Immunomagnetic Isolation of the Vascular Wall-Resident CD34+ Stem Cells from Mice
Summary
This study has established a stable and efficient method for the isolation, culture, and functional determination of vascular wall-resident CD34+ stem cells (CD34+ VW-SCs). This easy-to-follow and time-effective isolation method can be utilized by other investigators to study the potential mechanisms involved in cardiovascular diseases.
Abstract
Resident CD34+ vascular wall-resident stem and progenitor cells (VW-SCs) are increasingly recognized for their crucial role in regulating vascular injury and repair. Establishing a stable and efficient method to culture functional murine CD34+ VW-SCs is essential for further investigating the mechanisms involved in the proliferation, migration, and differentiation of these cells under various physiological and pathological conditions. The described method combines magnetic bead screening and flow cytometry to purify primary cultured resident CD34+ VW-SCs. The purified cells are then functionally identified through immunofluorescence staining and Ca2+ imaging. Briefly, vascular cells from the adventitia of the murine aorta and mesenteric artery are obtained through tissue block attachment, followed by subculturing until reaching a cell count of at least 1 × 107. Subsequently, CD34+ VW-SCs are purified using magnetic bead sorting and flow cytometry. Identification of CD34+ VW-SCs involves cellular immunofluorescence staining, while functional multipotency is determined by exposing cells to a specific culture medium for oriented differentiation. Moreover, functional internal Ca2+ release and external Ca2+ entry is assessed using a commercially available imaging workstation in Fura-2/AM-loaded cells exposed to ATP, caffeine, or thapsigargin (TG). This method offers a stable and efficient technique for isolating, culturing, and identifying vascular wall-resident CD34+ stem cells, providing an opportunity for in vitro studies on the regulatory mechanisms of VW-SCs and the screening of targeted drugs.
Introduction
The vascular wall plays a pivotal role in vascular development, homeostatic regulation, and the progression of vascular diseases. In recent years, numerous studies have unveiled the presence of various stem cell lineages in arteries. In 2004, Professor Qingbo Xu's group first reported the existence of vascular stem/progenitor cells in the periphery of the adult vascular wall, expressing CD34, Sca-1, c-kit, and Flk-11. These vascular stem cells exhibit multidirectional differentiation and proliferation potential. Under normal conditions, they remain relatively quiescent; however, when activated by specific factors, they can differentiate into smooth muscle cells, endothelial cells, and fibroblasts. Alternatively, they can regulate the perivascular matrix and microvessel formation through paracrine effects to promote the repair or remodeling of injured vessels2,3,4,5,6. Recently, resident CD34+ stem cells in the vascular wall were found to play a role in endothelial cell regeneration after femoral artery guidewire injury2. Consequently, the isolation and quantification of CD34+ VW-SCs and the examination of the basic biological characteristics of CD34+ stem cells are crucial for further studying the signal pathways involved in the regulation of CD34+ VW-SCs.
Various methods for cell separation are currently available, including techniques based on cell culture characteristics or physical properties of cells such as density gradient centrifugation, which results in sorted cells containing many non-target cells and relatively low purity7,8,9,10,11,12. Another commonly used technique is fluorescence/magnetic-assisted cell sorting. Fluorescence-activated cell sorting (FACS) is a complex system with high technical requirements, and it is relatively expensive, time-consuming, and potentially affects the activity of sorted cells13,14. However, magnetic-activated cell sorting (MACS) is more efficient and convenient, with a high recovery rate and cell activity and less impact on downstream applications8. Therefore, in this protocol, we applied MACS to purify CD34+ VW-SCs and further identified the cells by flow cytometry. The establishment of MACS-based isolation methods for studying vascular wall stem cells would be invaluable. Firstly, it permits experimental genetic and cell biological studies. Secondly, efficient isolation and culture of vascular wall resident stem cells allow systematic assessment and screening of signaling factors regulating stem cell functions. Thirdly, identification of crucial phenotypes in stem cells provides important 'quality control' in assessing cell status. Thus, identifying methods to purify could be useful for similar applications to analogous stem cells derived from vessels.
This report provides a detailed demonstration of a stable and reliable method for the culture of CD34+ VW-SCs, including cell identification and functional assessment performed by flow cytometry, immunofluorescence staining, and Ca2+ signaling measurement. This study provides a basis for further in-depth research on the function of CD34+ VW-SCs and their regulatory mechanisms in physiological and pathological conditions.
Protocol
This study was approved, and the animals were handled in accordance with the Guidelines for the Management and Use of Laboratory Animals in China. The research strictly adhered to the ethical requirements of animal experiments, with approval from the Animal Ethics Committee (Approval Number: SWMU2020664). Eight-week-old healthy C57BL/6 mice of either gender, weighing between 18-20 g, were utilized for the present study. The animals were housed at the Laboratory Animal Center of Southwest Medical University (SWMU).
1. Tissue block culture of adventitia from Aorta and mesenteric arteries
- Resident CD34+ VW-SCs isolation
- Anesthetize two mice with 3% isoflurane inhalation (following institutionally approved protocols). Confirm adequate anesthesia by toe-pinch. Position the mouse supine with medical tape and spray 70% ethanol sanitizer to disinfect the fur of the mouse. Open the thoracic and abdominal cavities using sterile ophthalmic scissors and forceps to expose the heart and intestines. After dissecting the isolated aorta and mesenteric arteries, pin the aorta and mesenteric bed in a petri dish containing silicon elastomer and filled with a physiological salt solution. With another set of sterilized scissors and forceps, rapidly dissect off the fat around the aorta and mesenteric arteries.
- Transfer the dissected tissues to a 6 cm Petri dish containing 1% Penicillin-Streptomycin-Amphotericin B solution (see Table of Materials). After rinsing twice, transfer the arteries to a tissue culture hood under a stereo microscope.
- Cut the arteries longitudinally and use fine forceps to peel off the outer layer of the aorta and the first branch of mesenteric arteries. Transfer the outer layers to a 3.5 cm Petri dish containing 0.1-0.2 mL of culture medium (see Table of Materials). Cut the outer layers into tiny pieces (about 1 mm3).
- Transfer the tissue pieces into a gelatin-coated T25 flask, ensuring even distribution on the flask's bottom. Maintain a moderate distance between tissue pieces to facilitate cell crawling and growth. Place the flask vertically in an incubator (37 °C, 5% CO2) for 3 h to allow tissue blocks to adhere to the flask's bottom.
- After 3 h, add 5 mL of DMEM high glucose growth medium to submerge the tissue blocks. Orient the T25 flask horizontally. Monitor cell migration around tissue blocks through a microscope at 3-day intervals.
NOTE: The medium should contain 20% fetal bovine serum, 0.2% Leukemia Inhibitory Factor (LIF), 0.2 mM β-Mercaptoethanol, and 1% penicillin/streptomycin (see Table of Materials). Fetal bovine serum supports cell proliferation, while LIF and β-Mercaptoethanol impede cell differentiation7,8. - When cells in the T25 flask reach about 80% confluence, passage and culture them into one or two T75 flasks. Subculture the cells over five passages to ensure proper growth and health.
- Magnetic separation of resident CD34+ stem cells
- When the cells in one or two T75 flasks reach 80% confluence, dissociate the cells with Trypsin, and resuspend them in 1 mL of sorting buffer (2 mM EDTA and 0.5% FBS). Determine the cell number (107/T75 flask) and centrifuge the cell suspension at 300 x g for 5 min (at room temperature).
- Discard the supernatant completely and resuspend the cell pellet in 98 µL of sorting buffer per 107 total cells. Add 2 µL CD34 antibody (see Table of Materials). Mix well and incubate for 30 min at 4°C in the dark with occasional shaking.
- Wash cells by adding 2 mL of sorting buffer and centrifuge at 300 x g for 10 min at room temperature.
- Remove the supernatant carefully and completely, and resuspend the cells in 80 µL of sorting buffer.
- Add 20 µL Anti-FITC MicroBeads (per 107 total cells, see Table of Materials) into the buffer. Mix well by repetitive pipetting and incubate for 15 min at 4 °C in the dark with intermittent shaking.
- Wash the cells twice by adding 2 mL of buffer and centrifuge at 300 x g for 5 min to repellet cells. Discard the supernatant completely and resuspend the cells in 1 mL of buffer.
- Position one MS column within the magnetic field of an appropriate separator, ensuring that a collection tube (e.g., a 15 mL centrifuge tube) is placed beneath the MS column to collect the separated components. (see Table of Materials). Rinse the column with 500 µL buffer and wait until it has completely run through.
- Load the cell suspension into the column and collect the flow-through containing unlabeled cells into the 15 mL tube.
- Remove the column from the separator and place it on a new 15 mL centrifuge tube. Introduce 500 µL of buffer into the column, then promptly flush out the magnetically labeled cells by pushing the plunger into the column.
- To enhance the purity of CD34+ cells, the eluted fraction can undergo enrichment using a second MS column.Repeat the separation process as mentioned above. Assess the purity of the sorted cells via flow cytometry.
NOTE: Typically, 10%-20% CD34+ cells are obtained, with around 70%-80% negative cells, and approximately 10% loss due to staining and centrifuge procedures. - To further confirm the purity of vascular wall CD34+ stem cells, identify the sorted cells by flow cytometry. The present study showed a purity of more than 90%.
2. Cell identification by immunofluorescence
- Seed cells on coverslips (diameter 8 mm) pre-coated with 0.04% gelatin.
- When the cells reach about 60%-70% confluence, rinse two times with PBS and fix the cells with 4% paraformaldehyde for 10 min.
- Wash cells with PBS for 3 min (3 times), and permeabilize the cells with 0.2% Triton X-100 (in PBS) for 5 min at room temperature.
- Wash cells with PBS for 3 min (3 times), and block the non-specific binding of the antibodies with 5% donkey serum (in PBS) for 1 h.
- Remove the blocking buffer by holding each coverslip on its edge with forceps and draining it onto a sheet of lint free wipe.
- Incubate the cells in 100 times diluted primary antibodies (CD34, Flk-1, c-kit, Sca-1, Ki67, see Table of Materials) in 1% BSA (in PBS) overnight at 4 °C.
- Decant the solution and wash the cells with PBS for 3 min (3 times). Incubate the cells with Alexa Fluor 488 labeled secondary antibody (1:200 in 1% BSA) (see Table of Materials) for 1 h at room temperature in the dark.
- Perform DAPI counterstaining by diluting DAPI stock solution to 0.5 µg/mL in PBS and incubating cells for 5 min.
- Rinse with PBS and seal the coverslips with antifade reagent. Capture images under the laser scanning confocal microscope.
3. Induced differentiation and characterization of CD34+ VW-SCs
- Seed CD34+ stem cells at an appropriate density (40%-50% confluence) on coverslips and 6-well plates, respectively.
- Induce endothelium cell-oriented differentiation from CD34+ cells by culturing cells in endothelial cell culture medium EBM-2 (see Table of Materials) for 1 week.
- Induce fibroblast cell-oriented differentiation from CD34+ cells by culturing cells in fibroblast cell culture medium FM-2 (see Table of Materials) for 3 days.
- Subject the undifferentiated and differentiated cells to immunofluorescence staining (as mentioned in step 2) at laser excitation of 488 nm and a 40x objective. Detect the expression of endothelial cell markers (CD31, VWF) and fibroblast cell markers (PDGFRα, Vimentin) (see Table of Materials).
- Dissociate the cells with Trypsin and obtain the cell pellet by centrifugation (300 x g, 5 min, room temperature). Resuspend cells with 100 µL of sorting buffer. Preincubate the cell suspension with purified anti-mouse CD16/CD32 mAb (1 µg) (see Table of Materials) at 4 ˚C for 5 min.
- Add 2 µL of PE Rat anti-Mouse CD34 (1:50), CD31 (PECAM-1) Monoclonal Antibody APC (2 µL, 1:50), and CD140a (PDGFRa) Monoclonal Antibody FITC (2 µL, 1:50) directly to preincubated cells in the presence of Mouse Fc Block and further incubate at 4 °C for 15 min.
- Wash the cells twice by adding 2 mL of buffer and centrifuge at 300 x g for 5 min (room temperature) to repellet the cells. Discard the supernatant completely and resuspend the cells in 300 µL of buffer. Put flow tubes with stained cells into an icebox ready for Flow cytometry.
4. Detection of intracellular Ca2+ signaling in vascular CD34+ stem cells
- Seed cells on coverslips 10 h before experiments at a density up to 70% confluence.
- Take out the coverslips and affix them to the bottom of a custom chamber with any petroleum jelly.
- Wash the coverslips once with PBS, replace with Fura-2/AM (5 µM) in Tyrode's solution (NaCl 137 mM, KCl 5.4 mM, MgCl2 1.2 mM, glucose 10 mM, HEPES 10 mM, CaCl2 2.4 mM) (see Table of Materials), and incubate cells for 30 min in the dark.
- Remove the dye by treating with Tyrode's solution for 10 min to allow for fura-2/AM de-esterification.
- Mount the chamber on the stage of an inverted fluorescence microscope equipped with a wide-field imaging system containing a monochromator and a CCD camera (see Table of Materials).
- Open the main window, go to the Grab Settings dialog and camera page, and press the live button for live image display.
- Choose the fluorescence image type, set the 340 nm/380 nm loop repeat times, image size, and camera exposure time at 380 nm and 340 nm to achieve optimal image brightness.
- Take single snapshots, and draw several freehand lines to circle the cells with different colors and predefine Region of Interests (ROIs), indicating the background as ROI 0.
- Re-edit an already embedded protocol in the workspace view and create a new workspace.
- Execute the protocol, and the "Fluorescence 340 nm div Fluorescence 380 nm (Live Kinetic)" will display the online kinetic graph.
- In the numerical view, a spreadsheet will be visible, presenting columns of gray-scale values and corresponding time information. Export the spreadsheet data by using the clipboard.
- Calculate F340 nm/F380 nm indicating intracellular Ca2+ changes after subtracting the background fluorescence (ROI 0).
Representative Results
Isolation and purification of CD34+ VW-SCs
High purity of CD34+ VW-SCs is obtained from the adventitia of the mouse aortic and mesenteric artery by tissue attachment and magnetic microbead sorting. The percentage of CD34+ cells in the vessel wall is generally 10%-30%. Flow cytometry confirms that the purity of CD34+ cells obtained by magnetic bead sorting is more than 90% (Figure 1A). Cellular immunofluorescence staining shows that CD34+ VW-SCs predominantly express CD34, c-kit, Flk-1, and Ki-67, with a relatively lower expression of Sca-1 (Figure 1B).
Differentiation of CD34+ VW-SCs
CD34+ VW-SCs are able to differentiate into endothelial cells and fibroblasts in vitro. CD34+ stem cells could be induced to differentiate into endothelial cells with increased expression of endothelial cell markers CD31 and VWF. Additionally, the differentiated fibroblast cells showed long spindle morphology and increased expression of fibroblast markers PDGFRα and Vimentin (Figure 2A,B). Furthermore, flow cytometry also confirmed the cells' differentiation ability; the percentage of CD34+CD31+ endothelial cells after differentiation increased 1.5 times compared with the undifferentiated cells (Figure 2C), and the percentage of CD34+PDGFRa+ fibroblast cells after differentiation increased 1.7 times compared with the undifferentiated cells (Figure 2D).
Characterization of Ca2+ signaling in CD34+ VW-SCs
Extracellular administration of ATP (10 µM) increased the intracellular Ca2+ level through the G-protein-coupled receptors (GPCR)-mediated signal pathway, and thapsigargin (TG) inhibited sarcoplasmic reticulum (SR) Ca2+ ATPase (SERCA) to deplete intracellular SR Ca2+ stores and triggered store-operated calcium entry (SOCE). Furthermore, caffeine stimulated the increased [Ca2+]i by activating ryanodine receptors (RyRs)-mediated Ca2+ release (Figure 3).
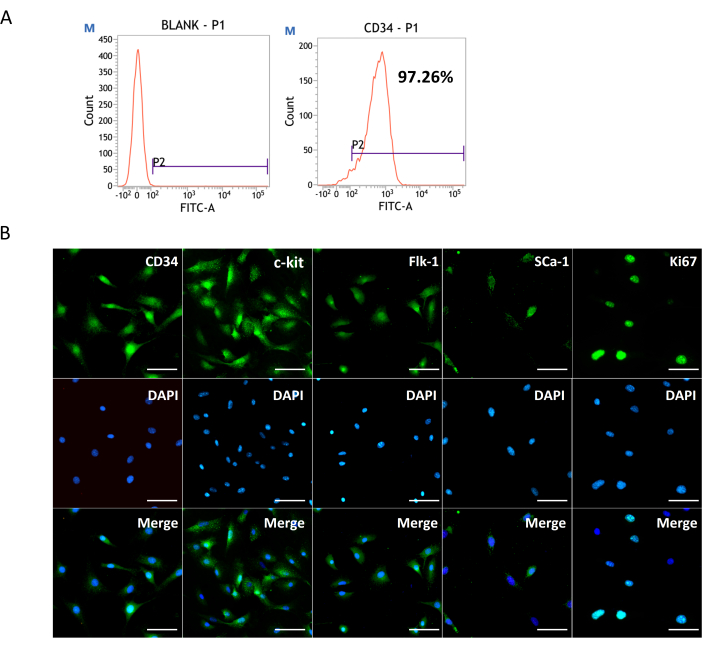
Figure 1: Harvested CD34+ VW-SCs by tissue culture and magnetically activated cell sorting (MACS). (A) Flow cytometric evaluation of purified CD34+ VW-SCs. (B) Representative images showing the expression of stem cell markers CD34, c-kit, Flk-1, Sca-1 and cell proliferation marker (Ki-67). Scale bars: 50 µm. Please click here to view a larger version of this figure.
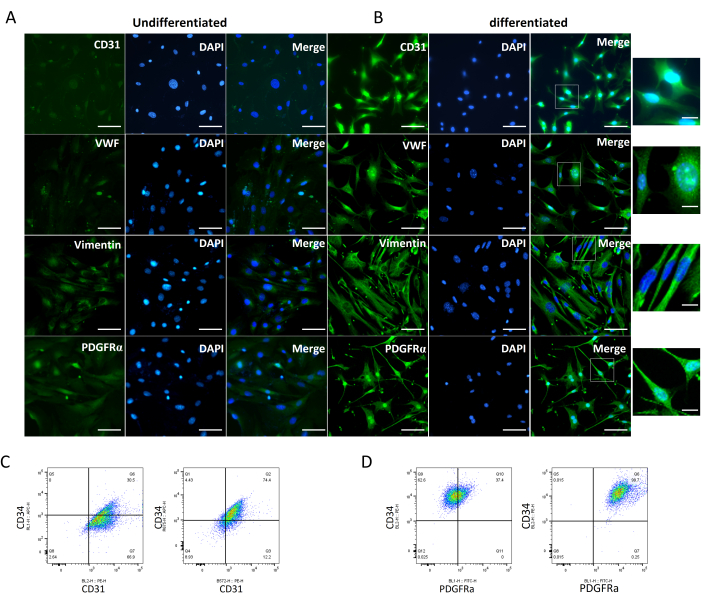
Figure 2: Detection of multidirectional differentiation ability of CD34+ VW-SCs. (A) Representative images showing the expression of endothelial markers CD31 and VWF, and fibroblast markers PDGFRα and Vimentin in CD34+ VW-SCs before differentiation. (B) Representative images showing the expression of endothelial markers CD31 and VWF, and fibroblast markers PDGFRα and Vimentin in CD34+ VW-SCs after differentiation. Scale bars: 50 µm. The inset scale bar is 10 µm. (C) The percentage of CD34+CD31+ endothelial cells before and after differentiation measured by Flow cytometry. (D) The percentage of CD34+PDGFRa+ fibroblast cells before and after differentiation measured by Flow cytometry. Please click here to view a larger version of this figure.
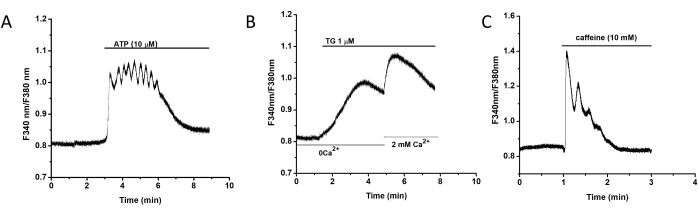
Figure 3: Intracellular Ca2+ signal in CD34+ VW-SCs. (A) Representative curve shows the effect of ATP (10 µM) on intracellular Ca2+ signaling. (B) Representative curve shows TG (1 µM)-induced intracellular Ca2+ release from SR store and the subsequent extracellular Ca2+ influx triggered by Ca2+ depletion after re-addition of 2 mM Ca2+. (C) Representative curve showing caffeine (10 mM)-activated intracellular Ca2+ signaling. Please click here to view a larger version of this figure.
Discussion
This study provides a quick and convenient method for obtaining functional CD34+ VW-SCs from the aorta and mesenteric arteries of mice. CD34+ VW-SCs obtained by this method have proliferative activity and multidirectional differentiation properties. Triphosphate inositol 1,4,5-trisphosphate receptors (IP3Rs), ryanodine receptors (RyRs), and store-operated calcium channels mediate Ca2+ release and entry in CD34+ VW-SCs. The establishment of this technique will lay the foundation for further investigation into the mechanisms involved in CD34+ VW-SCs participating in structural and functional remodeling in cardiovascular diseases.
The most common methods for primary cell isolation include tissue block attachment and enzymatic dissociation15,16. In the present report, tissue block attachment and magnetic bead sorting are combined to obtain high purity of CD34+ VW-SCs. The sorted vessel wall CD34+ stem cells are positive for CD34, and few express the endothelial cell marker CD31, which is consistent with a previous report2. Also, Jiang et al.2, using single-cell sequencing of freshly isolated femoral artery cells in mice, found that CD34 was mainly expressed in the endothelial and mesenchymal cell populations and not in the smooth muscle cell population. Experiments in vivo also confirm that CD34+ VW-SCs can differentiate into endothelial cells to participate in vascular repair post-injury. Since VW-SCs are heterogeneous, the CD34+ VW-SCs sorted by this method, along with other stem cell positive markers such as Flk-1, c-kit, and Sca-1, have different subpopulations, so their morphology may be different.
The most widely used methods for sorting stem cells are flow cytometry and immunomagnetic bead sorting17,18,19. The FACS method is characterized by high cell purity and recovery rate, but FACS is relatively time-consuming and expensive. Immunomagnetic bead separation is a highly specific cell sorting technology that integrates the theories of immunology, cell biology, and magnetic mechanics. This method is simple and fast and can be completed within 2 h. Different cell sorting methods affect cell purity and yield20. Similar to previous reports21, the sorting magnetic beads used in this experiment are only 50 nm in diameter, which exerts relatively less mechanical pressure on cells and does not cause cell damage and affect the biological activity of sorted cells. In addition, unlike the separation method reported in the literature8, we generally sort cells after being passaged for 5 generations, when the number of cells in a T75 flask can reach at least 1 × 107, and cell proliferation is active. Furthermore, after sorted cells are cultured for 3-5 passages, it is crucial to repeat the purification protocol and ensure whether auto-differentiation exists during culture and passages.
During the subculture of VW-SCs, DMEM high glucose medium with 0.2% LIF is used, in which FBS promotes the proliferation of VW-SCs, and LIF inhibits cell differentiation and supports the expansion of stem cells. In agreement with the existing study11, low-density culture favors the maintenance of stemness of VW-SCs, and the possibility of self-differentiation increases with passages. Furthermore, the selection of serum during culture is also crucial since some serum will potentially induce cell self-differentiation and affect the biological characteristics10.
Ca2+ is a major second messenger that controls various cellular functions, including cell contraction, migration, gene expression, cell growth, and apoptosis9. Previous reports8 only briefly described the basic characteristics of CD34+ VW-SCs, while in this study, we further detected the Ca2+ release from internal calcium stores and the extracellular Ca2+ influx triggered by ATP, caffeine, and TG, respectively. ATP is a nucleotide that is not only regarded as the major energy currency within cells but also acts as a transmitter/signaling molecule. ATP activates IP3Rs mediated Ca2+ release from the SR and regulates the activity of multiple downstream targets. Therefore, the cellular response to ATP may reflect the functional state of the cell22. Caffeine has long been used as a pharmacological probe for studying RyRs-mediated intracellular Ca2+ release. In this study, both ATP and caffeine rapidly increased intracellular Ca2+, and the elevated intracellular Ca2+ slowly decreased to the baseline within 1 min. Furthermore, SOCE is present in most non-excitable and partially excitable cells. In the present study, TG suppresses the Ca2+-ATP enzyme in CD34+ VW-SCs to induce Ca2+ depletion in the SR and mediate external Ca2+ influx23. The effect of ATP, caffeine, and TG on intracellular Ca2+ in CD34+ VW-SCs is consistent with other cells reported in previous studies24,25, suggesting that the CD34+ stem cells isolated in this study have normal intracellular Ca2+ release and external Ca2+ influx signaling properties.
In this study, functional CD34+ VW-SCs from mouse aortic and mesenteric arteries are efficiently cultured. The study of VW-SCs may provide significant insights into the mechanisms of vascular remodeling. The development of protocols with more markers and functional studies of CD34+ VW-SCs will further define the role of these cells in cardiovascular disease. Cell purification serves as a potent tool for delving into the cellular and genetic foundations of organ development and growth. MACS purification presents the advantage of being a convenient method for refining cell enrichment. When coupled with other techniques, such as RNA sequencing analysis, it has the potential to unveil new mechanisms underlying stem cell proliferation, migration, and differentiation in both physiological and pathological contexts12. This approach facilitates systematic screening of biological factors, extracellular matrix components, and small molecules that either promote or inhibit the fundamental functions of stem cells. Conducting such studies opens up unique opportunities for performing loss-of-function and gain-of-function experiments, ultimately contributing to a deeper understanding of the genetic basis of cardiovascular diseases. There are also some limitations within this method. For example, due to the phenotypic and functional heterogeneity of CD34+ cells in the vascular wall, it will need more markers to identify the specific subcellular type of resident vascular CD34+ cells.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was funded by grants from National Natural Science Foundation of China (No. 82070502, 31972909, 32171099), the Sichuan Science and Technology Program of Sichuan Province (23NSFSC0576, 2022YFS0607). The authors would like to thank Qingbo Xu from Zhejiang University for help with the cell culture, and the authors acknowledge the scientific and technical assistance of the flow cytometry platform in Southwest Medical University.
Materials
| 2% gelatin solution | Sigma | G1393 | |
| Anti-CD31 antibody | R&D | AF3628 | |
| Anti-CD34 antibody | Abcam | ab81289 | |
| Anti-c-kit antibody | CST | 77522 | |
| Anti-FITC MicroBeads | Miltenyi Biotec | 130-048-701 | |
| Anti-FITC MicroBeads MACS | Miltenyi Biotec | 130-048-701 | |
| Anti-Flk- 1 antibody | Abcam | ab24313 | |
| Anti-Ki67 antibody | CST | 34330 | |
| Anti-PDGFRα antibody | Abcam | ab131591 | |
| Anti-Sca- 1 antibody | Invitrogen | 710952 | |
| CD140a (PDGFRA) Monoclonal Antibody (APA5), FITC | eBioscience Invitrogen | 11-1401-82 | |
| CD31 (PECAM-1) Monoclonal Antibody (390), APC | eBioscience Invitrogen | 17-0311-82 | |
| CD34 Antibody, anti-mouse, FITC, REAfinity Clone REA383 | Miltenyi Biotec | 130-117-775 | |
| cell culture hood | JIANGSU SUJING GROUP CO.,LTD | SW-CJ-2FD | |
| Centrifuge | CENCE | L530 | |
| CO2 incubators | Thermofisher Scientific | 4111 | |
| Confocal laser scanning microscope | Zeiss | zeiss 980 | |
| DMEM High Glucose Medium | ATCC | 30-2002 | |
| EBM-2 culture medium | Lonza | CC-3162 | |
| FACSMelody | BD Biosciences | ||
| FACSMelody™ System | BD | ||
| Fetal bovine serum | Millipore | ES-009-C | |
| FM-2 culture medium | ScienCell | 2331 | |
| Fura-2/AM | Invitrogen | M1292 | |
| Goat anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 488 | Thermofisher Scientific | A32731 | |
| Leukemia inhibitory factor | Millipore | LIF2010 | |
| Microscope | Olympus | IX71 | |
| MiniMACS Starting Kit | Miltenyi Biotec | 130-090-312 | |
| Penicillin-Streptomycin-Amphotericin B Solution | Beyotime | C0224 | |
| Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block) | BD Pharmingen | 553141 | |
| Stereo Microscope | Olympus | SZX10 | |
| TILLvisION 4.0 program | T.I.L.L.Photonics GmbH | polychrome V | |
| VWF Monoclonal Antibody (F8/86) | Thermofisher Scientific | MA5-14029 | |
| β-Mercaptoethanol | Thermofisher Scientific | 21985023 |
Riferimenti
- Hu, Y., et al. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 113 (9), 1258-1265 (2004).
- Jiang, L., et al. Nonbone marrow CD34+ cells are crucial for endothelial repair of the injured artery. Circ Res. 129 (8), e146-e165 (2021).
- Tamma, R., Ruggieri, S., Annese, T., Ribatti, D. Vascular wall as a source of stem cells able to differentiate into endothelial cells. Adv Exp Med Biol. 1237, 29-36 (2020).
- Patel, J., et al. Functional definition of progenitors versus mature endothelial cells reveals key soxF-dependent differentiation process. Circulation. 135 (8), 786-805 (2017).
- Zhang, L., Issa Bhaloo, S., Chen, T., Zhou, B., Xu, Q. Roles of stem cells in vascular remodeling. Chin J Cell Biol. 43 (7), 1352-1361 (2021).
- Zhang, L., et al. Role of resident stem cells in vessel formation and arteriosclerosis. Circ Res. 122 (11), 1608-1624 (2018).
- Wu, Y., et al. Effects of estrogen on growth and smooth muscle differentiation of vascular wall-resident CD34+ stem/progenitor cells. Atherosclerosis. 240 (2), 453-461 (2015).
- Tang, J. M., et al. Isolation and culture of vascular wall-resident CD34+ stem/progenitor cells. Cardiol Plus. 4 (4), 116-120 (2019).
- Sukumaran, P., et al. Calcium signaling regulates autophagy and apoptosis. Cells. 10 (8), 2125 (2021).
- van der Sanden, B., Dhobb, M., Berger, F., Wion, D. Optimizing stem cell culture. J Cell Biochem. 111 (4), 801-807 (2010).
- Rotmans, J. I., et al. In vivo, cell seeding with anti-CD34 antibodies successfully accelerates endothelialization but stimulates intimal hyperplasia in porcine arteriovenous expanded polytetrafluoroethylene grafts. Circulation. 112 (1), 12-18 (2005).
- Qu, R., et al. The role of serum amyloid A1 in the adipogenic differentiation of human adipose-derived stem cells basing on single-cell RNA sequencing analysis. Stem Cell Res Ther. 13 (1), 187 (2022).
- Flynn, J., Gorry, P. Flow Cytometry Analysis to Identify Human CD8+ T Cells. Methods Mol Biol. 2048, 1-13 (2019).
- Bacon, K., Lavoie, A., Rao, B. M., Daniele, M., Menegatti, S. Past, present, and future of affinity-based cell separation technologies. Acta Biomater. 112, 29-51 (2020).
- Ma, H. G., Liu, H. Q., Liu, S. D., Tang, Y. Y. Primary culture and identification of rat glomerular microvascular endothelial cells. Acta Physiol Sin. 73 (6), 926-930 (2021).
- Liu, W. H., Wang, P., Yang, J. Isolation, culture and identification of rats hair follicle neural crest stem cells. Chin J Neuroanat. 35 (2), 207-211 (2019).
- Kumar, P., Garg, N. Flow cytometry approaches to obtain medulloblastoma stem cells from bulk cultures. Methods Mol Biol. 2423, 87-94 (2022).
- Haroon, M. M., Vemula, P. K., Palakodeti, D. Flow cytometry analysis of planarian stem cells using DNA and mitochondrial dyes. Bio Protoc. 12 (2), e4299 (2022).
- Catchpole, T., Nguyen, T. D., Gilfoyle, A., Csaky, K. G. A profile of circulating vascular progenitor cells in human neovascular age-related macular degeneration. PLOS One. 15 (2), e0229504 (2020).
- Wang, G., Yu, G., Wang, D., Guo, S., Shan, F. Comparison of the purity and vitality of natural killer cells with different isolation kits. Exp Ther Med. 13 (5), 1875-1883 (2017).
- Moore, D. K., Motaung, B., du Plessis, N., Shabangu, A. N., Loxton, A. G. Consortium SI. Isolation of B-cells using Miltenyi MACS bead isolation kits. PLOS One. 14 (3), e0213832 (2019).
- Jiang, L. H., Mousawi, F., Yang, X., Roger, S. ATP-induced Ca2+-signalling mechanisms in the regulation of mesenchymal stem cell migration. Cell Mol Life Sci. 74 (20), 3697-3710 (2017).
- Ong, H. L., Subedi, K. P., Son, G. Y., Liu, X., Ambudkar, I. S. Tuning store-operated calcium entry to modulate Ca2+-dependent physiological processes. Biochim Biophys Acta Mol Cell Res. 1866 (7), 1037-1045 (2019).
- Garcia-Carlos, C. A., et al. Angiotensin II, ATP and high extracellular potassium induced intracellular calcium responses in primary rat brain endothelial cell cultures. Cell Biochem Funct. 39 (5), 688-698 (2021).
- Reggiani, C. Caffeine as a tool to investigate sarcoplasmic reticulum and intracellular calcium dynamics in human skeletal muscles. J Muscle Res Cell Motil. 42 (2), 281-289 (2021).

