A Primary Human Trophoblast Model to Study the Effect of Inflammation Associated with Maternal Obesity on Regulation of Autophagy in the Placenta
PREPARAZIONE ISTRUTTORI
CONCETTI
Student Protocol
Placentae were collected from the Labor and Delivery Unit at University Hospital under a protocol approved by the Institutional Review Board of Oregon Health and Science University in Portland, Oregon, with informed consent from the patients.
1. Collection of Placental Tissue
- Preparation
NOTE: All equipment that encounters tissue must be sterile.- Sterilize the dissecting equipment by autoclaving for 60 min at 121 °C.
- Use personal protective equipment (PPE): lab coats/gowns/scrubs, gloves, and mask with a face shield or goggles.
- Turn on water bath and set to 37 °C.
- Warm two 50 mL conical tubes, each containing 25 mL of complete media (Iscove's Modified Dulbecco's Medium supplemented with 10% FBS and 1% Penicillin/Streptomycin, Table 3) in a 37 °C water bath.
- With informed patient consent, obtain a placenta immediately following delivery by cesarean section.
- Become familiar with the placenta. The umbilical cord is inserted on the fetal side (chorionic plate) and blood vessels can be seen radiating out from the umbilical cord insertion site. The opposite side is the maternal side, or basal plate. It includes the decidua and structures that contain vessel trees, called cotyledons.
- Sampling of Villous Tissue
NOTE: Villous tissue should be sampled from the placenta as soon as possible following delivery, preferably in 30 min or less.- With the chorionic plate facing upwards, excise 2-3 full-thickness sections (approximately 2.5 cm x 2.5 cm in size) 2-3 cm in from the periphery of the placenta at random, using forceps and scissors (Figure 1A).
NOTE: Avoid parts of the placenta that appear abnormal (i.e. white calcifications). - Trim away the chorionic and basal plates and any large blood vessels.
- Place the resulting villous tissue (Figure 1B, approximately 80 – 120 g total) in warm complete media and begin trophoblast isolation within 30 min of sampling.
NOTE: Some downstream assays and applications are affected by the length of latency period between delivery, sampling, and trophoblast isolation. It is best to keep these periods as short as possible and consistent between isolations. - Using the techniques described in steps 1.2.1 – 1.2.2, sample 5 random 1 cm x 1 cm sections of villous tissue from across the placenta (including the center).
- Cut each 1 cm x 1 cm villous tissue sample into 4-5 smaller pieces (approximately 30 mg each), place in 2 mL microcentrifuge tubes, and flash freeze in liquid N2. Store at -80 °C for use in later analyses.
- With the chorionic plate facing upwards, excise 2-3 full-thickness sections (approximately 2.5 cm x 2.5 cm in size) 2-3 cm in from the periphery of the placenta at random, using forceps and scissors (Figure 1A).
2. Isolation of Trophoblasts from Villous Tissue
- Preparation
NOTE: Use sterile equipment and perform procedures involving cells in a laminar flow hood.- Thaw four frozen density gradients (Table 1, see table of the essential supplies, reagents, and equipment, supplementary material) at 4 °C the night before the placenta arrives.
NOTE: Alternatively, density gradients can be made on the day of the isolation. - Turn on centrifuge and set to 20 °C.
NOTE: All centrifugation steps are at maximum acceleration and deceleration unless specified otherwise. - Prepare 1x HEPES-buffered salt solution (HBSS) supplemented with Ca+2 and Mg+2 (Table 3).
- Warm trypsin to 37 °C.
- Dilute DNase to approximately 2720 kilounits/mL in sterile supplemented HBSS.
- Warm 50 mL of Newborn Calf Serum (NCS) to 37 °C.
- Warm complete media to 37 °C.
- Warm freezing media (90% FBS, 10% DMSO, Table 3) to 37 °C.
CAUTION: DMSO is toxic and must be handled with gloves. - Prepare digestion buffer (Table 2 and 3) by mixing 308 mL of supplemented HBSS, 50 mL of Trypsin (3751.7 BAEE units/mL), and 0.5 mL of DNase (379.4 kilounits/mL) in a sterile bottle.
NOTE: The approximate time required to isolate cells from villous tissue is 7 h.
- Thaw four frozen density gradients (Table 1, see table of the essential supplies, reagents, and equipment, supplementary material) at 4 °C the night before the placenta arrives.
- Processing the Villous Tissue
- Rinse each piece of villous tissue in a 50 mL conical tube filled with room temperature phosphate buffered saline (PBS). Repeat and replace the PBS as necessary until the excess blood is removed (PBS rinse will be light red or pink when the tissue is thoroughly rinsed).
- Place the villous tissue in a sterile Petri dish and remove as many blood vessels as possible by gently scraping off the soft villous tissue from the vessels using a microscope slide.
- Finely mince the resulting villous tissue using scissors.
- Villous Tissue Digestion and Crude Isolation of Trophoblasts
- Transfer the minced villous tissue to a sterile bottle with digestion solution according to the calculated volumes based on the specific activity of trypsin and DNase (165 mL, Table 2).
- After 35 min of incubation in a 37 °C water bath with shaking at 70 revolutions per minute (rpm), tilt the digestion bottle on its side and allow the undigested pieces of tissue to settle at the bottom of the bottle. Carefully draw up the supernatant with a serological pipette, avoiding the settled tissue.
- Dispense the supernatant through a 100 µm cell strainer equally between 50 mL conical tubes.
NOTE: To save time, it is advisable to begin the second digestion by adding digestion solution (110 mL, Table 2) to the remaining settled tissue and resuming incubation as described in step 2.3.2. - Gently layer 3 – 5 mL of NCS underneath the strained supernatant by slowly dispensing from a serological pipette at the bottom of the tube. A meniscus between the strained supernatant (containing trophoblasts) and NCS should be visible (Figure 2A).
- Centrifuge the supernatants over NCS at 1,250 x g for 15 min at 20 °C. The resulting pellet will include red blood cells in the lower-most layer followed by a white layer containing the trophoblast cells (Figure 2B).
- Repeat steps 2.3.2 – 2.3.5 for each of the second and third digestions (adding 110 mL and 83.5 m, respectively, of digestion solution to the bottle of tissue, Table 2).
- Once all supernatants have been centrifuged, resuspend each pellet in 5 mL of warm complete media, and then pool the suspensions together.
- Split the cell suspension equally between two 50 mL conical tubes and centrifuge at 1,250 x g for 15 min at 20 °C.
- Gently remove the supernatant and resuspend each of the cell pellets in 6 mL of warm complete media.
- Density Centrifugation
- Divide the cell suspension equally between four density gradients (3 mL each) by slowly and carefully layering the cell suspension on the top of the density gradients with a transfer pipet.
- Centrifuge the density gradients at 1,250 x g for 20 min at 20 °C with minimum acceleration and deceleration. This should produce distinguishable bands of sedimented cells (Table 4).
- Slowly and carefully remove the top layers of density gradient media (DGM) until the opaque band(s) containing trophoblast cells (between 35 - 50% DGM) is reached (Table 4).
- Transfer the trophoblast bands into two 50 mL conical tubes and fill with warm complete media.
- Gently invert the tubes 3 – 6 times to mix and centrifuge at 1,250 x g for 15 min at 20 °C.
- Remove the supernatant and resuspend each cell pellet in 5 mL of warm complete media. Combine the cell suspensions and count viable cells using a hemocytometer and Trypan blue (or preferred cell counting method).
- Counting Cells with a Hemocytometer
- Mix the cell suspension by pipetting up and down with a serological pipet or by gently inverting the tube several times.
- Combine equal parts of cell suspension and Trypan blue (i.e. 20 µL each) in a separate tube and mix gently.
- Incubate for 1 – 2 min at room temperature.
- Gently dispense 15 – 20 µL of the cell-Trypan blue mixture in between the coverslip and the hemocytometer and allow the cells to diffuse across the grid by capillary action.
- Count viable cells (dead cells will be stained deep blue) in each of the four 4 x 4 quadrants with a tally counter. Employ a system of counting to ensure cells are not counted more than once (i.e. do not count cells that touch the bottom or left boundaries).
NOTE: Each 4 x 4 quadrant should contain between 50 – 150 cells. Too few or too many cells can lead to an over or underestimation of cell number. - Average the total cell counts from each of the 4 x 4 quadrants, multiply by 104, and then multiply by the dilution factor (cell suspension to Trypan blue) to calculate number of cells per mL.
- Multiply the number of cells per mL by the total volume of cell suspension to calculate the total cell yield.
NOTE: Approximately 100 million cells are expected from isolations starting with 80-120 g of villous tissue.
- Plating Cells
- Plate 3 million cells/well (3.3 x 105 cells per cm2) in a 6-well plate (2 mL of suspension per well) and gently rock back and forth and side to side to evenly distribute the cells.
NOTE: Trophoblasts require tissue culture treated plates to adhere properly. A monolayer of cells is required to promote syncytialization. - Leave the plated cells in the laminar flow hood for approximately 30 min to allow cells to evenly distribute, settle, and begin to adhere to the bottom of the wells before placing in the incubator.
- Culture cells for up to 72 h (with daily media changes) in a 37 °C incubator with 5% CO2 and 95% humidity.
NOTE: Examine the trophoblasts under a microscope at 10 – 20x every 24 h of culture. Trophoblasts do not proliferate and cannot be passaged. Over the course of 72 h of culture, the round individual trophoblasts fuse to form a syncytium (Figure 3A and B).
- Plate 3 million cells/well (3.3 x 105 cells per cm2) in a 6-well plate (2 mL of suspension per well) and gently rock back and forth and side to side to evenly distribute the cells.
- Freezing Cells
- Pellet unused cells by centrifugation at 1,250 x g for 10 min at 20 °C.
- Aspirate as much media as possible from the pellet.
- Resuspend the pellet in freezing media (Table 3).
- Freeze the aliquots at -80 °C in a freezing container filled with 100% isopropanol. Transfer the frozen aliquots to liquid N2 the following day for long term storage.
NOTE: Cells can be cultured after freezing.
- Thawing Cells
- Remove an aliquot of frozen cells and thaw in a 37 °C water bath while swirling. Remove the aliquot from the water bath just before it has fully thawed to a liquid.
- Immediately transfer the thawed cells to a 15-ml conical tube. Beginning slowing at first, add 10 mL of complete media with intermittent mixing.
- Invert tube several times to mix.
- Centrifuge at 200 x g for 10 min at 20 °C.
- Aspirate the supernatant and resuspend the pellet in 2 – 5 mL of warm complete media.
- Count the cells and plate as previously described.
3. Treatment of Primary Trophoblasts with TNFα, Collection of Cell Lysates, and Western Blotting
- Preparation
- Warm complete media to 37 °C.
- Make a 10 µg/mL stock of TNFα in complete media and store at -20 °C until ready for use.
- Make a 1 µg/mL working stock of TNFα in complete media when ready to treat the cells. Serial dilute the TNFα working stock to 104 pg/mL, 103 pg/mL, 500 pg/mL, 250 pg/mL, and 125 pg/mL in complete media.
NOTE: The TNFα concentration of 104pg/mL is moderately cytotoxic. Adjustment of the TNFα concentrations tested will depend on the specifics of the desired downstream applications.
- Treating Cells with TNFα
- After 24 h of culture, aspirate the culture media from the cells and replace with 2 mL of TNFα supplemented medias per well on 6-well plates (at least two wells per treatment and vehicle control).
- After 24 h of TNFα-exposure (48 h of culture), replace the TNFα supplemented medias with complete media.
- Harvesting Cells and Total Protein
- After 72 h of culture (24 h past removal of TNFα), aspirate media, gently rinse cells with room temperature PBS, and add 80 µL of ice cold Radioimmunoprecipitation Assay Buffer (RIPA) containing freshly added protease and phosphatase inhibitors (Table 3) directly to each well.
- Remove the cells from the plate with a cell scraper. Transfer the cells to a 1.5 mL microcentrifuge tube, pooling wells within treatment groups.
- Lyse the cells by vortexing the tubes on high for at least three intervals of 15 s.
- Incubate the cells at 4 °C with rocking for 15 min.
NOTE: Alternatively, after step 3.3.3., the cells can be incubated on ice for 15 min with intermittent agitation by flicking the tube, vortexing, or pipetting up and down. - Repeat step 3.3.3.
- Centrifuge the tubes at 10,000 x g for 5 min at 4 °C to pellet cellular debris.
- Transfer the supernatant (containing cellular protein) to a new 1.5 mL microcentrifuge tube and store at -80 °C.
NOTE: The protocol can be stopped here and resumed at a later time. It is advisable to make several aliquots of cellular protein samples to avoid multiple freeze-thaw cycles. - Determine total protein concentration by a preferred method, such as a bicinchoninic acid assay (BCA, see table of the essential supplies, reagents, and equipment, supplementary material).
- SDS-PAGE and Western Blotting for Rubicon or Protein of Interest
NOTE: Follow Western blotting protocols according to manufacturer's instructions using a preferred laboratory system.- Load between 20-40 µg of total protein in sample buffer (Table 3) per well on a 12% acrylamide gel. Separate proteins by SDS-PAGE in running buffer (Table 3).
- Wet-transfer the proteins from the gel to a polyvinylidene difluoride (PVDF) membrane according to manufacturer's instructions in transfer buffer (Table 3).
- Incubate the membrane in 5% milk powder in Tris-Buffered Saline with 0.1% Tween 20 (TBST, Table 3) for at least 1 h at room temperature with rocking.
- Incubate the membrane in a Rubicon primary antibody (see table of the essential supplies, reagents, and equipment, supplementary material) at 1:500 in 1% milk powder in TBST overnight at 4 °C with rocking.
- Gently wash the membrane 3 x 5 min in TBST and 1 x 5 min in TBS at room temperature with rocking.
- Incubate the membrane in 1:2000 – 1:5000 secondary antibody (HRP-linked or preferred visible conjugate, see table of the essential supplies, reagents, and equipment, supplementary material) in 5% milk powder in TBST for at least 1 h at room temperature with rocking.
- Repeat the washing procedure outlined in step 3.4.5 and visualize the blot with an appropriate visualization (i.e. chemiluminescent) substrate on an imaging system.
- Repeat the washing procedure outlined in step 3.4.5 and probe the membrane for β-actin or a preferred loading control according to manufacturer's instructions.
- On preferred image analysis software, analyze the expression of Rubicon by manual quantification of absorbance (band intensities), subtraction of background absorbance, and normalization to the corresponding loading control band intensities. Perform statistical analyses as appropriate to test for statistically significant changes in protein levels.
A Primary Human Trophoblast Model to Study the Effect of Inflammation Associated with Maternal Obesity on Regulation of Autophagy in the Placenta
Learning Objectives
Term human placentas from lean (pre-pregnancy body mass index (BMI) <25) mothers with uncomplicated pregnancies carrying female offspring were collected and sampled within 15 minutes of delivery by cesarean section (no labor). The placentas were examined for the absence of calcifications and typical development: weighing between 300-600 g with the umbilical cord and membranes removed, round in shape, between 15 – 25 cm in diameter, and umbilical cord inserted into the middle of the placenta. Villous tissue was dissected away from the basal and chorionic plates in 2-3 samples from across the placenta (Figure 1), yielding approximately 100 g of villous tissue as starting material for primary trophoblast isolation. Within 20 minutes of sampling villous tissue, the procedure to isolate primary trophoblasts was started as described here, yielding between 0.8 – 1 x 108 viable cells. The cells were seeded in 6-well culture plates at a density of 3 x 106 (3.3 x 105 cells per cm2). After 24 h of culture, the cells were examined under a microscope for attachment and proper trophoblast morphology was confirmed (individual round cells). The culture media was replaced with complete media containing a series of concentrations of TNFα between 125-104 pg/mL so that at least two wells were included per concentration and vehicle control (complete media only).
Twenty-four hours following TNFα-treatment (48 h of culture), all TNFα medias were replaced with complete media. No appreciable cell death was observed due to treatment with TNFα at concentrations at or below 103 pg/mL. Treatment with 104 pg/mL TNFα was moderately cytotoxic and the cytotoxic effects of this TNFα concentration did not persist after the media was changed as evidenced by Lactate Dehydrogenase (LDH) assays (data not shown). At 72 h of culture, cells were examined for syncytialization under a microscope. Immunocytochemistry for syncytialization and fibroblast contamination revealed relatively pure isolation of trophoblasts (Figure 3). Cellular lysates were harvested according to the protocol described here, yielding between 3-8 µg/µL of total protein per preparation as determined by a BCA assay (data not shown). Western blot analysis in cell lysates from female trophoblasts treated with TNFα showed an upregulation of Rubicon expression in response to concentrations of TNFα up to 250 pg/mL and subsequent downregulation of Rubicon expression at TNFα concentrations greater than 250 pg/mL (Figure 4A and B, 104 pg/mL excluded from analysis based on cytotoxic effects). Likewise, Rubicon is significantly upregulated in flash-frozen villous tissue biopsies from placentas from obese pregnancies with female fetuses compared to lean controls as evidenced by Western blot analysis (Figure 4C and D, n = 6 placentas per BMI class, ANOVA, P<0.05).
| Concentration (%) | 90% DGM (ml) | 1x HBSS (ml) | Layer Thickness (ml) |
| 4x gradient | 4x gradient | Total 34.5 ml | |
| 70 | 14 | 4 | 4.5 |
| 60 | 8 | 4 | 3 |
| 55 | 7.33 | 4.67 | 3 |
| 50 | 3.34 | 2.67 | 3 |
| 45 | 6 | 6 | 3 (13.5 ml mark) |
| 40 | 5.33 | 6.67 | 3 |
| 35 | 4.67 | 7.33 | 3 (19.5 ml mark) |
| 30 | 8 | 16 | 6 |
| 20 | 2.67 | 9.33 | 3 |
| 10 | 1.33 | 10.67 | 3 |
Table 1. Specifications for Making Density Gradients for Density Centrifugation of Primary Trophoblasts.
From left to right, column one specifies density gradient media (DGM, see table of the essential supplies, reagents, and equipment, supplementary material) concentration expressed as percentages of DGM in HBSS. Column two specifies the volume of DGM while column three specifies the volume of HBSS required for making the appropriate percentage of DGM solution. Column four specifies the volume to be added to the 50 mL conical tube to build the gradient, beginning with the most dense layer.
| Trypsin | HBSS | DNase | ||
| Digestion | (total activity; BAEE units) | volume (ml) | (total activity; Kunits) | Total Volume /digestion |
| 1 | 619037 (23.01 ml) | 141.76 ml | 62594 (0.230 ml) | 165 ml |
| 2 | 412691 (15.34 ml) | 94.51 ml | 41729 (0.154 ml) | 110 ml |
| 3 | 313270 (11.65 ml) | 71.74 ml | 31676 (0.116 ml) | 83.5 ml |
| Total | 1345000 (50 ml) | 308 ml | 136000 (0.5 ml) | 358.5 ml |
Table 2. Specifications for the Preparation of Digestion Solution for Primary Trophoblast Isolation Based on the Specific Activity of DNase and Trypsin.
From left to right, the first column specifies the number of the digestions, the second column specifies the trypsin activity required per digestion, the third column specifies the total volume of supplemented HBSS to be added for the appropriate digestion, the fourth column specifies the DNase activity required per digestion, and the final column specifies the volume of digestion solution to be added to the placental tissue for the appropriate digestion.
| HBSS (supplemented with Ca+2 and Mg+2) | Sample Buffer |
| 10% 10x HBSS | 90% 4X Laemmli dye |
| 1.26 mM CaCl2 (anhyd.) | 10% 2-Mercaptoethanol |
| 0.80 mM MgSO4 (anhyd.) | |
| 20.77 mM HEPES | |
| pH to 7.4 with 10N NaOH Make volume up to 1 L with sterile ddH2O Sterile filter into a sterile bottle |
|
| Complete Media | Running Buffer |
| Remove 11% v/v IMDM | 25 mM Tris Base |
| Add 10% v/v FBS | 190 mM Glycine |
| Add 1% 10,000 U/mL Penicillin/Streptomycin (100 U/mL final) | 0.1% SDS |
| pH to 8.3 | |
| Freezing Media | |
| 90% v/v FBS | Transfer Buffer |
| 10% v/v DMSO | 25 mM Tris |
| 190 mM Glycine | |
| Digestion Buffer | 20% Methanol |
| 50 mL Trypsin (26,900 BAEE units/mL) | pH to 8.3 |
| 0.5 mL DNAse (272,000 K units/mL) | |
| Bring to 358.5 ml in supplemented HBSS | TBS |
| 20 mM Tris | |
| RIPA Buffer | 150 mM NaCl |
| 25 mM Tris-HCl | pH to 7.6 |
| 5 mM EDTA | |
| 150 mM NaCl | TBST |
| 0.1% SDS | TBS with 0.1% Tween 20 |
| 0.5% Sodium deoxycholate | |
| 1% Triton X-100 | |
| 1 tablet of protease/phosphatase inhibitor per 10 ml RIPA Buffer |
Table 3. Solutions Required for Isolation and Culture of Primary Trophoblasts followed by Western Blotting.
| % DGM | ml mark | Cell type |
| 10 | 31.5-34.5 | Debris |
| 20 | 28.5-31.5 | |
| 30 | 22.5-28.5 | |
| 35 | 19.5-22.5 | Trophoblasts |
| 40 | 16.5-19.5 | |
| 45 | 13.5-16.5 | |
| 50 | 10.5-13.5 | Lymphocytes |
| 55 | 7.5-10.5 | |
| 60 | 4.5-7.5 | Red blood cells |
| 70 | Below 4.5 |
Table 4. Sedimentation of Trophoblasts by Density Centrifugation.
From left to right, the first column specifies the percentage of DGM (Table 1), the second column specifies the mL mark where the corresponding percentage of DGM is found on a 50 mL conical tube, and the third column specifies what cell type sediments at the corresponding percentage of DGM and mL mark on a 50 mL conical tube.Trophoblasts sediment between 50- 35% DGM, forming distinct opaque bands. Collecting DGM above or below this range will result in contamination of cellular debris and other cell types such as lymphocytes.
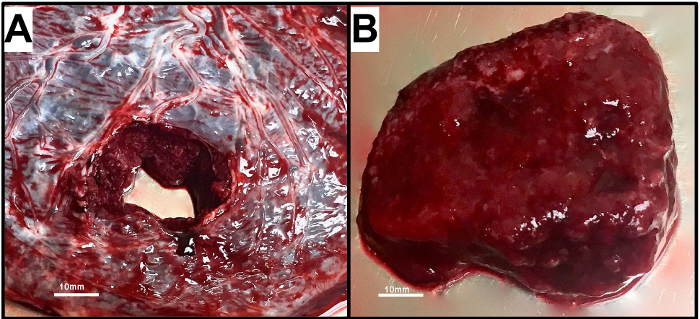
Figure 1. Villous Tissue is Isolated from the Term Human Placenta by Removing the Chorionic and Basal Plates.
A) With the chorionic plate (fetal side) facing upwards, a full thickness sample is excised from the placenta. B) A sample of villous tissue is obtained by removing the chorionic and basal plates. Please click here to view a larger version of this figure.
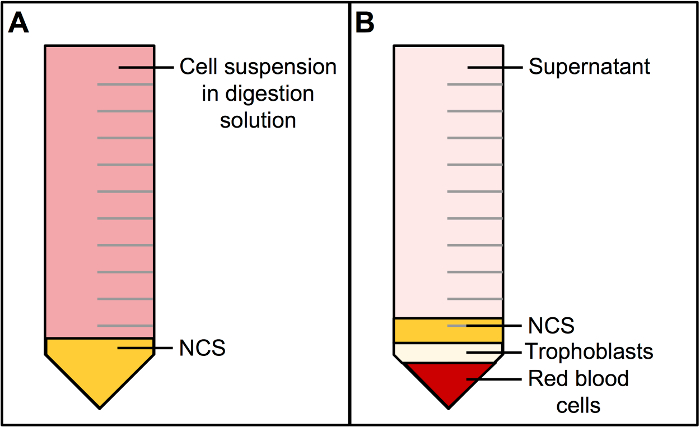
Figure 2. Centrifugation of Cells in Digestion Solution over Newborn Calf Serum results in a Multilayered Cell Pellet.
A) Newborn calf serum (NCS) is layered underneath the cell suspension in digest solution in a 50 mL conical tube. B) Centrifugation of (A) results in a multilayered cell pellet. The bottom-most layer is deep in red color and consists of red blood cells. The layer above includes trophoblasts and is white or beige in color. Above the trophoblast layer is NCS followed by digestion solution (supernatant) to the top of the tube. Please click here to view a larger version of this figure.
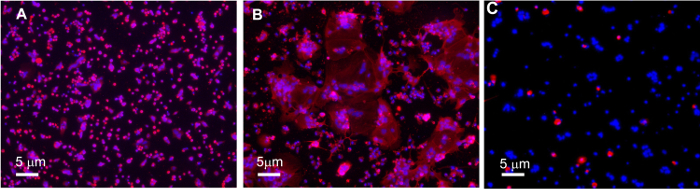
Figure 3. Immunocytological Analysis of Syncytialization and Fibroblast Content in Primary Human Trophoblast Cultures.
A) Representative image of Cytokeratin-7 (red) in cytotrophoblasts after 24 h of culture. B) Representative image of Cytokeratin-7 (red) in syncytiotrophoblasts after 72 h of culture shows multinucleated masses of cells that have fused. C) Representative image of Vimentin (red) in syncytiotrophoblasts after 72 h of culture. Images were acquired on a fluorescent microscope with DAPI (blue) nuclear counterstain. Visualized at 10X magnification. Please click here to view a larger version of this figure.
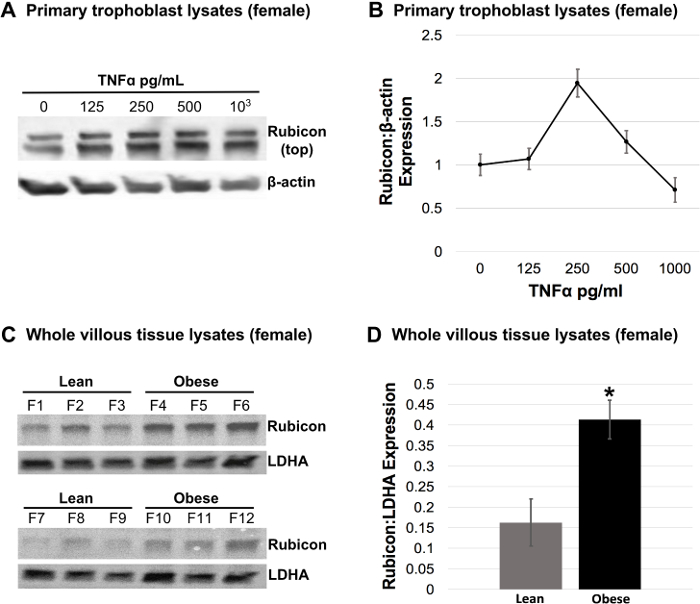
Figure 4. Regulation of Rubicon Expression in Response to TNFα-Treatment in Female Trophoblasts and Endogenous Rubicon Expression in Villous Tissue from Lean Versus Obese Pregnancies with Female Fetuses.
Primary trophoblasts from term placentas from lean mothers with healthy pregnancies carrying a female fetus were isolated and treated with 125, 250, 500, 103, and 104 pg/mL TNFα (or vehicle control). A) Representative Western blot for Rubicon in female trophoblast lysates treated with TNFα. β-actin was used as a loading control. B) Rubicon expression in response to TNFα-treatment in female trophoblasts was quantified from Western blots and normalized to β-actin. Values are mean Rubicon expression per TNFα concentration ± S.E. in n=3 placentas. C) Western blots for Rubicon in whole tissue lysates from flash frozen biopsies of villous tissue from lean versus obese pregnancies with female fetuses (F1 -F12). Lactate dehydrogenase A (LDHA) was used as a loading control. D) Rubicon expression in Western blots from (C) was quantified and normalized to LDHA. Values are mean Rubicon expression per BMI classification ± S.E. in n=6 placentas per BMI class (ANOVA, *P<0.05). Please click here to view a larger version of this figure.
List of Materials
| 10X HBSS | Gibco | 14185-052 | |
| CaCl2 (anhyd.) | Sigma-Aldrich | C1016-100G | |
| MgSO4 (anhyd.) | Sigma-Aldrich | M7506-500G | |
| Hepes | Fisher Scientific | BP310-500 | |
| Trypsin | Gibco | 15090-046 | |
| DNAse | Worthington Biochemical Corp. | LS002139 | |
| Protease/Phosphatase inhibitors | Thermofisher Scientific | 88668 | |
| Tris HCl | Invitrogen | 15506-017 | |
| EDTA | Invitrogen | 15576-028 | |
| NaCl | Sigma-Aldrich | S7653-1KG | |
| SDS | Fisher Scientific | BP166-600 | |
| Sodium deoxycholate. | Fisher Scientific | AAJ6228822 | |
| Triton X-100 | Sigma-Aldrich | X100-500ML | |
| Iscove’s Modified Dulbecco’s Medium (IMDM) | Gibco | 12440-046 | |
| Fetal Bovine Serum (FBS) | Corning | 35-010-CV | |
| Neonatal Calf Serum (NCS) | Gibco | 26010-074 | |
| Penicillin/Streptomycin (Pen/Strep) | Gibco | 15140-122 | |
| 10% Formalin | Fisher Scientific | 23-427-098 | |
| DMSO | Sigma-Aldrich | D2650-100ML | |
| TNFα | Sigma-Aldrich | SRP3177-50UG | |
| Phosphate Buffered Saline (PBS) | Gibco | 70013-032 | |
| K2EDTA vacutainer blood collection tubes | BD | 366450 | |
| Percoll (Density Gradient Media, DGM) | GE Healthcare | 17-0891-01 | |
| 6 well plates | Corning | 353046 | |
| Cell strainers | Fisher Scientific | 22363549 | |
| Eppendorf Safe-Lock Tubes 2.0 mL, natural | Fisher Scientific | 22363352 | |
| Trypan Blue | Corning | 25-900-Cl | |
| Bio-Rad Mini-PROTEAN Tetra System | Bio-Rad | 1658001FC | |
| Bio-Rad Mini Trans-Blot Cell | Bio-Rad | 1658033 | |
| TGX FastCast Acrylamide Kit, 12% | Bio-Rad | 1610175 | |
| Mini-Protean 3 Multi-Casting Chamber | Bio-Rad | 1654112 | |
| 4X Laemmli Sample Buffer | Bio-Rad | 1610747 | |
| 2-Mercaptoethanol | Sigma-Aldrich | M3148-100ML | |
| Glycine | Bio-Rad | 1610718 | |
| Tween-20 | Sigma-Aldrich | P7949-500ML | |
| Instant Nonfat Dry Milk | Carnation | ||
| Rubicon (D9F7) Rabbit mAb | Cell Signalling Technology | 8465S | |
| Monoclonal Anti-β-Actin antibody produced in mouse | Sigma-Aldrich | A2228-100UL | |
| Anti-rabbit IgG, HRP-linked Antibody | Cell Signalling Technology | 7074S | |
| Anti-mouse IgG, HRP-linked Antibody | Cell Signalling Technology | 7076S | |
| SuperSignal West Pico PLUS Chemiluminescent Substrate | Thermo Scientific | 34578 |
Lab Prep
Maternal obesity is associated with an increased risk of adverse perinatal outcomes that are likely mediated by compromised placental function that can be attributed to, in part, the dysregulation of autophagy. Aberrant changes in the expression of autophagy regulators in the placentas from obese pregnancies may be regulated by inflammatory processes associated with both obesity and pregnancy. Described here is a protocol for sampling of villous tissue and isolation of villous cytotrophoblasts from the term human placenta for primary cell culture. This is followed by a method for simulating the inflammatory milieu in the obese intrauterine environment by treating primary trophoblasts from lean pregnancies with tumor necrosis factor alpha (TNFα), a proinflammatory cytokine that is elevated in obesity and in pregnancy. Through the implementation of the protocol described here, it is found that exposure to exogenous TNFα regulates the expression of Rubicon, a negative regulator of autophagy, in trophoblasts from lean pregnancies with female fetuses. While a variety of biological factors in the obese intrauterine environment maintain the potential to modulate critical pathways in trophoblasts, this ex vivo system is especially useful for determining if expression patterns observed in vivo in human placentas with maternal obesity are a direct result of TNFα signaling. Ultimately, this approach affords the opportunity to parse out the regulatory and molecular implications of inflammation associated with maternal obesity on autophagy and other critical cellular pathways in trophoblasts that have the potential to impact placental function.
Maternal obesity is associated with an increased risk of adverse perinatal outcomes that are likely mediated by compromised placental function that can be attributed to, in part, the dysregulation of autophagy. Aberrant changes in the expression of autophagy regulators in the placentas from obese pregnancies may be regulated by inflammatory processes associated with both obesity and pregnancy. Described here is a protocol for sampling of villous tissue and isolation of villous cytotrophoblasts from the term human placenta for primary cell culture. This is followed by a method for simulating the inflammatory milieu in the obese intrauterine environment by treating primary trophoblasts from lean pregnancies with tumor necrosis factor alpha (TNFα), a proinflammatory cytokine that is elevated in obesity and in pregnancy. Through the implementation of the protocol described here, it is found that exposure to exogenous TNFα regulates the expression of Rubicon, a negative regulator of autophagy, in trophoblasts from lean pregnancies with female fetuses. While a variety of biological factors in the obese intrauterine environment maintain the potential to modulate critical pathways in trophoblasts, this ex vivo system is especially useful for determining if expression patterns observed in vivo in human placentas with maternal obesity are a direct result of TNFα signaling. Ultimately, this approach affords the opportunity to parse out the regulatory and molecular implications of inflammation associated with maternal obesity on autophagy and other critical cellular pathways in trophoblasts that have the potential to impact placental function.
Procedura
Maternal obesity is associated with an increased risk of adverse perinatal outcomes that are likely mediated by compromised placental function that can be attributed to, in part, the dysregulation of autophagy. Aberrant changes in the expression of autophagy regulators in the placentas from obese pregnancies may be regulated by inflammatory processes associated with both obesity and pregnancy. Described here is a protocol for sampling of villous tissue and isolation of villous cytotrophoblasts from the term human placenta for primary cell culture. This is followed by a method for simulating the inflammatory milieu in the obese intrauterine environment by treating primary trophoblasts from lean pregnancies with tumor necrosis factor alpha (TNFα), a proinflammatory cytokine that is elevated in obesity and in pregnancy. Through the implementation of the protocol described here, it is found that exposure to exogenous TNFα regulates the expression of Rubicon, a negative regulator of autophagy, in trophoblasts from lean pregnancies with female fetuses. While a variety of biological factors in the obese intrauterine environment maintain the potential to modulate critical pathways in trophoblasts, this ex vivo system is especially useful for determining if expression patterns observed in vivo in human placentas with maternal obesity are a direct result of TNFα signaling. Ultimately, this approach affords the opportunity to parse out the regulatory and molecular implications of inflammation associated with maternal obesity on autophagy and other critical cellular pathways in trophoblasts that have the potential to impact placental function.
