Applications of Liquid-Chromatography Tandem Mass Spectrometry in Natural Products Research: Tropane Alkaloids as a Case Study
Summary
We present a method for rapid mass spectrometry (MS)/mass spectrometry (MS)-based annotation and classification of tropane alkaloids, useful for both preliminary dereplication of tropane-containing samples and discovery of novel alkaloids for isolation.
Abstract
Although many drugs utilized today are synthetic in origin, natural products still provide a rich source of novel chemical diversity and bioactivity, and can yield promising leads for resistant or emerging diseases. The challenge, however, is twofold: not only must researchers find natural products and elucidate their structures, but they must also identify what is worth isolating and assaying (and what is already known – a process known as dereplication). With the advent of modern analytical instrumentation, the pace of natural product discovery and dereplication has accelerated. Liquid chromatography-tandem mass spectrometry (LC-MS/MS) has become an especially valuable technique for identifying and classifying chemical structures. Tropane alkaloids (TAs) are plant-derived compounds of great medicinal and toxicological significance. In this study, we developed an LC-MS/MS-based screening workflow utilizing the multiple MS/MS configurations available on a triple-quadrupole (QQQ) mass spectrometer to annotate and classify TA structures based on their distinct fragmentation patterns. By using a combination of data-dependent (DD) product ion scans, precursor ion scans (PrIS), and neutral loss scans (NLS), we applied this method to TA-rich extracts of the nightshades Datura stramonium and Datura metel. This method is rapid, sensitive, and was successfully employed for both preliminary dereplication of complex TA-containing samples and for the discovery of a novel candidate for isolation, purification (and eventual bioassay).
Introduction
Although fully synthetic molecules have become more prominent in drug discovery in recent decades, nearly two-thirds of all approved drugs from the last 39 years are natural products or natural-product-inspired,1,2 underscoring the continued importance of natural products research. Alkaloids, certain nitrogen-containing natural products, are especially prized for their medicinal properties. Tropane alkaloids (TAs) containing the [3.2.1.]-bicyclic nitrogen-containing system, are produced mostly by plants in the Solanaceae (nightshade), Erythroxylaceae, and Convolvulaceae families. Examples include atropine, scopolamine, and cocaine; multiple semi-synthetic or synthetic tropanes are also used clinically3. TAs and their derivatives are used to treat many conditions3,4 and several of these drugs appear on the WHO's 2023 List of Essential Medicines5. Because of their potent activities, TAs are also used recreationally (as stimulants or deliriants) and can cause poisoning upon ingestion of plants (or preparations) that contain them6,7. TAs are undesirable in human and animal food8 and can taint teas, spices, grains, honey, and herbal supplements9,10. Because of both their medicinal promise and ability to poison, analytical methods that can aid in the discovery of new TAs (and identification of known TAs) are useful.
In tandem mass spectrometry (MS/MS), "mass filters" (e.g., quadrupoles, time-of-flight tubes) are coupled together physically ("in-space"), or an instrument employs additional "in-time" reaction/separation steps. In-space MS/MS uses different modes to select and fragment different ions at the different mass filters (e.g., the quadrupoles of a triple-quadrupole or QQQ instrument). These different modes can be used to determine which specific fragments are made by a given ion (product ion scan), which ions in a sample yield certain fragments (precursor ion scan or PrIS) or undergo losses of a characteristic mass (neutral loss scan or NLS), or which specific compounds possess which specific fragments (multiple reaction monitoring). MS/MS, therefore, provides fragments that are useful for proposing structures for new compounds or confirming an existing compound's presence. MS/MS is increasingly used in the drug discovery, natural products chemistry, and metabolomics fields11,12, and has been used to profile alkaloid-containing species (for phytochemical characterization or chemotaxonomic analysis) and to detect and quantify specific alkaloids in food or medicinal plants10,13,14,15,16.
Despite the many mass spectrometry techniques available, there are challenges in finding new alkaloids. In addition to finding a candidate organism to screen, a full structural confirmation of an alkaloid is an arduous process that may include many different analytical techniques. Additionally, researchers could isolate a compound that is already known, wasting labor, time, and resources. This is especially difficult for TAs, where hundreds, if not thousands of TAs, many of which are isomeric with one another, are reported. The process of "identifying the knowns and distinguishing them from the unknowns" is known as dereplication. Databases of the retention times (r.t.s) and mass fragments of different TAs and other compounds are published to aid with this process17,18. Nonetheless, dereplication is laborious; merely annotating (i.e., assigning putative structures to) the alkaloids in a sample's entire LC-MS/MS chromatogram is time-consuming. Recently, both molecular networking19,20 and manual dereplication18,21,22 have been used for benzylisoquinoline, monoterpene indole, and tropane alkaloids, and PrISs have been used for "structural filtering" of spectra to identify pyrrolizidine and solanine-type alkaloids23,24. There are no specific methods or workflows available for rapid LC-MS/MS-based dereplication of TA-containing samples, however, even though TAs possess common, easily-identifiable fragments (Figure 1). The method described here uses a combination of data-dependent (DD) product ion scans, PrISs, and NLSs to annotate and classify TA structures in plants based on both the distinct fragmentation patterns for mono-, di-, and trisubstituted tropanes (Figure 1A) and the losses of common ester groups found in these alkaloids (Figure 1B). The study organisms are several species in the nightshade genus Datura. A rich source of diverse TAs, Datura has been used throughout the world's history for medicinal and cultural purposes17– and is a challenging matrix to dereplicate because of its numerous, structurally similar TAs, providing us with appealing samples upon which to test our method.
Protocol
CAUTION: Please consult all relevant material safety data sheets (MSDS) before using the listed chemicals.
1. Sample preparation
CAUTION: Liquid nitrogen can cause cryogen burns. Use cryogen gloves and eye protection in a well-ventilated area. Alkaloid-containing plant samples can be irritating to the skin; always handle them with gloves. Methanol is toxic and flammable and should be handled in a fume hood away from potential ignition sources.
NOTE: In theory, cultivated or wild plant tissue can be used (dried or ground fresh); the below procedure is just that utilized during method development.
- If the plant tissue of interest is fresh, freeze it by placing it in a polypropylene conical tube and immersing it in liquid nitrogen for 2-3 min.
- Place the frozen plant tissue into a pre-chilled mortar (in a polystyrene cooler with liquid nitrogen), and using a pre-chilled pestle, grind the tissue to a uniform powder.
- Quickly weigh the desired amount of tissue into a tared polypropylene microcentrifuge tube using a pre-chilled spatula, and immediately add 20% methanol (at room temperature [RT]) at a concentration of 1 mL per every 100 mg of tissue.
NOTE: Methanol (20%) is commonly used to extract TAs25. Occasionally, 0.1% formic acid is added, although no difference in extraction efficiency was observed during the development of this method and others. - Place the capped tubes on a rocking shaker (medium speed) for a minimum of 3 h at RT.
- Centrifuge the tubes at 9464 x g for 10 min. Pipette off the supernatant into an LC-MS autosampler vial, or, if still cloudy, filter through a 0.45 µm syringe filter first.
NOTE: The protocol can be paused here, although processed fresh plant samples and extracts should be stored in a -80 ˚C freezer prior to analysis to avoid any potential alkaloid degradation.
2. LC-MS instrument configuration and data collection
CAUTION: Acetonitrile is toxic and flammable; keep away from ignition sources and control vapors using a fume hood. Formic acid is corrosive; avoid skin and eye contact and wear appropriate personal protective equipment.
- Use an LC-MS instrument with an electrospray ionization (ESI) source and a reversed-phase HPLC column (C18, 4.6 x 100 mm).
- For HPLC, use 0.1% formic acid in H2O as Solvent A and 0.1% formic acid in acetonitrile as Solvent B; equilibrate the column with 99% A and 1% B. Configure a 30 min gradient of 1%-50% B over 26 min, returning to 1% B at 26.01 min, and holding at 1% B for 4 min. Use a column oven temperature of 45 ˚C and a flow rate of 0.5 mL/min.
- In the LC-MS method, use the following operating parameters for the mass spectrometer: interface voltage: 4.0 kV, nebulizing gas flow: 3 L/min, heating gas flow: 10 L/min, DL temperature: 250 ˚C, heat block temperature: 400 ˚C, interface temperature: 300 ˚C, drying gas flow: 10 L/min, and collision-induced dissociation (CID) gas (argon) pressure of 17 kPa.
- Build an MS method in ESI positive mode that includes both a Q3 scan and a Q1 scan (100-1000 Da) that functions as a survey event (set to the length of the LC method), with automatic isotope exclusion enabled. Include a product ion scan as a dependent event of the Q1 scan (DD analysis), with a mass window of 50-1000 Da, a collision cell energy of -20 V, and an event time of <0.2 s.
NOTE: The count threshold for triggering Q1's DD product ion scan can be variable, but typically, a level of 7,000-10,000 counts is used. On some instruments, such as the one used to develop the method, the Q3 scan provides greater mass accuracy and sensitivity than Q1 and is included to confirm ions observed in the Q1 scan, although it can be omitted from step 2.4 without issue. - To the above MS method, add positive-mode PrISs equal to the length of the LC method with collision cell energies of -20 V and event times of 0.75 s. Make sure in all cases, Automatic Isotope Exclude, or De-isotoping functions are enabled. Ensure that the m/z values of interest for TA fragments (in Da, see Figure 1A) are 124.1 (for monosubstituted TAs, mass window of 125-1000 Da), 122.1 and 140.1 (for disubstituted TAs, mass windows beginning at 123 and 141 Da, respectively), and 156.1 and 138.1 (for trisubstituted TAs, mass windows beginning at 157 and 139 Da, respectively).
NOTE: During method development, the PrIS were split over two different methods, one for mono- and disubstituted TAs and one for trisubstituted TAs, although they can be split up or combined any way. - Build a second MS method that includes the parameters in step 2.4.
- To that MS/MS method, add positive-mode NLS equal to the length of the LC method, with collision cell energies of -20 V and event times of 0.75 s. Make sure in all cases, Automatic Isotope Exclude or De-isotoping functions are enabled.
- Ensure that the neutral loss masses of interest for esters on TAs (in Da, see Figure 1B) are 100.05 (for esters derived from tiglic acid, mass window of 110-1000 Da), 60.03 (for acetyl groups, mass window of 100-1000 Da), and 166.06 (phenyllactic or tropic acid esters, mass window of 170-1000 Da).
- Download the LC-MS method and create data files for the samples of interest. Once the HPLC column is equilibrated at 45 ˚C, run the samples of interest in a batch or project file with appropriate extraction solvent blanks between different species or tissue types. A typical injection volume is 10-20 µL.
NOTE: At especially high volumes, the mass spectrometer detector may be saturated. If running very concentrated samples, be sure that the ESI source is cleaned and the instrument is tuned regularly. The protocol can be paused here after all data is collected.
3. Data analysis
- Examine the total ion chromatogram of the Q1 and Q3 scans (and the DD product ion scan), and note the parent mass of any abundant ions which have TA-like features: a) mass <500 Da for the [M+H]+ ion, usually an even-mass, b) typical r.t.s between 2-22 min using the above LC method, and c) fragments from the following list: m/z 93, 124, 142, 140, 122, 138, 156, 174, 110, or 128 Da.
- Examine the PrIS chromatogram/channel for m/z 124, and note which peaks/ions are at which r.t.s (recorded in step 3.1) produce this fragment. Click scan-by-scan through the chromatogram as well as examine the full MS/MS spectra obtained in the DD product ion scan, especially for lower-abundance species.
NOTE: A true species containing this fragment will exist for multiple scans and appear in both the Q1 and Q3 scans (if the latter is used). A spreadsheet is attached in the Supporting Information (Supplementary File 1) to aid with annotations. - Repeat step 3.2 with the other PrIS chromatograms. As both m/z 122 and 140 are indicative of disubstituted TAs and both m/z 138 and 156 are indicative of trisubstituted TAs, examine these chromatograms/channels together.
- Examine the NLS chromatogram/channels for m/z 100, 60, and 166, and note which peaks/ions at which r.t.s (recorded in step 3.1) produce these neutral losses. As with the PrIS, click scan-by-scan through the chromatogram and compare with the fragmentation obtained in the DD product ion scan, especially for lower-abundance species.
- Using the combination of PrIS and NLS data, supported by the DD product ion scan results, make putative annotations of the observed alkaloids by adding the smallest tropane mass (e.g., 124, 122, or 138) and the neutral loss and then accounting for the remaining leftover mass.
NOTE: Typical groups substituting TAs (and their masses in Da) are hydroxyl (18), acetyl (60), propionyl (74), isobutyryl (88), tigloyl (100), saturated tigloyl/2-methylbutyryl (102), or phenyllactate/tropic acid (166). - Compare annotations for alkaloids to those reported in the literature17 and databases (e.g., MoNA)26 to determine which TA substitution patterns are reported (and which are potentially novel). Additionally, use standards of some common tropane alkaloids (e.g., atropine, littorine, scopolamine) that are commercially available for confirmation.
- For additional structural information for low-abundance samples (the PrIS and NLS may pick up ions below the dependent event thresholds), collect a specific product ion scan (utilizing the mass of interest as the precursor ion) on a more concentrated sample.
NOTE: This method was developed on a low-resolution QQQ instrument. For all putative new compounds, a high-resolution MS instrument should be used to obtain accurate mass spectra.
Representative Results
To demonstrate the method's effectiveness, a standard mix of TAs (10 µg/mL each of an acetyltropine/acetylpseudotropine mix [monosubstituted], 10 µg/mL each of a mixture of two anisodamine isomers [disubstituted], along with hyoscyamine [monosubstituted], littorine [monosubstituted], and scopolamine [trisubstituted]) was analyzed as a positive control (Figure 2). A full Q1 scan chromatogram (displayed in the base peak chromatogram view) is shown in Figure 2A, with the TA standard structures indicated by their corresponding peaks. The PrIS for m/z 124 (Figure 2B) displays peaks in its chromatogram for the monosubstituted TAs; ergo, the acetyltropine/pseudotropine mix, hyoscyamine, and littorine are displayed. The peaks corresponding to acetyltropine and pseudotropine in the m/z 124 chromatogram are small (possibly because they are at a lower concentration than the other standards and possibly because they might produce a less abundant m/z 124 fragment than the other monosubstituted TAs). Nonetheless, they are still detectable in both this chromatogram and in the corresponding PrIS channel. Figure 2C and Figure 2D are PrIS chromatograms for m/z 122 and 140, respectively. Detecting disubstituted TA fragments, they both flag the two isomers of anisodamine, with a stronger signal visible on the m/z 140 channel. As different TAs may possess fragments of different intensities, the m/z 122 and 140 channels should be examined together. Finally, the m/z 156 (Figure 2E) and m/z 138 (Figure 2F) channels, diagnostic for trisubstituted TAs, both display one peak for scopolamine (the only trisubstituted TA in the standard mix).
NLS chromatograms were then used to assign the ester groups present. Figure 2G shows the NLS chromatogram for 60 Da (loss of acetic acid from acetate esters): the two acetylated monosubstituted TA isomers are visible. The NLS chromatogram for 166 Da (Figure 2I), meant to identify phenyllactic or tropic esters, correctly identifies all the alkaloids containing either of these groups (hyoscyamine, littorine, the two anisodamine isomers, and scopolamine). Finally, as predicted, the chromatogram (Figure 2H) for the loss of 100 Da (diagnostic of tigloyl esters) is blank for the exception of a minor impurity at r.t. 1.75 min, as none of the standard alkaloids contain this group.
The method was then tested for false positives. A water/methanol extract of tomato (Solanum lycopersicum) leaf served as a negative control. Despite being nightshades, tomatoes do not produce TAs. A full Q1 scan base-peak chromatogram is shown in Figure 3A. The PrIS for m/z 124 (Figure 3B) is mostly blank, with small baseline fluctuations that are likely detector noise (no signals matched those masses in the Q1 or Q3 scans). Two features with r.t.s of 2.6 and 3.4 min do yield a 124 fragment, but examination of the DD product-ion scan reveals that these features do not produce any other monosubstituted tropane fragments (m/z 93 or 142), suggesting that they are not TAs. This example also highlights the utility of including the DD product ion scan in this method to rule out false positives. Similarly, the m/z 122 channel for disubstituted TAs (Figure 3C) also just shows noise. The PrIS chromatograms for m/z 140, m/z 138, and m/z 156, and the three NLSs, which suggest only non-TA features on the DD scans, are shown in Supplementary Figure 1A-F.
To demonstrate the uses of this method in dereplicating a TA-containing sample, an extract of D. metel var. 'fastuosa' root was analyzed. The roots are the principal site of TA biosynthesis in nightshades, and many diverse TAs were predicted to be present. The full Q1 scan chromatogram is shown in Figure 4A. As expected, numerous features of varying abundance were detected on the PrISs for m/z 124 (Figure 4B), m/z 122 (Figure 4C), m/z 140 (Figure 4D), m/z 156 (Figure 4E), and m/z 138 (Figure 4F). Many of these TAs were also acetylated or tigloylated, or derived from phenyllactic/tropic acid, as shown by the numerous signals in the NLS chromatograms in Figure 4G-I, respectively.
A spreadsheet, provided as a .xls file (an empty sheet for reader use is Supplementary File 1, D. metel root is in Supplementary File 2), was used to first document which features (masses or r.t.s) appeared TA-like (using criteria in step 3.1). Next, fragments and neutral losses belonging to these features were noted. The method is extremely sensitive: the PrIS channels even readily detected low-abundance TAs that did not yield visible peaks on the corresponding chromatograms. Using the fragments and neutral losses, a structure for the alkaloid could then be proposed by following step 3.5.
An example of using the spectral data to annotate alkaloids is provided in Figure 5. The full Q1 scan chromatogram for D. metel root is in Figure 5A. A feature at 14.4 min was selected; the Q1 scan spectrum (Figure 5B) shows a parent mass of m/z 224 for this peak. The DD product ion scan spectrum is shown in Figure 5C. The PrIS channel for m/z 124 (Figure 5D) indicates that the 224 ion produces this fragment (also visible in the DD product ion scan). The NLS channel for 100 Da (to detect tigloylated TAs) also flags m/z 224 (also confirmed by the DD product ion scan). Adding up the neutral loss and lowest-mass TA fragment (100 + 124 Da) equals the parent mass, 224, confirming the tigloyl group is the only substitution; a likely structure for this alkaloid is indicated in Figure 5C. For di- and tri-substituted TAs, observed neutral loss masses were added to the lowest TA mass, and then another substitution was proposed to account for the remaining mass: for example, m/z 222, with disubstituted TA fragments of 140 and 122 and a neutral loss of 100 Da, has a leftover mass of 100 (222-122 = 100), likely to be a second tigloyl group. This workflow can intuitively be used for process of elimination: a feature lacking tropane fragments (even if it may lose 60, 100, or 166 Da) is unlikely to be a TA. In an example of thorough and rapid dereplication using this workflow, 71 distinct TAs of varying abundance and polarity were identified in D. metel root, including multiple isomeric compounds (Supplementary File 2). Some of these compounds had ester groups that could not be readily assigned, and some additional compounds were flagged as potential TAs, but could not be annotated with high confidence.
A methanol/water extract of ground D. stramonium seeds was also analyzed. The full Q1 scan base-peak chromatogram for D. metel root is shown in Figure 6A. The PrIS for m/z 124 is shown in Figure 6B and the PrIS for m/z 122 is shown in Figure 6C; the other chromatograms are shown in Supplementary Figure 2A-F. Figure 6B indicates a very abundant monosubstituted tropane alkaloid at a r.t. = 12.6 min, likely hyoscyamine. The spreadsheet showing seed TA annotations is included in Supplementary File 3. The performance of the method seemed lower with this particular sample: two very abundant features with a m/z of 259 at r.t.s of 8.5 and 10.2 mins yielded peaks on the NLS chromatograms for 60 and 100 Da (Supplementary Figure 2 D,E) with a mass of m/z 260 (a M+1 isotope) indicated on the corresponding spectra. These compounds are not TAs, but instead formed fragments consistent with beta-carboline alkaloids27. They are not acetylated or tigloylated, but lose 60 or 100 from their M+1 isotope, making their appearance on these two NLS channels incorrect. Additionally, scopolamine (r.t. = 10.5 min) yielded a m/z 122 fragment from its m/z 305 M+1 isotope peak, an isotope of scopolamine's expected m/z 121 fragment. Even though these methods employ de-isotoping functions, some isotope "leakage" is possible at these high sample concentrations. While this may be an occurrence unique to the instrument used in this study or this sample, it is worth noting.
Forty TAs were annotated in D. stramonium seeds using this method, including several that appear to be glycosylated, losing masses of 162 (hexose), 146 (deoxyhexose), or 132 Da (xylose or a similar sugar). While hexose-containing TAs have been reported in Merremia (Convolvulaceae)28, Duboisia29, and Atropa25, this is the first report of deoxyhexose or xylose-containing TAs. One TA had a r.t. of 13.8 min (Figure 7A) and a mass of m/z 436 (Q1 scan in Figure 7B). This feature produced a signal on the m/z 124 channel, indicating a monosubstituted TA (Figure 7D), but did not produce signals on any other PrIS or NLS channel. The DD product ion scan (Figure 7C) indicated the expected m/z 124, a loss of 312 Da, possibly 166 + 146 Da, from the parent ion. High-resolution MS/MS was obtained on a quadrupole time-of-flight instrument (spectrum in Figure 7E), which indicated a m/z 290 fragment, suggesting hyoscyamine or littorine glycosylated on the ester hydroxyl portion with rhamnose or a similar sugar; the measured accurate mass was also very close to the exact mass calculated for rhamnosyl-hyoscyamine or littorine (structure in Figure 7E). Because of its unprecedented structure and abundance, this alkaloid merits isolation from D. stramonium seeds, an example that demonstrates the use of this method in new alkaloid discovery.
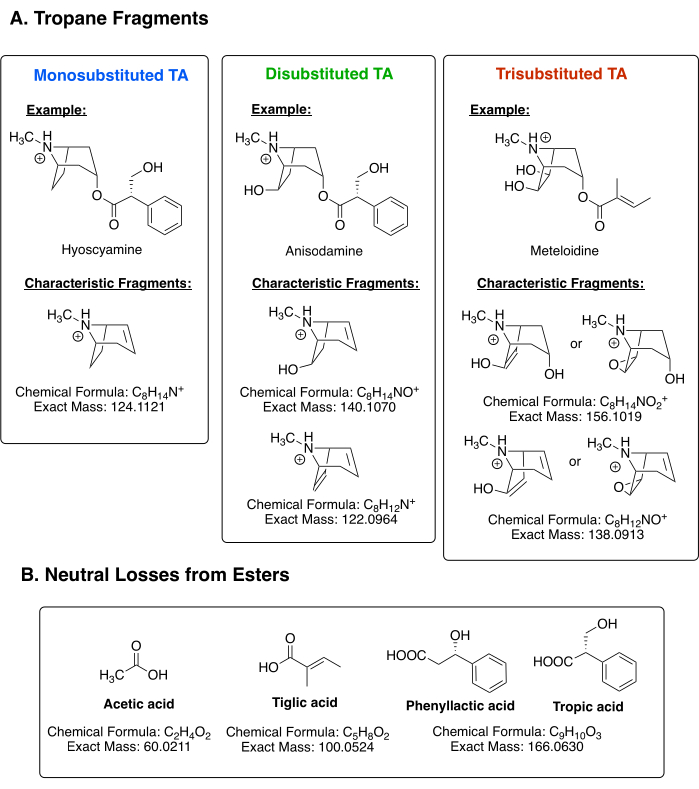
Figure 1: Relevant fragments and losses observed in MS of TAs. (A) Characteristic fragments (and their diagnostic masses) formed from monosubstituted, disubstituted, and trisubsituted TAs, as well as representative examples of alkaloids in each class. (B) Common neutral losses observed from ester groups in TAs (and their diagnostic masses). Please click here to view a larger version of this figure.
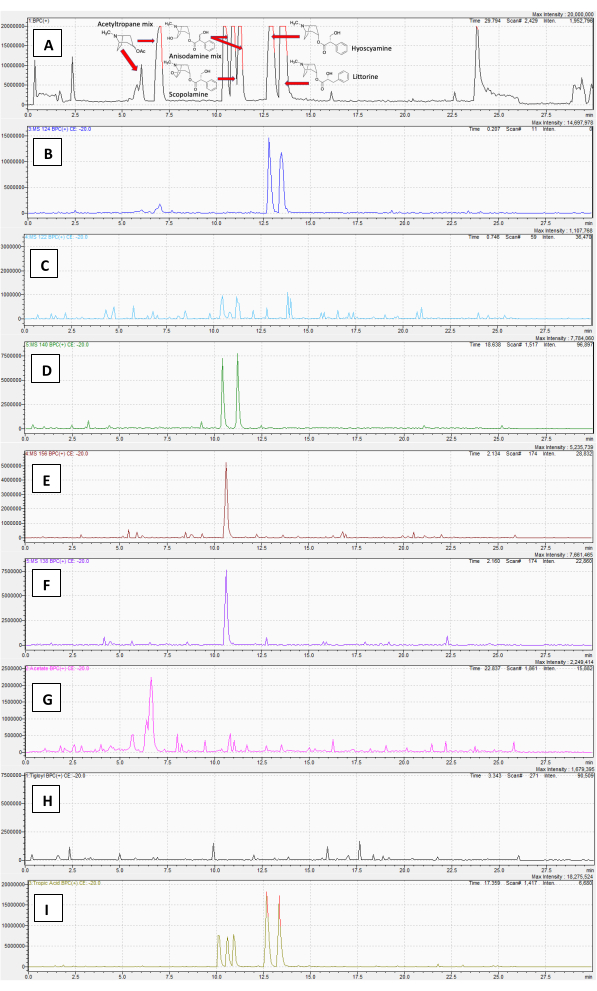
Figure 2: Positive control precursor ion and neutral loss scans. For all chromatograms, the X-axis is retention time, and the Y-axis is counts (ion abundance). (A) The total ion chromatogram, in base-peak chromatogram view, of the alkaloid standard mix. Structures of the standards are indicated; the corresponding peaks are denoted by red arrows. (B–F) are PrIS chromatograms for (B) m/z 124, (C) 122, (D) 140, (E) 156, and (F) 138. (G–I) NLS chromatograms for 60 Da (acetyl, G), 100 Da (tigloyl, H), and 166 Da (phenyllactic/tropic acid, I). Please click here to view a larger version of this figure.
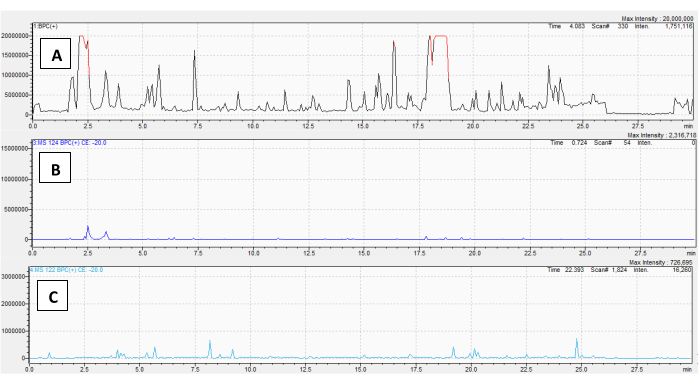
Figure 3: Negative control precursor ion and neutral loss scans. For all chromatograms, the X-axis is retention time, and the Y-axis is counts (ion abundance). (A) The total ion chromatogram, in base-peak chromatogram view, of a methanol/water extract of S. lycopersicum leaf. (B) The PrIS chromatogram for m/z 124 showing only two non-tropane features at r.t. 2.6 and 3.4 min. (C) The PrIS chromatogram for m/z 122, blank except for detector noise. The scales used as the same as in Figure 2; the remaining chromatograms are in Supplementary Figure 1. Please click here to view a larger version of this figure.
Figure 4: Application of the MS/MS method to D. metel var. 'fastuosa' roots. For all chromatograms, the X-axis is retention time, and the Y-axis is counts (ion abundance). (A) The total ion chromatogram, in base-peak chromatogram view, of a methanol/water extract of D. metel var. 'fastuosa' roots. (B–F) are PrIS chromatograms for m/z (B) 124, (C) 122, (D) 140, (E) 156, and (F) 138. (G–I) are NLS chromatograms for 60 Da (acetyl, G), 100 Da (tigloyl, H), and 166 Da (phenyllactic/tropic acid, I). Note the abundant signals on each chromatogram corresponding to numerous TAs. Please click here to view a larger version of this figure.
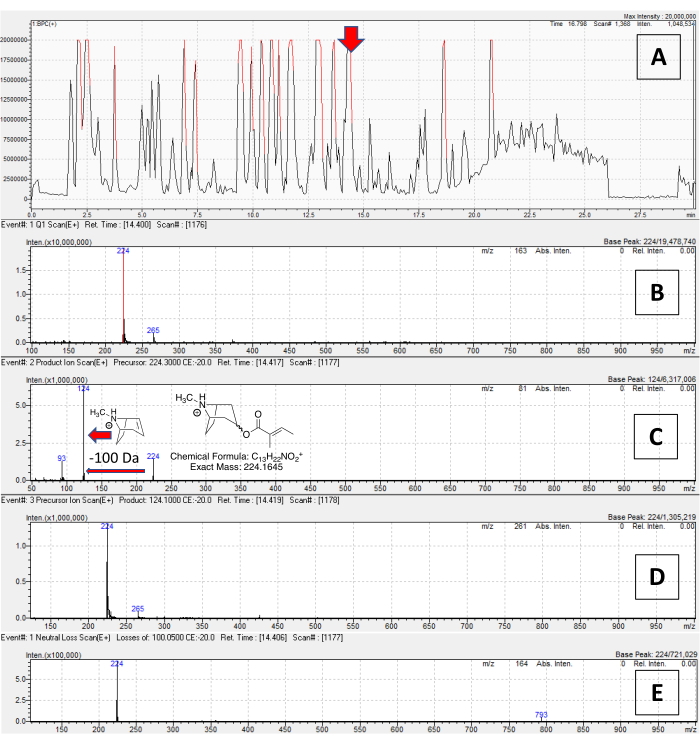
Figure 5: Application of combined DD product ion scan, precursor ion scan, and neutral loss scan method for proposing the structure for a TA. (A) The total ion chromatogram, in base-peak chromatogram view, of a methanol/water extract of D. metel var. 'fastuosa' roots. A peak at r.t. = 14.4 min is indicated by a red arrow. (B) The Q1 (survey scan), with an abundant mass of m/z 224 for this peak. For all spectra, the X-axis is mass, and the Y axis is intensity. (C) The DD product ion scan, showing the fragmentation of the m/z 224 ion, indicating a loss of 100 Da and a m/z 124 fragment (red arrows). This compound appears on both the (D) m/z 124 PrIS channel and (E) the 100 Da NLS channel, indicating it is both monosubstituted and tigloylated (structure in panel C). Please click here to view a larger version of this figure.
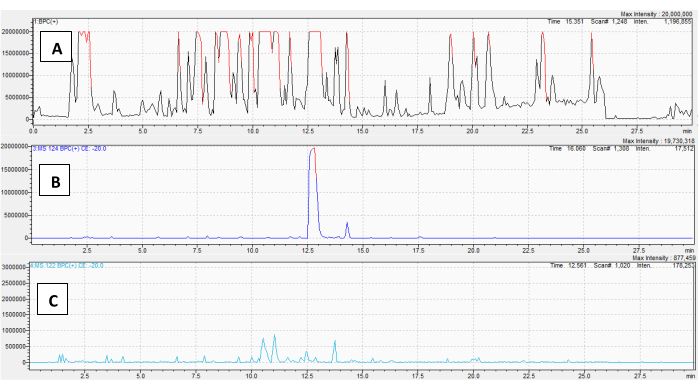
Figure 6: Application of the MS/MS method to D. stramonium seeds. For all chromatograms, the X-axis is retention time, and the Y-axis is counts (ion abundance). (A) The total ion chromatogram, in base-peak chromatogram view, of a methanol/water extract of D. stramonium seeds. (B) The PrIS chromatogram for m/z 124; (C) depicts the PrIS chromatogram for m/z 140. The remaining chromatograms are in Supplementary Figure 2. Please click here to view a larger version of this figure.
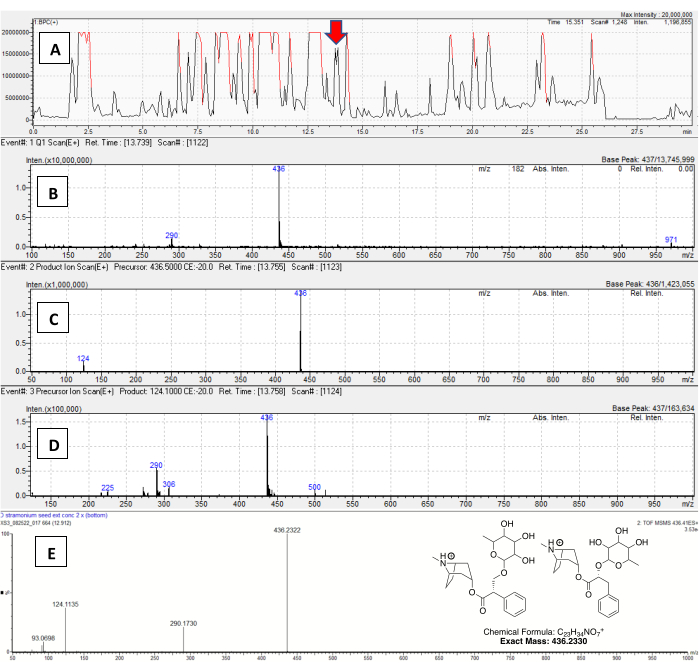
Figure 7: Annotating a new alkaloid in D. stramonium seeds. For all chromatograms, the X-axis is retention time, and the Y-axis is counts (ion abundance). (A) The total ion chromatogram, in base-peak chromatogram view, of a methanol/water extract of D. stramonium seeds. A feature at r.t. = 13.7 min is indicated. (B) The Q1 (survey scan) showing a mass of m/z 436 for this peak. For all spectra, the X-axis is mass, and the Y axis is intensity. (C) The DD product ion scan showing the fragmentation of the m/z 436 ion. (D) This monosubstituted TA compound appears on the m/z 124 PrIS channel. (E) The high-resolution MS/MS spectrum for this new TA, along with proposed structures and exact mass. Please click here to view a larger version of this figure.
Supplementary Figure 1: Additional negative control precursor ion and neutral loss scans. (A–C) PrIS chromatograms for m/z (A) 140, (B) 156, and (C) 138. (D–F) NLS chromatograms for 60 Da (acetyl, D), 100 Da (tigloyl, E), and 166 Da (phenyllactic/tropic acid, F). The scales used are the same as in Figure 2. Please click here to download this File.
Supplementary Figure 2: Application of the MS/MS method to D. stramonium seeds; additional chromatograms. (A–C) are PrIS chromatograms for m/z (A) 140, (B) 156, and (C) 138. (D–F) NLS chromatograms for 60 Da (acetyl, D), 100 Da (tigloyl, E), and 166 Da (phenyllactic/tropic acid, F). Please click here to download this File.
Supplementary File 1: Blank spreadsheet used for alkaloid annotations. Please click here to download this File.
Supplementary File 2: Spreadsheet of alkaloid annotations and structures for D. metel var. 'fastuosa' roots. Please click here to download this File.
Supplementary File 3: Spreadsheet of alkaloid annotations and structures for D. stramonium seeds. Please click here to download this File.
Discussion
Although the instrument parameters provided in the protocol allow for satisfactory performance, the successful use of this method may require careful attention to or optimization of several critical steps. While the HPLC solvent gradient provided in step 2.2 is generally appropriate for tropane alkaloids, it may need to be modified depending on the tropane alkaloid profile of the sample or plant species being examined. The sample injection volume can also be changed depending on the sensitivity of the instrument and how the method is used. "Deep" metabolic profiling or dereplication should use a larger injection volume (15-20 µL was used in this study), while simple detection of alkaloids could use smaller volumes. Care should be taken when using large sample volumes to (a) keep the ion source cleaned regularly, (b) monitor the HPLC column for pressure changes or clogs (an appropriate guard column should always be used), (c) prevent carry-over between samples, and (d) examine chromatograms for potential "isotope leakage" as described above. The interface temperatures and voltages should not be changed significantly from those specified in the method to avoid premature in-source fragmentation. Additionally, although a collision cell voltage of 20 V is recommended, it may not provide optimal chromatogram signal (or diagnostic DD product ion scan fragments) for all TAs. Some MS instruments and functions have a "collision energy ramping" feature, or the ability to collect scans at multiple or optimized collision energies, which could also find utility in a method like this30.
This MS method itself is also readily modifiable: while tiglic, acetic, and phenyllactic/tropic acid esters are common in Datura species, the TA ester substitution patterns may be different in other plants or samples. The NLS masses can be changed to detect other ester groups (e.g., a loss of 74 Da for propionic esters) or potentially detect glycosylations (e.g., a loss of 162 Da for hexoses). The PrIS masses can also be changed. For example, a TA in both Datura samples (r.t. = 12.5 min; see Supplementary File 1 and Supplementary File 2) were annotated as norhyoscyamine or norlittorine (hyoscyamine/littorine minus the tropane N-methyl group) based on its DD product ion scan fragments (m/z 110 and 93) and match to the literature spectra31; this TA did not appear on any of the tropane fragment PrIS channels. A channel could be added for these two masses to detect nortropanes. The m/z 93 fragment is also present in most monosubstituted TAs17; adding this channel could provide additional confirmation for a monosubstituted TA's presence and help reduce false positives.
In addition to its adaptability for different types of TAs, one major advantage of this protocol (over existing alkaloid annotation methods) is its speed. Depending on how the MS method is configured (and the HPLC run time used), all MS/MS data for a sample can be collected within 60-90 min. For example, during our studies, the mono- and di-substituted TA PrISs were built into one method, the trisubstituted TA PrISs were in another method, and the NLSs were in a third (all methods contained the DD product ion scan), for a total of three injections at thirty minutes apiece. Complete annotation of the sample utilizing the combined MS/MS data is much faster than manual examination of every peak or collecting individual product ion scans for numerous peaks of interest. This method can be performed on any instrument with product ion scan, PrIS, and NLS modes and can be used for rapid qualitative assessment (i.e., are TAs present or absent?), for distinguishing or "fingerprinting" different plant genera, species, varieties or cultivars, organs, or growth stages, and for comparing observed TAs to those reported (as part of a dereplication workflow). As demonstrated, the protocol can also be used for the discovery of new TAs as leads for isolation, purification, or bioassay, as well as identification of candidate species and tissues to isolate them from. This method could be used in the natural products and metabolomics fields, including to aid in understanding plant biochemistry and for early-stage drug discovery investigation. Additional applications could be found in food safety or forensics (e.g., detection of TAs in food, supplements, or biofluids).
The method does have several drawbacks that warrant discussion. First, depending on the instrument used and the purpose of employing the protocol, a large sample injection volume could be required, which could cause instrument performance issues and the potential for false positives in some cases (as described above). Second, this method was developed on a low-resolution QQQ instrument, and false positives could result from the low resolution (e.g., the feature containing a m/z 124 fragment in the negative control that is not a TA). A higher-resolution instrument would provide better diagnostic ability, and accurate-mass MS/MS spectra should be obtained for any proposed new alkaloid. Additional drawbacks to this method are simply the disadvantages of MS/MS analysis as a whole. While MS/MS is useful for proposing structures ("annotation" as designated by the Metabolomics Standards Initiative32), it does not provide concrete assignments of molecular connectivity ("identification"). For example, in both Datura samples, we found several isomers of a m/z 306 TA, which seemed to have both m/z 124 and m/z 140 fragments (Supplementary File 2), thus making definitive assignment of the substitution pattern difficult. This could represent a yet-undiscovered fragmentation mechanism, and another technique (such as nuclear magnetic resonance spectroscopy) may be helpful in resolving ambiguities. MS/MS-based methods frequently struggle with isomerism: the phenyllactic acid ester littorine (or its derivatives) is difficult to distinguish from hyoscyamine (tropic acid ester), and other Solanaceous plants contain TAs substituted with the isomeric tigloyl, senecioyl, and angeloyl groups33,34; in the absence of standards, these compounds might be difficult, if not impossible, to tell apart. The method also will not differentiate enantiomers (e.g., 3,6- vs. 3,7-dihydroxytropane; see Figure 1 for numbering) or regioisomers (e.g., distinguishing 3-acetyl from 7-acetyl without a standard). We have assigned most monosubstituted tropanes as 3-substituted based on prior patterns reported in the genus Datura and other Solanaceous plants17,35. Ergo, any biochemical knowledge or literature about the organism studied (if available) should always be consulted alongside any instrumental analysis.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was funded by a Faculty Research Grant (Northern Michigan University, awarded to M.A.C.), an undergraduate research fellowship (Northern Michigan University, awarded to J.C), and the Department of Chemistry. The authors wish to thank John Berger (NMU) for assistance with plant tissue preparation, Hannah Hawkins (NMU) for LC-MS maintenance and troubleshooting assistance, and Dr. Ryan Fornwald and his CH 495 (Natural Products Synthesis) students for their preparation the acetyltropine mix. The authors also wish to thank Dr. Daniel Jones (Michigan State University) for acquiring high-resolution MS/MS spectra.
Materials
| Acetonitrile, For UHPLC, suitable for mass spectometry | Sigma-Aldrich | 900667 | HPLC solvent |
| Argon gas | AirGas | AR UHP300 | CID gas |
| Formic acid, 99% for analysis | Thermo Scientific | AC270480010 | HPLC additive |
| Guard column holder | Restek | 25812 | |
| HPLC, Shimadzu LC-2030C 3D Plus | Shimadzu | 228-65802-58 | HPLC column |
| LCMS, Shimdazu LCMS-8045 | Shimadzu | 225-31800-44 | Mass spectrometer; we ran LabSolutions software, which is standard for Shimadzu instruments |
| Liquid nitrogen | AirGas | NI 180LT22 | |
| Methanol, for HPLC/UHPLC/LCMS | VWR | BDH 85800.400 | For making extraction solvent |
| Microcentrifuge | VWR | 2400-37 | |
| Microcentrifuge tubes, 1.5 mL | Fisher Scientific | 05-408-129 | |
| Mortar | Fisher Scientific | FB961C | For grinding plant tissues |
| Pestle | Fisher Scientific | FB961M | For grinding plant tissues |
| Pipette 1000 mL | Gilson | F144059M | |
| Pipette tip 1000 mL | Fisher scientific | 02-707-404 | |
| Plant tissues | Various sources | N/A | Can be anything wild or cultivated |
| Polypropylene conical tubes, 15 mL | Fisher Scientific | 05-539-4 | |
| Polystyrene cooler | ULINE | S-18312 | The type of coolers that reagents for molecular biology are shipped in would be appropriate |
| Roc C18 3 µm, 100 mm x 4.6 mm | Restek | 9534315 | HPLC column |
| Roc C18, 10 mm x 4 mm | Restek | 953450210 | Guard column |
| Rocking shaker | Themo Scientific | 11-676-680 | |
| Screw thread vial convenience kit (9 mm) | Fisher scientific | 13-622-190 | LCMS autosampler vials |
| Syringe, 3 mL | Fisher Scientific | 03-377-27 | |
| Syringe filter 0.45 µm | Avantor/VWR | 76479-008 | |
| Water, for use in liquid chromatography and mass spectrometry | JT Baker | 9831-03 | For making extraction solvent |
| Water solution, contains 0.1% v/v formic acid, For UHPLC, suitable for mass spectometry | Sigma-Aldrich | 900687-1L | HPLC solvent |
Riferimenti
- Newman, D. J., Cragg, G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 75, 311-335 (2012).
- Newman, D. J., Cragg, G. M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 83 (3), 770-803 (2020).
- Kohnen-Johannsen, K., Kayser, O. Tropane alkaloids: Chemistry, pharmacology, biosynthesis and production. Molecules. 24 (4), 796 (2019).
- Shim, K. H., Kang, M. J., Sharma, N., An, S. S. A. Beauty of the beast: anticholinergic tropane alkaloids in therapeutics. Nat Prod Bioprospect. 12 (1), 33 (2022).
- Web Annex A. World Health Organization Model List of Essential Medicines – 23rd List, 2023. The Selection and Use of Essential Medicines 2023: Executive Summary of the Report of the 24th WHO Expert Committee on the Selection and Use of Essential Medicines. , 24-28 (2023).
- Kerchner, A., Farkas, A. Worldwide poisoning potential of Brugmansia and Datura. Forensic Toxicol. 38, 30-41 (2020).
- Hanna, J. P., Schmidley, J. W., Braselton, W. E. Datura delirium. Clin Neuropharmacol. 15, 109-113 (1992).
- Alexander, J., et al. Scientific opinion of the panel on contaminants in the food chain on a request from the European Commission on tropane alkaloids (from Datura sp.) as undesirable substances in animal feed. EFSA J. 691, 1-55 (2008).
- González-Gómez, L., Morante-Zarcero, S., Pérez-Quintanilla, D., Sierra, I. Occurrence and chemistry of Tropane alkaloids in foods, with a focus on sample analysis methods: A review on recent trends and technological advances. Foods. 11 (3), 407 (2022).
- Cirlini, M., Cappucci, V., Galaverna, G., Dall’Asta, C., Bruni, R. A sensitive UHPLC-ESI-MS/MS method for the determination of tropane alkaloids in herbal teas and extracts. Food Control. 105, 285-291 (2019).
- Amorim Madiera, P. J., Florencio, M. H., Prasain, J. Applications of Tandem Mass Spectrometry: From Structural Analysis to Fundamental Studies. Tandem Mass Spectrometry – Applications and Principles. , (2012).
- Xing, J., Xie, C., Lou, H. Recent applications of liquid chromatography-mass spectrometry in natural products bioanalysis. J Pharm Biomed Anal. 44 (2), 368-378 (2007).
- Negrin, A., Long, C., Motley, T. J., Kennelly, E. J. LC-MS metabolomics and chemotaxonomy of caffeine-containing holly (Ilex) species and related taxa in the Aquifoliaceae. J. Agric. Food Chem. 67 (19), 5687-5699 (2019).
- Och, A., Szewczyk, K., Pecio, L., Stochmal, A., Zaluski, D., Bogucka-Kocka, A. UPLC-MS/MS profile of alkaloids with cytotoxic properties of selected medicinal plants of the Berberidaceae and Papaveraceae families. Oxid Med Cell Longevity. 2017, 9369872 (2017).
- Li, Y., Pang, T., Shi, J., Liu, X., Deng, J., Lin, Q. Simultaneous determination of alkaloids and their related tobacco-specific nitrosamines in tobacco leaves using LC-MS-MS. J Chromatogr Sci. 53 (10), 1730-1736 (2015).
- González-Gómez, L., Morante-Zarcero, S., Pereira, J. A. M., Câmara, J. S., Sierra, I. Improved analytical approach for determination of tropane alkaloids in leafy vegetables based on µ-QuEChERS combined with HPLC-MS/MS. Toxins. 14 (10), 650 (2022).
- Cinelli, M. A., Jones, A. D. Alkaloids of the Genus Datura: Review of a rich resource for natural product discovery. Molecules. 26, 2629 (2021).
- Gonçalves Dantas, C., et al. Dereplication of tropane alkaloids from four Erythroxylum species using liquid chromatography coupled with ESI-MSn and HRESIMS. Rapid Commun Mass Spectrom. 37 (21), e9629 (2023).
- Santos, C. L. G., et al. Molecular networking-based dereplication of strictosidine-derived monoterpene indole alkaloids from the curare ingredient Strychnos peckii. Rapid Commun Mass Spectrom. 34 (3), e8683 (2020).
- Qin, G. F., et al. MS/MS-based molecular networking: An efficient approach for natural products dereplication. Molecules. 28, 157 (2023).
- Du, N., Zhou, W., Jin, H., Liu, Y., Zhou, H., Liang, X. Characterization of tropane and cinnamamide alkaloids from Scopolia tangutica by high-performance liquid chromatography with quadrupole time-of-flight tandem mass spectrometry. J Sep Sci. 42 (6), 1163-1173 (2019).
- Agnes, S. A., et al. Implementation of a MS/MS database for isoquinoline alkaloids and other annonaceous metabolites. Sci Data. 9 (1), 270 (2022).
- Sixto, A., Pérez-Parada, A., Niell, S., Heinzen, H. GC-MS and LC-MS/MS workflows for the identification and quantitation of pyrrolizidine alkaloids in plant extracts, a case study: Echium plantagineum. Rev Bras Farmacog. 29 (4), 500-503 (2019).
- Wang, H., Xu, X., Wang, X., Guo, W., Jia, W., Zhang, F. An analytical strategy for discovering structural analogues of alkaloids in plant food using characteristic structural fragments extraction by high resolution orbitrap mass spectrometry. LWT- Sci Technol. 154, 112329 (2022).
- Parks, H. M., et al. Redirecting tropane alkaloid metabolism reveals pyrrolidine alkaloid diversity in Atropa belladonna. New Phytol. 237 (5), 1810-1825 (2023).
- . MassBank of North America Available from: https://mona.fiehnlab.ucdavis.edu (2024)
- Maier, I., et al. Fluorodaturatin und Homofluorodaturatin – zwei neue β-carbolinderivate in Samen von Datura stramonium L. var. stramonium. Monatsh Chem. 112, 1425-1439 (1981).
- Jennett-Siems, K., et al. Chemotaxonomy of the pantropical genus Merremia (Convolvulaceae) based on the distribution of tropane alkaloids. Phytochemistry. 66, 1448-1464 (2005).
- Kohnen, K. L., Sezgin, S., Spiteller, M., Hagels, H., Kayser, O. Localization and organization of scopolamine biosynthesis in Duboisia myoporoides R. Br. Plant Cell Physiol. 59 (1), 107-118 (2018).
- Guan, P., et al. Full collision energy ramp-MS2 spectrum in structural analysis relying on MS/MS. Anal Chem. 93 (46), 15381-15389 (2021).
- Al Balkhi, M. H., Schlitz, S., Lesur, D., Lanoue, A., Wadouachi, M., Boitel-Conti, M. Norlittorine and norhyoscyamine identified as products of littorine and hyoscyamine metabolism by 13C-labeling in Datura innoxia hairy roots. Phytochemistry. 74, 105-114 (2012).
- Sumner, L. W., et al. Proposed minimum reporting standards for chemical analysis. Metabolomics. 3, 211-221 (2007).
- Gambaro, V., Labbe, C., Castillo, M. Angeloyl, Tigloyl and Senecioyloxytropane Alkaloids from Schizanthus hookerii. Phytochemistry. 22 (8), 1838-1839 (1983).
- Christen, P., Cretton, S., Humam, M., Bieri, S., Muñoz, O., Joseph-Nathan, P. Chemistry and biological activity of alkaloids from the genus Schizanthus. Phytochem Rev. 19, 615-641 (2020).
- Lounasmaa, M., Tamminen, T. The Tropane Alkaloids. The Alkaloids: Chemistry and Pharmacology. , (1993).

.
