning saw for preparing the inner dosator needles, but a belt sander can be used in place. If a belt sander is used, it is important to slide a smaller needle or wire through the inner stainless-steel needle to ensure the needle is open and was not occluded in the process. This has not been noticed as an issue when using the precision sectioning saw.
The low cost and ease of preparation of the dosators facilitate their use as single-use disposable delivery devices, where re-loading the device and cleaning/sterilizing between uses is not required. Beds of the dry powder can be pre-filled and stored based on the API and formulation storage requirements, necessitating the user to only tamp the needle into the powder prior to assembly and actuation. Filling of the powder bed tube can be performed in a laboratory setting where a balance and hood are available, requiring minimal equipment to be present in the animal procedure laboratory10. The dosators are designed for preliminary, proof-of-concept studies in mice and demonstrate accurate and reproducible loading.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors wish to acknowledge funding from the National Institutes of Health (R01AI155922). Microscopy was performed at the Chapel Hill Analytical and Nanofabrication Laboratory (CHANL), a member of the North Carolina Research Triangle Nanotechnology Network, RTNN, which is supported by the National Science Foundation, Grant ECCS-1542015, as part of the National Nanotechnology Coordinated Infrastructure, NNCI.
Materials
| 0.6 mL microcentrifuge tubes | Fisher Scientific | 05-408-120 | |
| Analytical balance | Mettler Toledo | AR1140 | Any analytical balance with sufficient range can be used |
| Blunt stainless-steel needle, 1 inch, 21 G | McMaster-Carr | 75165A681 | |
| Blunt stainless-steel needle, 1 inch, 22 G | McMaster-Carr | 75165A683 | |
| Blunt stainless-steel needle, 1 inch, 25 G | McMaster-Carr | 75165A687 | |
| Disposable syringe with luer lock (1 mL) | Fisher Scientific | 14-823-30 | 3-mL syringes can also be used |
| Female BALB/c mice | Charles River, Wilmington, MA, USA | ||
| High-performance cascade impactor | Next Generation Impactor | Apparatus 5 | |
| Lab film (e.g., Parafilm) | Fisher Scientific | S37440 | |
| Low-lint wiper (e.g., Kimwipes) | Kimberly-Clark Professional | 34133 | |
| Low-resistance dry powder inhaler | RS01 mod 7 | ||
| Polypropylene needle, 1.5 inch, 16 G | McMaster-Carr | 6934A111 | |
| Polypropylene needle, 1.5 inch, 18 G | McMaster-Carr | 6934A53 | |
| Polypropylene needle, 1.5 inch, 20 G | McMaster-Carr | 6934A55 | |
| Precision sectioning saw | TedPella | 812-300 | Belt sander can be used as an alternative |
| PTFE needle, 2 inch, 20 G | McMaster-Carr | 75175A694 | |
| USP General Chapter <601> | http://www.uspbpep.com/usp31/v31261/usp31nf26s1_c601.asp |
Riferimenti
- Wu, X., Li, X., Mansour, H. M. Surface analytical techniques in solid-state particle characterization for predicting performance in dry powder inhalers. KONA Powder and Particle Journal. 28, 3-18 (2010).
- Maloney, S. E., Mecham, J. B., Hickey, A. J. Performance testing for dry powder inhaler products: towards clinical relevance. KONA Powder and Particle Journal. 40, 172-185 (2023).
- Maloney, S. E., et al. Spray dried tigecycline dry powder aerosols for the treatment of nontuberculous mycobacterial pulmonary infections. Tuberculosis. 139, 102306 (2023).
- Kaur, J., et al. A hand-held apparatus for "nose-only" exposure of mice to inhalable microparticles as a dry powder inhalation targeting lung and airway macrophages. European Journal of Pharmaceutical Sciences. 34 (1), 56-65 (2008).
- Yi, J., et al. Whole-body nanoparticle aerosol inhalation exposures. Journal of Visualized Experiments. (75), e50263 (2013).
- Chung, Y. H., Han, J. H., Lee, Y. -. H. A study on subchronic inhalation toxicology of 1-chloropropane. Toxicological Research. 31 (4), 393-402 (2015).
- Kuehl, P. J., et al. Regional particle size dependent deposition of inhaled aerosols in rats and mice. Inhalation Toxicology. 24 (1), 27-35 (2012).
- Manser, M., et al. Design considerations for intratracheal delivery devices to achieve proof-of-concept dry powder biopharmaceutical delivery in mice. Pharmaceutical Research. 40, 1165-1176 (2023).
- Stewart, I. E., et al. Development and characterization of a dry powder formulation for anti-tuberculosis drug spectinamide 1599. Pharmaceutical Research. 36 (9), 136 (2019).
- Durham, P. G., et al. Disposable dosators for pulmonary insufflation of therapeutic agents to small animals. Journal of Visualized Experiments. (121), e55356 (2017).
- Miwata, K., et al. Intratracheal administration of siRNA dry powder targeting vascular endothelial growth factor inhibits lung tumor growth in mice. Molecular Therapy: Nucleic Acids. 12, 698-706 (2018).
- Duret, C., et al. Pharmacokinetic evaulation in mice of amorphous itraconazole-based dry powder formulations for inhalation with high bioavailability and extended lung retention. European Journal of Pharmaceutics and Biopharmaceutics. 86 (1), 46-54 (2014).
- Maloney, S. E., et al. Preparation strategies of the anti-mycobacterial drug bedaquiline for intrapulmonary routes of administration. Pharmaceuticals. 16 (5), 729 (2023).
- Price, D. N., Kunda, N. K., Muttil, P. Challenges associated with the pulmonary delivery of therapeutic dry powders for preclinical testing. KONA Powder and Particle Journal. 36, 129-144 (2019).
- Qiu, Y., Liao, Q., Chow, M. Y. T., Lam, J. K. W. Intratracheal administration of dry powder formulation in mice. Journal of Visualized Experiments. (161), e61469 (2020).
- Fiegel, J., et al. Preparation and in vivo evaluation of a dry powder for inhalation of capreomycin. Pharmaceutical Research. 25 (4), 805-811 (2008).

