Saggio per la morte cellulare: saggio di rilascio di cromo della capacità citotossica
English
Condividere
Panoramica
Fonte: Frances V. Sjaastad1,2, Whitney Swanson2,3e Thomas S. Griffith1,2,3,4
1 Programma di laurea in microbiologia, immunologia e biologia del cancro, Università del Minnesota, Minneapolis, MN 55455
2 Centro di Immunologia, Università del Minnesota, Minneapolis, MN 55455
3 Dipartimento di Urologia, Università del Minnesota, Minneapolis, MN 55455
4 Masonic Cancer Center, Università del Minnesota, Minneapolis, MN 55455
Una delle funzioni principali delle cellule del sistema immunitario è quella di rimuovere le cellule bersaglio che sono state infettate da virus o hanno subito la trasformazione in una cellula tumorale. I saggi in vitro per misurare la capacità citotossica delle cellule immunitarie sono stati un punto fermo nei laboratori per molti anni. Questi saggi sono stati utilizzati per determinare la capacità delle cellule T, delle cellule NK o di qualsiasi altra cellula immunitaria di uccidere le cellule bersaglio in modo antigene-specifico o non specifico. I ligandi di morte (ad esempio, ligando fas o TRAIL), le citochine (ad esempio, IFNg o TNF) o i granuli citotossici (ad esempio, perforina / granzima B) espressi dalle cellule emotrici sono alcuni modi in cui la morte delle cellule bersaglio può essere indotta. Con l’esplosione della ricerca sull’immunoterapia tumorale negli ultimi anni, c’è un crescente interesse nella ricerca di agenti per aumentare l’attività citotossica delle cellule immunitarie per migliorare i risultati dei pazienti. Al contrario, alcune malattie sono caratterizzate dall’attività esuberante dell’attività citotossica delle cellule immunitarie, con conseguenti sforzi per identificare gli agenti per temperare queste risposte. Pertanto, avere un test in cui l’utente può facilmente integrare qualsiasi numero di diverse cellule emotrici, cellule bersaglio e / o modificatori di risposta nel progetto sperimentale può servire come mezzo prezioso per valutare rapidamente la capacità citotossica delle cellule emotrici e / o la reattività della cellula bersaglio.
Questi saggi in vitro comportano la miscelazione di diverse popolazioni cellulari, oltre a utilizzare un numero relativamente basso di cellule emotrici e bersaglio. Pertanto, una necessità del test è quella di etichettare le cellule bersaglio in un modo che possa essere facilmente rilevato e quantizzato, consentendo all’utente di determinare quindi la “lisi specifica percentuale” mediata dalle cellule emotrici. La radioattività – in particolare, il cromo 51 (51Cr) sotto forma di Na251CrO4– è un modo economico per etichettare rapidamente e in modo non specifico le proteine cellulari all’interno delle cellule bersaglio (1). La breve etichettatura e i tempi totali del test riducono il potenziale di cambiamenti significativi nel numero e/o nel fenotipo delle cellule bersaglio, che potrebbero influenzare l’esito del test. Dopo la perdita dell’integrità della membrana delle cellule bersaglio a causa dell’attività citotossica delle cellule emotrici, le 51proteine cellulari marcate con Cr all’interno delle cellule bersaglio vengono rilasciate nel surnatante di coltura, diventando disponibili per la quantificazione. Come con qualsiasi test che esamina la funzione delle cellule immunitarie in vitro, ci sono una serie di considerazioni importanti da considerare per migliorare le prestazioni dell’esperimento. Una delle caratteristiche più critiche è quella di utilizzare cellule emocromatrici sane (per la massima attività citotossica) e target (per la massima reattività e la minima morte spontanea /rilascio di 51Cr). È necessario il contatto tra effettore e cellula bersaglio (che porta all’uso comune di piastre a 96 pozzi a fondo tondo per incoraggiare il contatto cellula-cellula) (2). Infine, l’analisi dei dati dipende dall’inclusione di popolazioni di cellule bersaglio di controllo positive e negative.
Il seguente protocollo delineerà i passaggi per l’esecuzione di un test standard di rilascio di 51Cr per misurare la capacità citotossica di una popolazione di cellule emotrici, sebbene sia stata recentemente sviluppata una versione non radiativa che utilizza Europium. 51 anni Cr è un potente emettitore di radiazioni γ. Di conseguenza, l’uso di questo test richiede un’adeguata formazione sulla sicurezza delle radiazioni, uno spazio di laboratorio dedicato, un contatore gamma e lo smaltimento di campioni radioattivi.
La sequenza generale degli eventi in questo test sono: 1) preparare 51bersagli etichettati con Cr; 2) preparare le cellule emotrici e aggiungerle alla piastra mentre le cellule bersaglio sono etichettate; 3) aggiungere bersagli etichettati alla piastra; 4) piastra di incubazione; 5) raccogliere supernatanti; e 6) analizzare i dati dopo aver eseguito i campioni sul contatore. I campioni sono comunemente preparati in triplice copia e quindi mediati per tenere conto di eventuali sottili differenze di pipettaggio.
Un DPI adeguato è importante per questo test. In particolare, l’utente deve indossare un camice da laboratorio e guanti. Gli occhiali di sicurezza possono essere richiesti in base al laboratorio o all’istituzione. Ci dovrebbe essere un’ampia schermatura al piombo per la conservazione sicura e l’uso del 51Cr durante tutte le fasi. Infine, ci dovrebbero essere spazi di laboratorio dedicati e attrezzature riservate all’utilizzo di 51Cr, compresa tutta la segnaletica appropriata per indicare dove vengono conservati i campioni con 51Cr e un contatore Geiger dotato di sonda gamma per esaminare lo spazio per possibili contaminazioni.
In questo esercizio di laboratorio, determineremo la capacità delle cellule mononucleate del sangue periferico umano (PBMC) (CpG stimolate vs. non stimolate) di uccidere le cellule di melanoma, utilizzando la linea cellulare del melanoma umano WM793 come modello e il test di rilascio del cromo.
Procedura
Risultati
In this example, effector cells stimulated with CpG (Figure 1, black circles) killed the target cells more effectively, as the ratio of effector cells to target cells increased. This increase was not observed in the unstimulated PBMCs (white circles), indicating that CpG stimulation is necessary for the observed increase in target cell lysis.
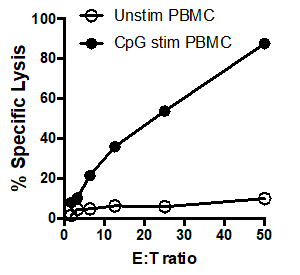
Figure 1: 51Cr assay scatter plot: Tumoricidal activity by human PBMCs, unstimulated (white circles) and after stimulation with CpG (black circles), tested at different effector: target cell ratios (E: T) ratios (ranging from. 50:1 to 1.5:1).
Applications and Summary
The assay described here has considerable flexibility, as a variety of effector and target cells can be used depending on the question being asked. For example, effector cell specificity can be determined by using different target cells or the mechanism of effector cell killing can be determined by using cells deficient in specific proteins or using protein specific inhibitors. A major problem with the 51Cr release assay is the potential for a high spontaneous release rates by the target cells. When cultured alone (without effector cells), the spontaneous release of 51Cr by the target cells should ideally be no more than 30% of the total ("maximal") release by the target cells immediately lysis. Higher spontaneous release rates may be due to using unhealthy target cells, either due to poor health (e.g., extended culture of a cell line) or an overly long labelling period.
Riferimenti
- Brunner, K. T., Mauel, J., Cerottini, J. C. and Chapuis. B. Quantitative assay of the lytic action of immune lymphoid cells on 51Cr-labelled allogeneic target cells in vitro; inhibition by isoantibody and by drugs. Immunology, 14 (2):181-196, (1968).
- Kemp, T. J., B. D. Elzey, and T. S. Griffith. Plasmacytoid dendritic cell-derived IFN-alpha induces TNF-related apoptosis-inducing ligand/Apo-2L-mediated antitumor activity by human monocytes following CpG oligodeoxynucleotide stimulation. The Journal of Immunology, 171 (1): 212-218, (2003).
Trascrizione
In this video you will observe how to perform the chromium release assay and determine the cytotoxic potential of the effector cells.
Immune cells are responsible for identifying and removing potentially harmful cells, like cancer or virus-infected cells, from the body, which is an integral part of the immune response. Several immune cells, like T-cells and NK cells, possess a property known as cytotoxic potential, which is the ability to identify target cells and secrete proteins that induce protein degradation, lysis, and death of those target cells. Quantifying cytotoxic potential is critical for measuring immune cell activation and potency, and the chromium release assay is commonly used for this purpose.
This method enables users to compare cytoxicity induced by specific types of immune cells under different conditions, which is valuable for studying cancer immunotherapy and immunity related diseases. To begin, the target cells, like cancer cells, are incubated with a radioactive isotope, chromium 51, which is taken up by the cells. Next, these radio labeled cells are co-cultured with the isolated immune cells of interest, also called the effector cells, in a round bottom, 96- well plate to facilitate interaction between the two cell types.
The overall setup of the assay involves incubating a specific number of target cells with different concentrations of the immune cells, along with appropriate controls. The co-culture allows the effector cells to induce apoptosis and lysis in the target cells, resulting in the release of the intracellular chromium 51 into the supernatant. Then, at a preoptimized time point, the supernatant containing the released chromium is harvested from all the wells. The chromium 51, being radioactive, spontaneously undergoes radioactive decay to emit gamma radiation. The gamma radiation levels in the supernatants from all the wells in the assay plate represent a quantifiable output of the lysis of the target cells. This is measured using a gamma counter, which is then used to determine the cytotoxic potential of the immune cells.
To begin, the target cells, human melanoma cell line WM793 in this example, are prepared into a single cell suspension. To do this, first remove the media from the tissue culture flask and wash the cells with five milliliters of 1X PBS. Decant the PBS and then add one milliliter of trypsin to the plate for approximately two minutes. Gently tap the flask to loosen the cells from the flask surface and then add five milliliters of RPMI media to the flask. Pipette the media up and down to collect the cells and add this suspension to a 15 milliliter conical tube.
Place the tube in the centrifuge for five minutes at 1200 RPM. Next, remove the media from the tube making sure not to disrupt the cell pellet. Gently flick the bottom of the tube to disrupt the cell pellet and add 10 milliliters of media to the tube. Then, gently pipette the media up and down to bring the cells into suspension. Next, determine the cell concentration using a hemocytometer and transfer two milliliters of the original cell suspension to a new 15 milliliter conical tube. Place the tube into a centrifuge and pellet the cells at 12 hundred RPM for five minutes. After centrifugation, pour the excess media out of the tube into a waste container. Briefly vortex the tube to resuspend the cell pellet in the small volume of medium left behind.
Next, prepare to use Chromium 51 by moving to a lab space dedicated for this particular radioactivity. There should be ample lead shielding for safe storage and use of the Chromium 51 during all steps, as well as proper signage to indicate where samples with Chromium 51 are being kept. A Geiger counter equipped with a pancake probe is also necessary to serve in the space for possible contamination.
Once set up for the proper use of radioactivity, add 100 microcuries of Chromium 51 directly to the target cell suspension. Then, add a small piece of radioactive tape to the tube to indicate that the sample and tube are now radioactive. Place the tube in a 37 degree celsius incubator with a lead shield and incubate for an hour, flicking the tube every 15 to 20 minutes.
While the target cells are labeling, prepare a single cell suspension of effector cells. In this example, human peripheral blood mono nuclear cells, or PDMCs, were isolated from whole blood by standard density gradient centrifugation to a concentration of 5 times 10 to the 6th. Transfer this effector cell suspension into a disposable reagent reservoir and then add 200 microliters of this suspension into each well of row B in a 96-well round-bottom plate. Next, add 100 microliters of RPMI to each well in row C through G of the plate.
Now, begin performing serial dilutions of the PBMCs to have a range of effector cell numbers by first removing 100 microliters of the cells in the wells in row B and adding this to row C. Then, further dilute the effector cells by transferring 100 microliters of cells from row C to row D. Continue the serial dilution. Once row G is reached, move 100 microliters from the wells to leave a final volume of 100 microliters in each well in that row. Next, add 100 microliters of tissue culture medium to the wells in row A to serve as a control for the spontaneous release of Chromium 51 from the target cells, as no effector cells should be added to this row. Then, place a plate into a 37 degree celsius incubator until the target cells are ready to be added.
After the incubation period, remove the target cells from the incubator and wash with 5 milliliters of FBS to remove any excess Chromium 51. Then, place the tube in a designated centrifuge and spin at 1200 rpm for 5 minutes. Remove the radioactive FBS wash into an appropriate waste container and repeat the wash step by resuspending the pellet in a fresh 5 milliliters of FBS. Place the tube in a designated centrifuge and spin the cells again at 1200 rpm for 5 minutes. Remove the second wash and check the pellet for incorporated radioactivity using a Geiger counter. Finally, Resuspend the pellet in 10 milliliters of complete medium and pour the Chromium 51 labeled, target cell suspension into a disposable reagent reservoir. Then, add 100 microliters of these labeled target cells to every well of the 96-well effector cell plate. Next, add 100 microliters of 1% NP-40 in water to the wells in row H to lyse all the target cells this each row. These wells will be used as a control to determine the total counts per minute, or cpm.
Now that the plate is prepared, secure the lid by adding a small piece of tape to the each side of the plate and place a piece of radioactive tape on the lid to indicate it contains chromium 51. Then, place the plate in a centrifuge marked to handle radioactive samples. If only one experimental plate is being used, add a balance plate to the centrifuge. Set the centrifuge to 1200 rpm, and bring the plate up to speed. Once at the speed, stop the machine. Remove the plate from the centrifuge. Then, place the plate in a 37 degree celsius incubator with a small piece of lead shielding over the plate for additional safety. Incubate for 16 hours to allow the target cells to lyse.
At the end of the incubation period, carefully remove the tape around the edge of the plate, and remove the lid. Next, place the harvesting frame on the plate making sure to confirm the small filter discs are in place for each of the cotton plugs. Now, slowly and gently press the cotton plugs into the wells. After approximately ten seconds, release the pressure on the cotton plugs, and then transfer the cotton plugs to tube strips. Place each of these tubes into a secondary FACS tube. Finally, load the FACS tubes onto a gamma counter and run the samples to quantitate the amount of chromium 51 released in each condition. Carefully record the order in which the tubes were loaded into the counter.
Here, unstimulated PBMCs were added to the first 3 lanes and CPG stimulated PMBCs were added to lanes 4 through 6. In this example, the counts per minute were entered into the cells of a spreadsheet in the same manner as the samples were laid out in the original plate and the averages of the triplicates were calculated. For example, for the first condition, cells A1, A2, and A3 were averaged in cell I3. Once the averages are determined, the percent of specific lysis for each condition can be calculated using this formula. For example, to calculate the percent specific lysis for the unstimulated cells that had a ratio of 50 to 1 effector cells to target cells the spontaneous CPM, which in this example, is 1164.67, was subtracted from the experimental CPM, 1129. 67. This number can then be divided by the difference between the maximum CPM and the spontaneous CPM, and then multiplied by 100 to give the percent specific lysis. This is then calculated for each condition. These data can then be graphed to show comparison of the E to T ratio with the percent specific lysis for both the unstimulated PBMCs, and the CPG stimulated PBMCs. In this example, effector cells stimulated with CPG more effectively killed target cells as the ratio of effector cells to target cells increased. This increase was not observed in the unstimulated PBMCs, indicating that CPG stimulation is necessary for the observed increase in target cell lysis.
