Establishment and Evaluation of a Risk Prediction Model for Pathological Escalation of Gastric Low-Grade Intraepithelial Neoplasia
Summary
Here, we performed a systematic evaluation of patients diagnosed with gastric low-grade intraepithelial neoplasia by previous endoscopic biopsy and obtained a pathological diagnosis by complete resection of the lesion by endoscopic submucosal dissection (ESD), analyzing factors that potentially increase the risk of pathological escalation.
Abstract
The study aims to explore the risk factors for pathological escalation after endoscopic surgery for gastric low-grade intraepithelial neoplasia (LGIN) and to establish and evaluate a risk prediction model for LGIN. A total of 120 patients diagnosed with gastric LGIN by biopsy and endoscopic submucosal dissection (ESD) between November 2020 and June 2022 were retrospectively analyzed. Gender, age, Helicobacter pylori (HP) infection, lesion size, lesion location, morphology, gastric mucosal congestion, nodules status, surface ulceration and erosion, and ME-observation of all patients were collected and divided into upgraded and non-upgraded groups according to the biopsy and ESD postoperative pathological diagnosis results. Independent risk factors for pathological escalation after ESD surgical treatment were screened by logistic regression analysis, and a risk prediction model was established. Among the 120 patients with gastric LGIN, 49 patients developed postoperative pathological upgrading; the rate of pathological upgrading was 40.83%. Among them, 42 patients were upgraded to high-grade intraepithelial neoplasia (HGIN), 1 case was upgraded to advanced gastric cancer, and 6 cases were upgraded to early gastric carcinoma (EGC). Univariate analysis showed that age, lesion size, gastric mucosal congestion, surface ulcers, and erosion were significantly different between the groups (p < 0.05). Multivariate Logistic regression analysis revealed that age ≥60 years, focal length ≥2 cm, gastric mucosal congestion, and surface ulceration and erosion were independent risk factors for postoperative pathological escalation in patients with gastric LGIN. Final joint probability forecasting model for P = 1/[1 + e(26.515-0.161 x β1-0.357 x β2+0.039 x β3-0.269 x β4)]. Age, lesion size ≥2 cm, gastric mucosal congestion, and lesion surface ulceration and erosion are risk factors for postoperative pathological upgrading in patients with gastric LGIN. The risk prediction model established in this study based on risk factors has predictive value and can provide a scientific reference for the clinical treatment of patients with gastric LGIN.
Introduction
Gastric cancer is one of the most common malignant tumors, especially in East Asia, with high incidence and mortality rates. It is one of the most common cancers in China, with new diagnoses and deaths accounting for about half of the global total1. It is a major cause of morbidity and mortality in the Chinese population2. Gastric cancer ranks third in the world in terms of the highest mortality rate among tumors, and its prognosis is highly dependent on the stage of the lesion1. The 5-year survival rate for advanced-stage patients is less than 30%, while the 5-year survival rate for early-stage patients is usually more than 90%. Therefore, early diagnosis and treatment of gastric cancer are essential to prevent and control the disease3.
The Correa cascade reaction is widely recognized as one of the major patterns of gastric cancer development, suggesting that the carcinogenic process of gastric cancer gradually progresses from atrophic gastritis to intestinal metaplasia, intraepithelial neoplasia, and finally to adenocarcinoma4. With the application of modern technology and the increasing popularity of gastroscopy, an increasing number of gastric mucosal precancerous lesions have been detected, including magnifying endoscopy (ME), staining endoscopy, and narrow-band imaging (NBI)5.
Low-grade intraepithelial neoplasia (LGIN) is one of the precancerous lesions of gastric cancer and is closely related to gastric cancer. However, some patients with low-grade intraepithelial neoplasia showed pathological upgrading after endoscopic submucosal dissection (ESD) compared with biopsy findings6. Therefore, there is some controversy in clinical practice regarding the choice of follow-up or treatment for patients with biopsy-proven LGIN. This article explores the risk factors for pathological upgrading after ESD treatment in patients with gastric LGIN establishes and evaluates a risk prediction model for LGIN occurrence, and provides more scientific and valuable reference opinions for the clinical management of gastric LGIN patients.
Protocol
The protocol described in this study has been reviewed and approved by the ethics committee of Longyan First Affiliated Hospital of Fujian Medical University in accordance with the ethical guidelines established by the Declaration of Helsinki. The safety and well-being of human participants are our topmost concern, and all procedures have been designed to minimize potential risks and discomfort. All data collected will be treated confidentially and used solely for the purposes of this research. Subjects will be free to withdraw from the study at any time, and their decision to participate or withdraw will not affect their relationship with the researchers or the institution.
1. General Information
- Select patients diagnosed with gastric LGIN. In this retrospective analysis, a total of 120 patients diagnosed with gastric LGIN by histopathological examination and treated with ESD at Longyan First Hospital, the Third Hospital of Xingtai, and Gansu Cancer Hospital from November 2020 to June 2022 were included.
2. Inclusion and exclusion criteria
- Inclusion criteria
- Include patients diagnosed with gastric LGIN by histopathological examination and treated with ESD within 3 months of diagnosis.
- Ensure that all biopsy and surgical specimens are diagnosed according to the World Health Organization (WHO) pathological diagnostic criteria for tumors of the digestive system7, and all pathological sections are reviewed by two pathologists before and after surgery.
- Complete clinical data.
- Exclusion criteria
- Exclude patients who have recently received antibiotics, proton pump inhibitors (PPIs), bismuth preparations, and acid suppressants for the treatment of Helicobacter pylori (HP) infection.
- Exclude patients diagnosed with gastric high-grade intraepithelial neoplasia (HGIN), gastric cancer, or other metastatic tumors by histopathological examination.
- Exclude patients who underwent surgery.
- Exclude patients who underwent radiotherapy or chemotherapy.
- Exclude patients with incomplete clinical data.
3. Research methods
- Endoscopic examination and surgery
- Let all patients diagnosed with gastric LGIN by histopathological examination undergo routine white light endoscopy (WLE).
- Prior to performing endoscopy, ensure that the patient undergoes some preparations, including fasting for more than 6 h.
NOTE: The examination usually involves local anesthesia of the throat to minimize discomfort. - Insert the endoscope through the patient's mouth and gradually advance it through the esophagus into the stomach. The interior of the digestive tract is observed under WLE, including the color, shape, texture, and distribution of blood vessels in the mucosa, looking for possible lesions.
- Take tissue samples or perform other therapeutic operations depending on the observation results. After completing the examination, gradually withdraw the endoscope and end the examination.
- Prior to performing endoscopy, ensure that the patient undergoes some preparations, including fasting for more than 6 h.
- Use ME-NBI technology to observe the microstructure of the gastric mucosal surface to determine the extent of the lesion further.
NOTE: ME-NBI is a formation mode that is switched by a button on the endoscope after observing the approximate mucosa in the WLE mode. It can observe microvascular (MV) and microstructure (MS) that cannot be observed with conventional endoscopy.- After finding the suspicious lesion in WLE, zoom in from far to near, from normal to the center of the lesion.
- Through ME-NBI examination, judge whether the demarcation line (DL) exists, the anisotropy of the MV and MS, and some special signs on the surface of the lesion to better observe the microstructure of the surface of the GI mucosa, and then make a judgment about the nature of the lesion, which may be lesions.
- Next, make an electrocoagulation mark with a disposable mucosal incision knife 3-5 mm outside the lesion boundary in the patient, with a distance of approximately 2 mm between the mark points.
- Inject the prepared injection solution (250 mL of saline + 3 mg of epinephrine + 2 mL of indigo rouge) into the submucosa through a special endoscopic injection needle.
NOTE: Saline is mainly used to keep the submucosa moist and separate the mucosal layer from the muscular layer. Epinephrine constricts blood vessels and reduces bleeding. Indigo Carmine is a dye that helps doctors better visualize the distribution of blood vessels in the submucosa. The ingredients dispensed include 250 mL of saline + 3 mg of epinephrine + 2 mL of indigo rouge. - Once the lesion is completely elevated, make a circumferential incision with a disposable mucosal incision knife at a point approximately 3 mm from the lesion marking point, followed by submucosal dissection until the lesion is completely removed.
- After dissection, carefully observe the wound and perform electrocoagulation using electrocoagulation forceps. If necessary, use metal hemostatic clips to clamp the wounds.
- Let all patients diagnosed with gastric LGIN by histopathological examination undergo routine white light endoscopy (WLE).
- Pathological examination
- Remove the tissue sample after complete excision using suction or snare.
- Fix and preserve the removed tissue sample and mark the oral and anal sides for pathological examination and diagnosis. Fix the samples in 10% formalin for 24-48 h at 20-25 °C.
NOTE: The pathological description included information on the general morphology of the lesion, volume and size, margins, histological classification, depth of infiltration, and gastric mucosal lesions.
- Fix and preserve the removed tissue sample and mark the oral and anal sides for pathological examination and diagnosis. Fix the samples in 10% formalin for 24-48 h at 20-25 °C.
- Remove the tissue sample after complete excision using suction or snare.
- HP detection method
- Use either the rapid urease test or the 13C breath test.
- In the rapid urease test, take a sample of the patient's stomach lining with biopsy forceps under endoscopy and place it in a reagent containing urease.
- Check for the presence of H. pylori in the stomach lining. The urease enzyme breaks down the urea to produce ammonia, which makes the reagent alkaline and turns it red. Observe the change in color of the reagent and determine the presence of H. pylori infection.
NOTE: The 13C-breath test is a non-invasive, painless, and side-effect-free test performed by orally administering urea containing 13C-labeled urea and using an isotope ratio mass spectrometer to detect the amount of 13C-labeled carbon dioxide in the patient's exhaled breath. If Helicobacter pylori is present in the stomach, the bacteria will break down the 13C-labeled urea to produce 13C-labeled carbon dioxide, and the presence or absence of Helicobacter pylori infection can be determined by measuring the amount of 13C-labeled carbon dioxide in the exhaled gas. It is important to note that patients must discontinue proton pump inhibitors (PPIs) for at least 2 weeks and antibacterial drugs, bismuth, and certain herbal medicines with antibacterial properties for at least 4 weeks before performing the rapid urease test or 13C breath test. These medications can affect the accuracy of the test results. Patients must also fast or refrain from eating for at least 2 h during the 13C breath test to avoid the influence of food on the test results.
- Use either the rapid urease test or the 13C breath test.
- Data collection and grouping
- Compile the general clinical data of the patients, including gender, age, Helicobacter pylori (HP) infection, lesion size, lesion location, morphology, gastric mucosal congestion, nodules status, surface ulceration and erosion, and ME observation.
- Divide all patients in the study into upgraded and non-upgraded groups according to whether the post-ESD pathological diagnosis was upgraded based on the evaluations performed.
NOTE: The upgraded group had a postoperative pathological diagnosis of gastric HGIN, early gastric cancer (EGC), or advanced gastric cancer, while the non-upgraded group had a postoperative pathological diagnosis of gastric LGIN or inflammation.
4. Statistical methods
- Use statistical software to organize and analyze all data. Analyze categorical data statistically as frequencies (in percentages) and compare using chi-square tests or Fisher's exact tests.
NOTE: SPSS software was used in this study. - Employ logistic regression analysis to identify risk factors associated with post-ESD pathological upgrading in patients diagnosed with gastric LGIN. A P-value less than 0.05 indicates a statistically significant difference.
Representative Results
Incidence of pathological upgrade in gastric LGIN patients after ESD
A total of 120 gastric LGIN patients were included in this study, of which 49 (40.83%) experienced pathological upgrades after ESD. Among them, 42 cases were upgraded to HGIN, 1 case was upgraded to advanced gastric cancer, and 6 cases were upgraded to EGC. A total of 71 cases did not experience pathological upgrade, among which 2 cases were downgraded to inflammation, and 69 cases remained as LGIN, with a rate of pathological downgrade of 1.67%.
Univariate analysis of pathological upgrade after ESD in gastric LGIN patients
As shown in Table 1, there were no statistically significant differences (p > 0.05) in gender, HP infection, lesion location, surface nodules, presence of a demarcation line (DL) in the ME layer, lesion morphology, microstructure (MS), and microvascular (MV) morphology between the postoperative pathological upgrade group and the non-upgrade group of gastric LGIN patients. However, there were statistically significant differences (p < 0.05) in age, lesion size, gastric mucosal congestion, and surface ulceration and erosion.
Multivariate logistic regression analysis of pathological upgrade after ESD in gastric LGIN patients
Factors with p < 0.05 screened out by univariate analysis, including age, lesion size, gastric mucosal congestion, and surface ulceration and erosion, which were used as independent variables, and pathological upgrade after ESD in gastric LGIN patients was used as the dependent variable for logistic regression analysis (Table 2). Table 3 showed that age ≥60 years, lesion diameter ≥2 cm, gastric mucosal congestion, and surface ulceration and erosion were independent risk factors for pathological upgrade after ESD in patients with gastric LGIN. The final joint probability prediction model was P = 1 / [1 + e(26.515-0.161xβ1-0.357xβ2+0.039xβ3-0.269xβ4)] based on the prediction formula for logistic regression: P(y = 1/x) = 1/(1+e-(β0+β1×1+β2×2+β3×3+β4×4)), where y represents the dichotomous dependent variable, x represents the vector of independent variables, β represents the regression coefficient, and e represents the base of the natural logarithm. Additionally, technical term abbreviations will be defined when first used. This formula indicates that given the independent variable x, the probability of event y = 1 occurring is P(y = 1/x). When the sum of β0 + β1 x 1 + β2 x 2 + β3 x 3 + β4 x 4 increases, so too does the value of P(y = 1/x), indicating a higher probability of the event y = 1 occurring; and conversely, a lower probability of the event y = 1 occurring. Here, β1 is the regression coefficient of age, β2 is the regression coefficient of lesion size, β3 is the regression coefficient of gastric mucosal congestion, and β4 is the regression coefficient of surface ulceration and erosion.
Case presentation
As a representative case, a 50-year-old female patient presented with rough mucosa and erosion at the gastric angle during a physical examination. The endoscopic biopsy confirmed low-grade intraepithelial neoplasia (LGIN). Further investigations using magnification endoscopy, narrow band imaging (NBI), and pigment endoscopy suggested the patient was at risk of developing high-grade intraepithelial neoplasia (HGIN). The patient was advised to undergo endoscopic submucosal dissection (ESD) treatment, and the final pathological diagnosis was HGIN (as indicated in Figure 1).
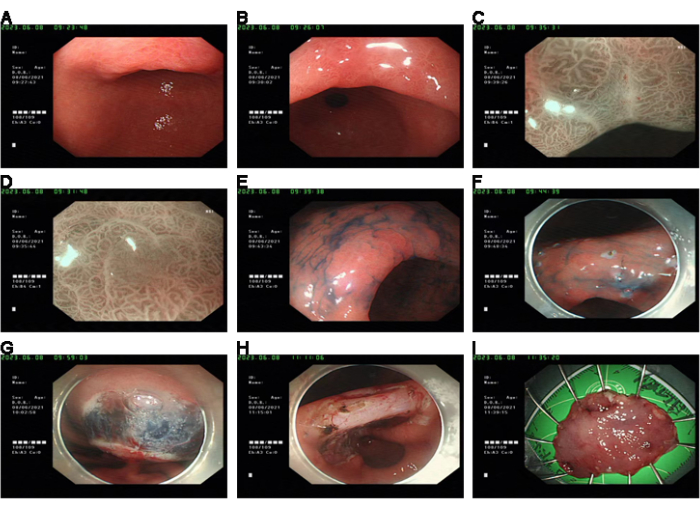
Figure 1: Case presentation. (A,B) Pre-operation, WLE showed rough gastric mucosa with erosion. (C,D) Irregular MV and MS were observed in the lesions observed by ME + NBI. (E) The contours of the lesions could be seen by indigo blush staining. (F) The lesions were labeled under the endoscope. (G) Stripped after submucosal water injection. (H) Wound hemostatic treatment. (I) Fixed specimens were sent for pathological examination. Please click here to view a larger version of this figure.
Table 1: Univariate analysis of post-ESD pathological upgrade (%). Please click here to download this Table.
Table 2: Multivariate logistic regression analysis variable assignment table for post-ESD pathological upgrade. Please click here to download this Table.
Table 3: Multivariate logistic regression analysis of post-ESD pathological upgrade. Please click here to download this Table.
Discussion
Gastric LGIN is a common gastrointestinal disease, and as the number of patients continues to increase, the prevention and treatment of the disease become increasingly crucial. Establishing a risk prediction model is essential in guiding clinical treatment and preventing disease occurrence8. Pathological upgrading of gastric LGIN refers to the deterioration of LGIN properties within a certain period, which can lead to the transformation of LGIN into HGIN or even more severe conditions. In clinical practice, the prediction of pathological upgrading has become a focus of attention for medical personnel. Currently, some prediction models based on clinical manifestations, imaging findings, and biomarkers have been studied, but these have the disadvantages of complex indicators and long-term consumption. Therefore, it is necessary to develop a simple and effective predictive model. According to the current domestic consensus recommendations, ESD should be actively adopted for the treatment of gastric LGIN patients with high-risk factors for pathological upgrading. However, an increasing number of studies have shown significant differences between biopsy pathological results and postoperative pathological results in gastric LGIN patients9.
The study revealed that out of the 120 gastric LGIN patients observed, 49 underwent pathological upgrading post-surgery, resulting in a pathological upgrading rate of 40.83%. This rate is relatively elevated when compared to other relevant studies. Possible reasons for these findings include underestimation of the results obtained from pathological biopsy. Variations in endoscopists’ qualifications may lead to variations in patients’ condition and biopsy site assessment, resulting in superficial or inadequate sampling and site deviations. The accuracy of results may also be influenced by the cognitive ability and knowledge of pathologists. Additionally, biopsy accuracy may be affected by different endoscopic techniques. For instance, the application of high-definition gastroscopy to observe the microvascular and microstructure morphology of lesions and determine the most conspicuous site for targeted biopsy can considerably enhance the precision of biopsy.
This study demonstrated that the older the age of gastric LGIN patients, the higher the risk of pathological upgrading after surgery, which is similar to other domestic research results10. Consequently, for gastric LGIN patients over 60 years of age, close follow-up should be performed, and if the patient has more risk factors, timely surgery is recommended. Regarding the size of the lesion, this study showed that lesion size ≥2 cm is an independent risk factor for pathological upgrading, which is basically consistent with other research results11. The critical value of lesion size that may affect the occurrence of pathological upgrading after surgical treatment of gastric LGIN patients is still controversial, and clinical research is needed to find the most suitable critical value. Large sample size methods should be used for further research. The results showed that mucosal surface congestion, ulceration, and erosion are independent risk factors for pathological upgrading after surgery in patients with gastric LGIN, which is similar to relevant research12. Lesion surface ulceration and erosion are more likely to cause pathological upgrading after surgery, which may be because repeated damage to the gastric mucosa can promote dysplasia and intestinal metaplasia, thereby increasing the risk of gastric cancer.
However, this study encountered certain limitations. Firstly, being a retrospective study made it impossible to fully control for bias and other confounding factors. In the future, prospective studies ought to be carried out to validate the conclusions and strengthen objectivity and inference ability. Secondly, due to the limited number of samples, the samples could not be segregated into a validation set for external validation of the model. Therefore, it is essential to gather additional patient data from several centers to augment the number of samples and expand the scope of observed variables. This will improve the model’s predictive efficacy and the credibility of its outcomes. Furthermore, conducting external validation will reinforce the model’s reliability.
In conclusion, our research reveals that the risk prediction model, generated through analysis of independent risk factors for pathological upgrading in patients with gastric LGIN who underwent surgery, offers significant predictive value and can provide beneficial guidance for LGIN treatment in clinical contexts.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study is funded by the Longyan City Science and Technology Plan Project (Grant number 2020LYF17029).
Materials
| Disposable mucosal incision knife | Olympus (Japan) | KD-650Q | |
| Endoscopic image processing device | Olympus (Japan) | CV-290 | |
| Hemostasis Clips | MICRO-TECH(Nanjing) | ROCC-D-26-195 | |
| High-frequency hemostatic forceps | Olympus (Japan) | FD-410LR | |
| Indicarminum | MICRO-TECH(Nanjing) | MTN-DYZ-15 | |
| Injection Needles | MICRO-TECH(Nanjing) | IN02-25423230 | |
| Magnifying gastroscope | Olympus (Japan) | GIF-H290Z | |
| Orthodontic rubber band | 3M Unitek Corporation | 6.4 mm 3.5 oz | |
| Therapeutic gastroscopy | Olympus (Japan) | GIF-2TQ260M | |
| Transparent cap | Olympus (Japan) | D-201-11804 |
Riferimenti
- Bray, F., et al. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 68 (6), 394-424 (2018).
- Migita, K., et al. Rnf126 as a marker of prognosis and proliferation of gastric cancer. Anticancer Res. 40 (3), 1367-1374 (2020).
- Zhang, H., Yang, X., Zhang, X., Huang, X. The significance of endoscopic kyoto classification of gastritis in the gastric cancer risk assessment: A systematic review and meta-analysis. Medicina. 102 (22), e33942 (2023).
- Cheng, H. C., et al. Evolution of the correa’s cascade steps: A long-term endoscopic surveillance among non-ulcer dyspepsia and gastric ulcer after H. pylori eradication. J Formos Med Assoc. 122 (5), 400-410 (2023).
- Kim, B., Cho, S. J. Endoscopic screening and surveillance for gastric cancer. Gastrointest Endosc Clin N Am. 31 (3), 489-501 (2021).
- Choi, C. W., et al. The risk factors for discrepancy after endoscopic submucosal dissection of gastric category 3 lesion (low grade dysplasia). Dig Dis Sci. 59 (2), 421-427 (2014).
- Nagtegaal, I. D., et al. The 2019 who classification of tumours of the digestive system. Histopathology. 76 (2), 182-188 (2020).
- Zou, L., et al. Endoscopic characteristics in predicting prognosis of biopsy-diagnosed gastric low-grade intraepithelial neoplasia. Chin Med J (Engl). 135 (1), 26-35 (2022).
- Ryu, D. G., et al. Clinical outcomes of endoscopic submucosa dissection for high-grade dysplasia from endoscopic forceps biopsy. Gastric Cancer. 20 (4), 671-678 (2017).
- Xu, G., et al. Risk factors for under-diagnosis of gastric intraepithelial neoplasia and early gastric carcinoma in endoscopic forceps biopsy in comparison with endoscopic submucosal dissection in Chinese patients. Surg Endosc. 30 (7), 2716-2722 (2016).
- Kim, M. K., et al. Is lesion size an independent indication for endoscopic resection of biopsy-proven low-grade gastric dysplasia. Dig Dis Sci. 59 (2), 428-435 (2014).
- Zhao, G., et al. How commonly is the diagnosis of gastric low grade dysplasia upgraded following endoscopic resection? A meta-analysis. PLoS One. 10 (7), e0132699 (2015).

