Amnesia Anterógrada
English
Condividere
Panoramica
Fuente: Laboratorios de Jonas T. Kaplan y Sarah I. Gimbel, University of Southern California
La amnesia anterógrada es la pérdida de la capacidad de formar nueva memoria. Esto puede distinguirse de la amnesia retrógrada, que es la pérdida de viejos recuerdos. La amnesia anterógrada puede resultar de daño a las estructuras en el cerebro que están involucradas en la formación de nuevos recuerdos. Los pacientes con daño a las estructuras del lóbulo temporal medial, incluyendo el hipocampo, la amígdala y las cortezas circundantes, a menudo tienen graves déficits en la formación de ciertos tipos de recuerdos. Estos casos pueden ser informativos como a cómo se organiza la memoria en el cerebro, y cómo diferentes sistemas de soportan diferentes tipos de memorias.
En este video, vamos a probar a un paciente con daño intermedio del lóbulo temporal en una serie de tareas de memoria diseñado para distinguir entre diferentes formas de memoria. En primer lugar, vamos a probar memoria a corto plazo o de trabajo, que es el proceso que utilizamos para mantener la información en cuenta temporalmente. A continuación, probamos dos tipos de memoria a largo plazo: Memoria explícita e implícita. Memorias explícitas son fáciles de verbalizar y consciente. Por ejemplo, recuerdos de hechos o episodios de nuestra vida son recuerdos explícitos. Fácilmente podemos decir alguien lo que hemos comido para desayunar, o lo que la ciudad es la capital de Francia. Memoria implícita implica el conocimiento que obtenemos de la experiencia pero que no es fácilmente expresable. Por ejemplo, saber cómo hacer las cosas, o se acostumbra a un estímulo son formas de memoria implícita.
Estos procedimientos se basan en parte en estudios del paciente famoso Henry Molaison, también conocido por sus iniciales H.M., que tenía amnesia anterograde severa como resultado de una cirugía para la epilepsia insuperable en el que las partes de ambos lóbulos temporales fueron resecadas. 1 realizará una prueba de span de dígitos, memoria a corto plazo, una prueba de aprendizaje asociado emparejado, que mide memoria explícita o declarativa, y el espejo plano, una prueba de aprendizaje implícito de la habilidad. 2
Procedura
Risultati
On the Digit Span Test, the patient successfully repeated a sequence of six digits in forward order, and five digits in reverse order. This level of performance shows some degree of intact short-term memory; average performance on this task for the healthy controls was seven forward, six reverse (Figure 2A). On the Verbal Paired-associate Test, the patient was not able to recall a single word pair. This demonstrates a severe deficit in the formation of explicit long term memories (Figure 2B). On the Mirror Drawing task, the amnesic patient shows fewer errors with practice, evidencing an ability to learn a motor task (Figure 2C).
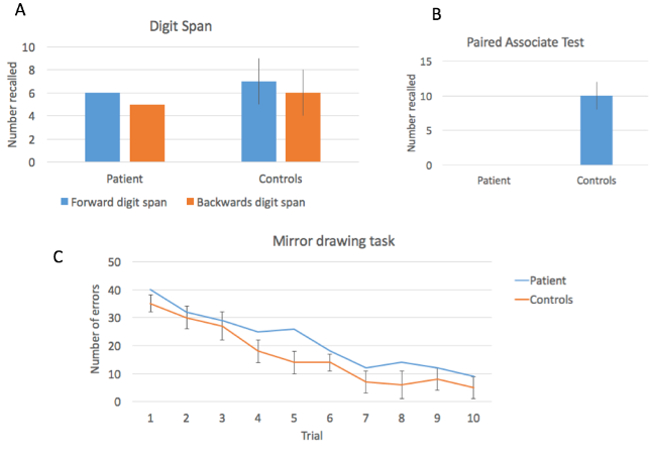
Figure 2: Performance on three memory tests. The patient showed relatively intact performance on the Digit Span Test, but was severely impaired in the Verbal Paired-associate Learning task. Performance on the motor learning task showed improvement over time.
These results demonstrate two important dissociations. The first is a dissociation between short-term memory, which requires active rehearsal to maintain, and long-term memory, the process that allows us to hold on to information without needing continual rehearsal. Patients with medial temporal lobe damage generally do not have major difficulties with short-term memory, as demonstrated by an intact digit span. However, after a few seconds, if information is not rehearsed, it is not maintained. This is demonstrated by the complete inability to retain the paired word associations in our patient. The second dissociation is between explicit and implicit memory. While this patient cannot remember the words recently seen, the Mirror Drawing test shows that they are able to learn a motor skill, a kind of learning which does not depend on medial temporal lobe structures.
Applications and Summary
Cases like these have been incredibly important in the history of cognitive neuroscience for learning associations between brain structures and function. While this video has demonstrated a general effect of medial temporal lobe damage on memory function, it is important to note that a deeper understanding requires an examination of the relationship between the specifics of which structures were damaged, and memory performance. In the case of Henry Molaison, it was many years before the technology of brain imaging allowed for a clear understanding of the nature of his lesion. After he died in 2008, a post-mortem examination allowed a precise reconstruction of the lesion, showing that in addition to large portion of the hippocampus, there was also damage to the surrounding cortex and white matter fibers that carry signals into and out of the hippocampus.6
Memory loss is a consequential component of many forms of neural disease; in addition to resulting from focal brain damage, memory disturbances may result from degenerative diseases such as Alzheimer’s Disease and Fronto-Temporal Dementia. Those conditions typically affect explicit long-term memories. Alternatively, motor learning, like the kind tested here, may be affected in conditions that affect the basal ganglia like Parkinson’s Disease. Given the importance of memory in our lives, understanding the neural systems that underlie different forms of memory, and how memory is parcellated into different processes may also lead to techniques for improving memory performance.
Riferimenti
- Scoville, W.B. & Milner, B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 20, 11-21 (1957).
- Milner, B., Corkin, S. & Teuber, H.L. Further Analysis of Hippocampal Amnesic Syndrome – 14-Year Follow-up Study of Hm. Neuropsychologia 6, 215-& (1968).
- Drachman, D.A. & Arbit, J. Memory and the hippocampal complex. II. Is memory a multiple process? Arch Neurol 15, 52-61 (1966).
- Uttl, B., Graf, P. & Richter, L.K. Verbal Paired Associates tests limits on validity and reliability. Arch Clin Neuropsychol 17, 567-581 (2002).
- Milner, B. Memory and the medial temporal regions of the brain. in Biology of Memory (eds. Pribram, K.H. & Broadbent, D.E.) 29-50 (Academic Press, New York, 1970).
- Annese, J., et al. Postmortem examination of patient H.M.'s brain based on histological sectioning and digital 3D reconstruction. Nat Commun 5, 3122 (2014).
Trascrizione
For many people, creating new memories and remembering old ones happen seamlessly. However, in the case of amnesia, these processes are disrupted.
When individuals cannot remember old memories, say the name of their childhood pet or where they grew up, they suffer from retrograde amnesia. Contrast this with anterograde amnesia—an inability to form new memories—like the trip to the ocean today will not be recalled tomorrow.
This memory gradient—ranging from facts and episodes occurring long ago to the present—has led to important discoveries of how the brain organizes and stores memories.
In this video, we will test a patient with specific brain damage to the medial temporal lobe, similar to the famous H.M. who suffered from severe anterograde amnesia. We will investigate a series of tasks designed to distinguish between different forms of memory, as well as other cases where memory loss varies with the extent of brain damage.
In this experiment, two groups of participants—patients with known damage to the hippocampus and surrounding temporal lobe and healthy controls—are asked to complete three distinct memory tasks: the Digit Span Test, Verbal Paired-associate Learning, and Mirror Drawing.
In the first task, the Digit Span Test, participants are asked to listen to and remember a series of digits, starting first with a randomized sequence of four numbers. At the end of every list, they are instructed to speak them back in order.
Sequences are grouped in blocks of three, with each line containing different numbers. If the participant is able to repeat any of the lines within a block without errors, another digit is added, creating a second block. This process is continued until the participant incorrectly repeats an order.
When that happens, the task is modified and participants must repeat sequences in reverse. For example, if 5, 6, 1, 4 are spoken, the correct response would be 4, 1, 6, 5. In both cases, the dependent variable is the maximum number of digits they were able to repeat correctly.
The Forward and Reverse Digit Span Tests assess short-term or working memory, which is the process we use to keep information in mind temporarily. This type of memory does not depend on the hippocampus, so performance levels are expected to be similar in amnesiacs and normal participants.
For the second task, Verbal Paired-associate Learning, participants first listen to 15 word pairs, including duos like bank-milk, during the study phase and then are asked to remember them. Later in the testing phase, one of each of the paired words are verbalized, and they must provide the other half.
This time, the dependent variable is how many word pairs are remembered correctly. It is hypothesized that there will be more incorrect trials—those that take longer than 10 s for a response or are deemed unknown—for patients compared to controls, because the Verbal Paired-associate Test assesses a form of long-term memory referred to as explicit, which is dependent on the hippocampus.
The third and final task is Mirror Drawing, where participants are asked to trace around the outline of a five-pointed star on a piece of paper while viewing their hand in the reflection of a mirror.
Here, the dependent variable is the total number of times the participant draws outside of the lines in each of the 10 trials. It’s expected that both groups will retain what they’ve learned over the course of several trials to show improvement because the form of long-term memory that mirror drawing assesses is procedural—implicit memory that involves knowledge that’s not easily expressible—and is hippocampal-independent.
To begin, first recruit patients with known medial temporal lobe damage. Verify the extent of their damage on MRI images taken post-surgery, most commonly for the treatment of epilepsy. In addition, recruit age-matched controls without any history of neurological impairments or disease.
After gaining consent from all participants, explain the instructions for the first task, the Digit Span Test, which involves repeating back a series of digits in the same order that they were stated. Elaborate that the sequence will get longer until they can no longer remember all of the numbers.
From a sheet of randomized sets, read the four-digit series 4-6-3-1, at a rate of 1 number per s, and have each participant repeat them back. [Participants say: 4-6-3-1]. Note that one digit is added to the length as long as one of the three sequences within a block is repeated back correctly.
Continue until participants fail to repeat any of the three series correctly, and subsequently record the maximum sequence lengths obtained for each. Afterwards, modify the task so that participants repeat the digits back in reverse order using the same procedure as before. Again, record the maximum lengths that were correctly repeated backwards.
Following a 2-min break, now explain rules for the next task, Verbal Paired-associative Learning: They will hear 15 word pairs and should try to remember them together. After a small break, they will be given one of the items and should state what was originally paired with it.
Using an established list, read 1 pair every 5 s during this study phase. After going through the entire list, give participants a 3-min break. Resume with the testing phase by reading the first word from each set in a random order. Wait for participants to state the other one. Mark whether responses are correct, or incorrect if either 10 s elapses or they do not know.
Finally, initiate the final task, mirror drawing. Explain that they are to trace around the outline of the five-pointed star figure, staying within the lines as much as possible. Indicate that they will only be able to see their hand through a mirror reflection. Next, allow participants to trace around the shape.
Once completed, provide them with a 5-min break and a new star to trace. Repeat until 10 stars have been outlined. For each one, record how many times participants crossed the borderlines.
To visualize the data for the Digit Span Test, plot the maximum number of digits recalled by the patient and controls across the forward and reverse versions. Notice that longer sequences were recalled in the forward direction than backwards, but there were no significant differences between groups.
Next, for the Verbal Paired-associate Test, graph the total number of word pairs that were recalled for both the patient and controls. The patient was not able to recall any of the word pairings, compared to an average of 10 for controls. These results suggest that the medial temporal lobe damage caused a severe deficit in the formation of long-term memories.
Finally, graph the number of tracing errors made across trials for both the patient and controls. Notice that both groups made a similar number of errors and improved over time, suggesting that performance on an implicit memory task is intact, and thus, not dependent on the hippocampus.
To summarize, the results demonstrate two important dissociations. The first one is between short- and long-term memory. Patients with medial temporal lobe damage generally do not have major difficulties with short-term memory, as demonstrated by an intact digit span.
However, after a few seconds, if information is not rehearsed, it is not maintained. This was demonstrated by the complete inability to retain the paired word associations in the patient.
The second dissociation is between explicit and implicit memory. While this patient couldn’t remember the words recently seen, the mirror drawing test showed that they were able to learn a motor skill, a kind of learning which does not depend on medial temporal lobe structures.
Now that you are familiar with using several tests to assess short- and long-term memory function, including explicit and implicit ones, let’s look at how memory loss can result from a number of disorders and vary with the extent of damage.
For example, Alzheimer’s Disease and Fronto-Temporal Dementia are known to affect explicit long-term memories, whereas Parkinson’s Disease affects implicit motor learning, which is dependent upon different brain regions, including the basal ganglia.
The severity of memory loss is linked to the amount of damage to the brain regions. This is exemplified by the progressive nature of disorders like Alzheimer’s Disease, where a patient’s memory loss becomes worse over time. This is why early diagnosis and treatment is such an important research field.
You’ve just watched JoVE’s video on anterograde amnesia. Now you should have a good understanding of how memories can be affected and how to implement multiple behavioral tests to assess function. You should also know how to evaluate the results and apply the approach to investigate other scenarios that suggest memory loss.
Thanks for watching!
