Aqueous Synthesis of Plasmonic Gold-Tin Alloy Nanoparticles
Summary
Here, the synthesis of gold (Au) seeds is described using the Turkevich method. These seeds are then used to synthesize gold-tin alloy (Au-Sn) nanoparticles with tunable plasmonic properties.
Abstract
This protocol describes the synthesis of Au nanoparticle seeds and the subsequent formation of Au-Sn bimetallic nanoparticles. These nanoparticles have potential applications in catalysis, optoelectronics, imaging, and drug delivery. Previously, methods for producing alloy nanoparticles have been time-consuming, require complex reaction conditions, and can have inconsistent results. The outlined protocol first describes the synthesis of approximately 13 nm Au nanoparticle seeds using the Turkevich method. The protocol next describes the reduction of Sn and its incorporation into the Au seeds to generate Au-Sn alloy nanoparticles. The optical and structural characterization of these nanoparticles is described. Optically, prominent localized surface plasmon resonances (LSPRs) are apparent using UV-visible spectroscopy. Structurally, powder X-ray diffraction (XRD) reflects all particles to be less than 20 nm and shows patterns for Au, Sn, and multiple Au-Sn intermetallic phases. Spherical morphology and size distribution are obtained from transmission electron microscopy (TEM) imaging. TEM reveals that after Sn incorporation, the nanoparticles grow to approximately 15 nm in diameter.
Introduction
Plasmonic metal nanoparticles1,2 have applications in catalysis, optoelectronics, sensing, and sustainability due to their ability to absorb light with great efficiency, concentrate light into sub-nanometer volumes, and enhance catalytic reactions3,4,5. Only a few metals display efficient localized surface plasmon resonances (LSPRs). Among them, one of the widely explored metals is Au3.
Au is an extensively studied noble metal known for its stable alloy formation with other metals. However, the Au LSPR is limited to the visible and infrared and cannot be tuned to higher energies6,7,8. Meanwhile, post-transition metals have a variety of interesting reactive and catalytic properties distinct from the noble metals6,9,10. By alloying Au with post-transition metals, the LSPR can be tuned toward higher energies toward the UV1. This protocol focuses on Au-Sn alloying. Sn is known to alloy readily with many metals, can have UV LSPRs, and has interesting catalytic applications, such as formic acid formation via carbon dioxide reduction6,7,8. Au and Sn alloys were synthesized using a seeded process through chemical reduction and diffusion of Sn into the seeds.
The primary goal of this method is to synthesize aqueous metal nanoparticle alloys quickly (i.e., in a few hours) and reproducibly at the benchtop using aqueous chemistry. Initially, Au seeds are prepared using the Turkevich method11, followed by seed-based diffusion synthesis, a common strategy when forming random alloy nanoparticles8. Notably, alloying of Sn requires a relatively short time (~30 min) in a mild environment with simple equipment compared to other methods7,8 that require higher temperature, higher vacuum instrumentation, or hazardous solvents. This process can be performed in mild, aqueous conditions without the need for burdensome environmental controls. The resulting Au-Sn alloys have consistent morphology, size, shape, and optical properties that can be controlled by manipulating the Sn content.
Protocol
The equipment and reagents used in the study are listed in the Table of Materials.
1. Turkevich synthesis method of citrate-capped Au nanoparticle seeds
- Cleaning of the glassware
- Clean glassware and stir bars using aqua regia (1:3 mole ratio of HNO3:HCl).
- Rinse with ultrapure water until no odor remains and dry before use.
- Preparation of reagent solutions
- Measure 39.4 mg of HAuCl4∙3H2O using an analytical balance into a clean and labeled 20 mL glass scintillation vial. To prepare a 10 mM solution, micropipette 10 mL of ultrapure water into the solution.
- Measure 58.8 mg of trisodium citrate dihydrate using an analytical balance into a clean and labeled 20 mL glass scintillation vial. To prepare a 100 mM solution, micropipette 10 mL of ultrapure water into the solution.
- Sonicate both the precursor solutions prior to use for 30 s; confirm visually that the total reagent dissolution has occurred.
- Synthesis of Au seeds
- Place a clean 25.4 mm polytetrafluoroethylene (PTFE) stir bar into a clean 250 mL round bottom flask.
- Add 58.56 mL of ultrapure water to the flask.
- Move this flask onto a 120 V 250 mL heating mantle, placed onto a stir plate.
- Assemble the heating mantle and set the attached heat controller to 138 °C, stirring at 640 rpm.
- Attach a condenser to the top of the round bottom flask, secure it onto a stand, and flow water through the condenser.
- When the water boils at 100 °C and the reaction is at reflux, directly pipette 1.2 mL of the 10 mM HAuCl4 into the solution by briefly removing the condenser.
- Allow the reaction to return to reflux, then detach the condenser.
- Quickly inject 480 µL of 100 mM trisodium citrate solution at once in a single addition.
NOTE: The 100 mM trisodium citrate should be added rapidly with a single injection to ensure consistent, monodisperse particle formation. - Immediately place the condenser back on the flask and allow the solution to reflux for 8 min.
NOTE: After about 2 min from the citrate injection, a visible color change to dark purple-red should be observed, with the final color being burgundy. - After 8 min, remove the round-bottom flask from the heating mantle and allow it to return to room temperature.
2. Synthesis of Au-Sn bimetallic nanoparticles
- Preparation of precursor solutions
- To prepare a 10 wt% polyvinylpyrrolidone (PVP) solution, perform the following steps:
- Accurately measure 0.1 g of PVP using an analytical balance into a clean and labeled 20 mL glass scintillation vial.
- Micropipette 1 mL of ultrapure water into the vial. Sonicate for 1 min to completely dissolve.
- To prepare 5 mM SnCl4 solution, follow the steps mentioned below:
- Using a micropipette, transfer 7.5 mL of ultrapure water into a clean 20 mL scintillation vial.
- Quickly inject 4.34 µL of SnCl4 into the vial using a micropipette and swirl the solution until completely dissolved.
CAUTION: The SnCl4 solution should be handled in a fume hood due to its corrosiveness, fumes, and reactivity at ambient conditions that may lead to the decomposition of the reagent.
- To prepare 260 mM NaBH4 solution, follow the step below:
- Accurately measure 20 mg of NaBH4 using an analytical balance into a clean and labeled 20 mL glass scintillation vial.
NOTE: The NaBH4 solution is prepared immediately before injection into the sample.
- Accurately measure 20 mg of NaBH4 using an analytical balance into a clean and labeled 20 mL glass scintillation vial.
- To prepare a 10 wt% polyvinylpyrrolidone (PVP) solution, perform the following steps:
- Formation of bimetallic nanoparticles
- Pipette 6 mL of Au seeds and the corresponding amount of ultrapure water to a clean 20 mL glass scintillation vial with a 12.7 mm PTFE stir bar.
NOTE: The amount of ultrapure water, PVP, SnCl4, and NaBH4 is dependent on the %Au-Sn solution being made and can be found in Table 1. - Place the vial on a stir plate and begin stirring at 1,500 rpm.
- Pipette the appropriate amount of 10 wt% PVP into the reaction vial.
- Add the corresponding amount of the 5 mM SnCl4 solution into the reaction vial.
- Remove and tightly cap the reaction vial and place into a 60 °C hot water bath for 10 min.
NOTE: The stir bars may remain in the vials during this step. - After 10 min, remove the vial from the hot water bath, uncap it, and place it back onto the stir plate at 1,500 rpm.
- Add 2.03 mL of ultrapure water to the vial containing solid NaBH4, cap tightly, and shake until dissolved.
- Immediately pipette the 260 mM NaBH4 solution into the reaction vial in one swift injection and allow it to stir for 30 s.
NOTE: The solution changes from burgundy to yellow-orange in color with bubble formation. - Remove the reaction vial from the stir plate, loosely cap it, and place it into a 60 °C hot water bath for 20 min.
- After 20 min, remove the vial from the hot water bath.
- Remove the stir bar from the reaction vial.
- Allow the solution to cool to room temperature prior to characterization.
- Pipette 6 mL of Au seeds and the corresponding amount of ultrapure water to a clean 20 mL glass scintillation vial with a 12.7 mm PTFE stir bar.
3. Optical characterization of plasmonic bimetallic nanoparticles
- Zero the instrument using ultrapure water in the quartz cuvette as a blank and run a background correction.
- Transfer the sample into a clean quartz cuvette with a path length of 1 cm to acquire the UV-visible spectrum with a range of 200-700 nm.
4. Structural characterization of plasmonic bimetallic nanoparticles
- Transfer an appropriate amount of sample into a 2.0 mL microcentrifuge tube using a micropipette and centrifuge at room temperature at 5,510 x g for 8 min.
- After 8 min, remove the supernatant from the tube using a pipette without disturbing the pellet. The pellets are left at the bottom of the tubes.
- Add 1.50 mL of ultrapure water to the tubes containing the pellet and vortex to resuspend.
- Centrifuge the samples again at 5,510 x g for 8 min.
- After completion, remove most of the supernatant, leaving a concentrated sample in each tube. About 200 µL of concentrated particle colloid should remain.
- Using a pipette, transfer the concentrated samples onto a zero-background silicon holder.
- Place the holder uncovered in a desiccator to fully dry.
- Once dried, place the sample into the X-ray diffractometer to collect data. A Cu Kα with a wavelength of 1.54 Å was used as the X-ray source with a scan rate at 1° min-1 in the 2θ range of 10°-90°.
5. Imaging of plasmonic bimetallic nanoparticles
- Transfer an appropriate amount of the sample into a 2.0 mL microcentrifuge tube using a micropipette and centrifuge at 5,510 x g for 8 min (at RT).
- After 8 min, remove the supernatant from the tube using a pipette without disturbing the pellet. The pellets are left at the bottom of the tubes.
- Add 1.5 mL of ultrapure water to the tubes containing the pellet and vortex to resuspend.
- Centrifuge samples again at 5,510 x g for 8 min.
- After completion, remove most of the supernatant and manually agitate the tube by rocking the sample until the pellet is homogeneously dispersed into the remaining supernatant. Leave behind the concentrated sample in each tube.
- Using a micropipette, pipette 10 µL of the concentrated sample onto a Cu Carbon Type-B transmission electron microscope (TEM) grid.
- Place the grid uncovered in a desiccator for approximately 2 h to dry.
NOTE: Sample imaging was performed using a TEM with a 100 kV accelerating voltage; additional analysis for polydispersity and size analysis was performed using ImageJ software.
Representative Results
Figure 1 shows representative results for Au seeds and Au-Sn alloy nanoparticles. Following the Au seeds synthesis protocol, a distinct, asymmetric absorption peak around 517 nm with an extinction maximum of approximately 0.7 is observed, corresponding to the LSPR. The peak blue shifts with the addition of Sn, correlating with an apparent optical color change in the sample from burgundy to orange to tan-brown. Further blue-shifting and broadening of the peak are observed with an increased percentage of Sn added. The expected LSPR maxima are around 514 nm, 502 nm, 475 nm, and 470 nm for additions of 10%, 20%, 30%, and 40% Sn, respectively. At 40% Sn added, a heavily dampened LSPR is observable. The extinction decreases with increasing amounts of Sn due to their lower extinction coefficients compared to pure Au nanoparticles.
13 nm spherical, monodisperse Au nanoparticles are obtained for the seeds. The nanoparticle size should grow slightly with a spherical shape maintained as Sn is incorporated. The size will differ depending on the amount of Sn added, as indicated in Table 2. The crystal structure data are compared with reference stick patterns from the Open Crystallographic Database for face-centered cubic (FCC) Au (9013036), trigonal Au5Sn (1510571), and hexagonal AuSn (1510301)1.
The Au seeds XRD pattern should show peaks only relating to FCC Au, with a primary peak around 38° for the (111) reflection1. No additional peak is observed other than the shifting of the (111) peak for 10% Sn-added nanoparticles. A faint reflection around 40°, representing the Au5Sn intermetallic alloy, is seen at 20% Sn added. More defined Au5Sn peaks can be seen at 30% and 40% Sn added. Additions above 40% Sn should also exhibit AuSn intermetallic peaks.
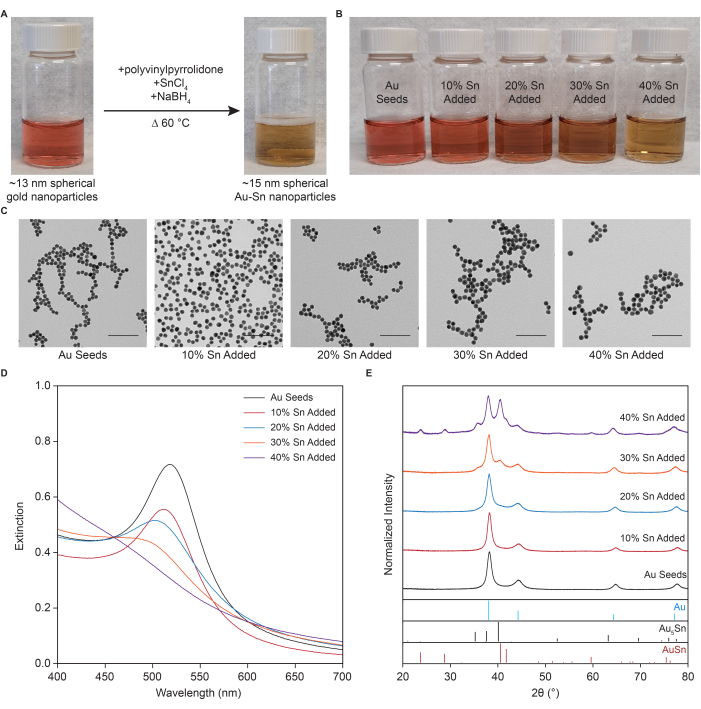
Figure 1: Representative results for Au seeds and Au-Sn alloy nanoparticles. (A) Typical visual change observed upon Sn reduction. (B) Different visual appearances of the Au seeds and nanoparticle solutions with 10%-40% Sn added. (C) Representative transmission electron micrographs of the nanoparticles. Scale bars are 100 nm. (D) UV-visible spectroscopy and (E) X-ray diffraction for the Au seeds and nanoparticles with 10%-40% Sn added. The figure is adapted from Branco et al.2. Please click here to view a larger version of this figure.
| Au seeds (mL) | Ultrapure water (mL) | 10% wt% PVP (µL) | 5 mM SnCl4 (µL) | 260 mM NaBH4 (µL) | ||
| 10% Au-Sn Addition | 6.00 | 1.82 | 31.9 | 26.6 | 153.2 | |
| 20% Au-Sn Addition | 6.00 | 1.77 | 35.9 | 59.8 | 172.4 | |
| 30% Au-Sn Addition | 6.00 | 1.70 | 41.0 | 102.5 | 197.0 | |
| 40% Au-Sn Addition | 6.00 | 1.61 | 47.8 | 159.4 | 229.9 | |
Table 1: Required precursor solution volumes for the synthesis of Au-Sn nanoparticles.
| Sample | Diameter (nm) | Standard deviation (nm) | Coefficient of variation (%) |
| Au seeds | 13.3 | ± 1.1 | 8.3 |
| 10% Sn Added | 13.3 | ± 1.0 | 7.6 |
| 20% Sn Added | 13.6 | ± 1.4 | 10.6 |
| 30% Sn Added | 14.4 | ± 1.0 | 7.1 |
| 40% Sn Added | 15.8 | ± 1.4 | 9.0 |
Table 2: Nanoparticle size distribution.
Discussion
In this study, Au seeds were prepared using the Turkevich method11. Regarding procedural limitations of this method, it is necessary to perform the 480 µL injection of 100 mM trisodium citrate rapidly. If the citrate solution is injected slowly, polydisperse particles may form with a large size distribution. Additionally, the cleanliness of the glassware can significantly impact the quality and consistency of Au seeds. If glassware is not cleaned well before use with aqua regia, the Au seeds can aggregate, which can be visually observed if a purple color persists. It is crucial to ensure that these seeds fully cool to room temperature before characterization.
Once the suitability of the Au seeds is determined, they can be used in the preparation of Au-Sn nanoparticles. While making the 5 mM Sn solution, the SnCl4 injection should be performed inside a fume hood, and care should be taken to minimize exposure of the SnCl4 solution to air to limit degradation. The solution is highly reactive at ambient conditions, corrosive, and emits fumes upon opening, so handling inside the fume hood is essential for safety reasons. Another critical step in this synthesis is the addition of the precursor solution 260 mM NaBH4. This is the reducing agent used to reduce Sn into the Au seeds, but NaBH4 degrades quickly once mixed with water. Hence, it is crucial to wait to add the water to the solid NaBH4 until right before it is needed and then inject it rapidly into the solution vial as soon as it has fully dissolved. Prolonged exposure of NaBH4 in solution may lead to a loss of effectiveness in fully reducing Sn into the Au seeds.
Despite the limitations of this method, it is capable of synthesizing nanoparticles with a prominent LSPR peak and high-quality plasmonic properties. With the limited versatility in strongly plasmon-active transition metals, post-transition metal alloying is a successful method to enable tunability to potentially more applicable plasmon energies1,2,3,6,7,8. By combining the post-transition metal, Sn, with a noble metal such as Au, an alloy can be created that exhibits a higher energy plasmon resonance, underscoring the functional impact of post-transition metal alloying in the future of UV-based applications. The hope is that these nanoparticles can be utilized in catalysis, drug delivery, and optoelectronics.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work relates to Department of Navy awards N00014-20-1-2858 and N00014-22-1-2654 issued by the Office of Naval Research. Characterization was supported in part by the National Science Foundation Major Research Instrumentation program under Grant 2216240. This work was also partially supported by the University of Massachusetts Lowell and the Commonwealth of Massachusetts. We are grateful to the UMass Lowell Core Research Facilities.
Materials
| Basix Microcentrifuge Tubes | Fisher Scientific | Cat#02-682-004 | |
| Cary 100 UV-visible Spectrophotometer | Agilent Technologies | Cat#G9821A; RRID:SCR_019481 | |
| Cary WinUV | Agilent Technologies | https://www.agilent.com/en/product/molecular-spectroscopy/uv-vis-uv-visnir-spectroscopy/uv-vis-uv-vis-nirsoftware/cary-winuv-softwar | |
| Crystallography Open Database | CrystalEye | RRID: SCR_005874 | http://www.crystallography.net/ |
| Cu Carbon Type-B Grids (200 mesh, 97 µm grid holes) |
Ted Pella | Cat#01811 | |
| Direct-Q 3 UV-R Water Purification System | MilliporeSigma | Cat#ZRQSVR300 | |
| Entris Analytical Balance | Sartorius | Cat#ENTRIS64I-1SUS | |
| Glass round-bottom flask (250 mL) | Fisher Scientific | Cat#FB201250 | |
| Glass scintillation vials | Wheaton | Cat#986548 | |
| Hydrochloric acid (HCl, NF/FCC) |
Fisher Scientific | CAS: 7647-01-0, 7732-18-5 | |
| Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4·3H2O, 99.99%) |
Alfa Aesar | CAS: 16961-25-4 | kept in a desiccator for consistency of purity and stability |
| ImageJ | National Institute of Health | RRID: SCR_003070 | https://imagej.nih.gov/ij/download.html |
| Isotemp GPD 10 Hot Water Bath | Fisher Scientific | Cat#FSGPD10 | |
| Isotemp Hot Plate Stirrer | Fisher Scientific | Cat#SP88857200 | |
| Mili-Q Ultrapure Water (18.2 MΩ-cm) |
Water purification system | ||
| Miniflex X-Ray Diffractometer | Rigaku | RRID:SCR_020451 | https://www.rigaku.com/products/xrd/miniflex |
| Model 5418 Microcentrifuge | Eppendorf | Cat#022620304 | |
| Nitric acid (HNO3, Certified ACS Plus) |
Fisher Scientific | CAS: 7697-37-2, 7732-18-5 | |
| On/Off Temperature Controller for Heating Mantle | Fisher Scientific | Cat#11476289 | |
| Optifit Racked Pipette Tips (0.5-200 µL) | Sartorius | Cat#790200 | |
| Optifit Racked Pipette Tips (10-1000 µL) | Sartorius | Cat#791000 | |
| Philips CM12 120 kV Transmission Electron Microscope | Philips | RRID:SCR_020411 | |
| Pipette Tups (1-10 mL) | USA Scientific | Cat#1051-0000 | |
| Poly(vinylpyrrolidone) (PVP; molecular weight [MW] = 40,000) |
Alfa Aesar | CAS: 9003-39-8 | kept in a desiccator for consistency of purity and stability |
| Practum Precision Balance | Sartorius | Cat# PRACTUM1102-1S | |
| PTFE Magnetic Stir Bar (12.7 mm) | Fisher Scientific | Cat#14-513-93 | |
| PTFE Magnetic Stir Bar (25.4 mm) | Fisher Scientific | Cat#14-513-94 | |
| Quartz Cuvette (length × width × height: 10 mm × 12.5 mm × 45 mm) |
Fisher Scientific | Cat#14-958-126 | |
| Round Bottom Heating Mantle 120 V 250 mL | Fisher Scientific | Cat#11-476-004 | |
| SmartLab Studio II | Rigaku | https://www.rigaku.com/products/xrd/studio | |
| Sodium borohydride (NaBH4, 97+%) |
Alfa Aesar | CAS: 16940-66-2 | kept in a desiccator for consistency of purity and stability |
| SureOne Pipette Tips (0.1-10 µL) | Fisher Scientific | Cat#02-707-437 | |
| Tacta Mechanical Pipette (P10) | Sartorius | Cat#LH-729020 | |
| Tacta Mechanical Pipette (P1000) | Sartorius | Cat#LH-729070 | |
| Tacta Mechanical Pipette (P10000) | Sartorius | Cat#LH-729090 | |
| Tacta Mechanical Pipette (P20) | Sartorius | Cat#LH-729030 | |
| Tacta Mechanical Pipette (P200) | Sartorius | Cat#LH-729060 | |
| Tin (IV) chloride (SnCl4, 99.99%) |
Alfa Aesar | CAS: 7646-78-8 | kept in the fume hood and sealed with Parafilm between uses to avoid exposure to ambient conditions |
| Trisodium citrate dihydrate (C6H5Na3O7·2H2O, 99%) |
Alfa Aesar | CAS: 6132-04-3 | kept in a desiccator for consistency of purity and stability |
| Zero-Background Si Sample Holder | Rigaku |
Riferimenti
- Fonseca Guzman, M. V., et al. Plasmon manipulation by post-transition metal alloying. Matter. 6 (3), 1-17 (2023).
- Branco, A. J., et al. Synthesis of gold-tin alloy nanoparticles with tunable plasmonic properties. STAR Protoc. 4 (3), 102410 (2023).
- Kelly, K. L., Coronado, E., Zhao, L. L., Schatz, G. C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J Phys Chem B. 107 (3), 668-677 (2003).
- Linic, S., Christopher, P., Xin, H., Marimuthu, A. Catalytic and photocatalytic transformations on metal nanoparticles with targeted geometric and plasmonic properties. Acc Chem Res. 46 (8), 1890-1899 (2013).
- Naldoni, A., Shalaev, V. M., Brongersma, M. L. Applying plasmonics to a sustainable future. Science. 356 (6341), 908-909 (2017).
- King, M. E., Fonseca Guzman, M. V., Ross, M. B. Material strategies for function enhancement in plasmonic architectures. Nanoscale. 14 (3), 602-611 (2022).
- Zhou, M., Li, C., Fang, J. Noble-metal based random alloy and intermetallic nanocrystals: Syntheses and applications. Chem Rev. 121 (2), 736-795 (2020).
- Cortie, M. B., McDonagh, A. M. Synthesis and optical properties of hybrid and alloy plasmonic nanoparticles. Chem Rev. 111 (6), 3713-3735 (2011).
- Leitao, E. M., Jurca, T., Manners, I. Catalysis in service of main group chemistry offers a versatile approach to p-block molecules and materials. Nat Chem. 5 (10), 817-829 (2013).
- Melen, R. L. Frontiers in molecular p-block chemistry: From structure to reactivity. Science. 363 (6426), 479-484 (2019).
- Turkevich, J., Stevenson, P. C., Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Farad Disc. 11, 55-75 (1951).

