Locomotor Assessment of 6-Hydroxydopamine-induced Adult Zebrafish-based Parkinson’s Disease Model
PREPARAZIONE ISTRUTTORI
CONCETTI
Student Protocol
The present study has been approved by the Committee on Animal Research and Ethics (CARE), Universiti Technologi MARA (UiTM) [Reference No: UiTM CARE 346/2021, dated 7 May 2021].
NOTE: The published protocols22,25,26 for standard husbandry and maintenance of the 6-OHDA-lesioned adult zebrafish PD model were utilized. Experiments were conducted with adult male zebrafish (Danio rerio) aged more than five months old with a standardized length of 3.2-3.7 cm.
1. Zebrafish maintenance and pre-ICV microinjection preparations
- Maintain the fish in an aerated water tank under a controlled temperature of 28 ± 1.0 °C. For zebrafish husbandry and maintenance, use distilled water mineralized with commercial sea salt (1 g/L) throughout the experiment27.
- House a maximum of 25 fish per 45 L tank or one fish per 1.8 L water and expose them to a schedule of 14 h light and 10 h dark photoperiod. Feed the fish at least twice per day with food pellets supplemented with freeze-dried worms.
- Prepare a concentrated stock solution of tricaine methanesulfonate (MS-222) by dissolving 2.5 g of MS-222 and 5 g of sodium bicarbonate in 250 mL of distilled water. Dilute 2 mL of stock solution to produce 200 mL of working anesthesia solution.
- Prepare 99.96 mM of 6-OHDA by first dissolving 0.2 mg of ascorbic acid in 1 mL of 0.9% w/v sterile-filtered NaCl. Filter the solution with 0.2-micron filter before adding 25 mg of 6-OHDA in powder form into the solution. Prepare the solution fresh before each injection and store it in dark at 4 °C.
CAUTION: Wear appropriate personal protective equipment (i.e., gloves, laboratory coat, and face mask) and practice good laboratory practices when handling the chemicals. All handlings of the chemicals should be done within a biosafety cabinet.
2. Anaesthetisation and ICV injection of zebrafish
- Fast the fish for 24 h to avoid regurgitation during anesthesia. Anaesthetise the fish by immersing it into a container containing 0.01% w/v of MS-222 solution for approximately 1 min or until all visible muscular movement ceases.
- Position the anesthetized fish on a water-soaked sponge placed under a stereomicroscope and wet the fish regularly.
- Identify the position for injection based on the intersection between the metopic suture (MS), coronal suture (CS), and sagittal suture (SS) that connects the frontal and parietal skull of the zebrafish brain.
- Make a small hole of 1.0 mm2 area using a sharp 27 G needle in the skull guided by the specific anatomical position on the zebrafish skull (Figure 1A,B).
- Lower the microcapillary injector at a 60° angle until it reaches a depth of 1,200 µm from the cranial roof of the zebrafish skull (Figure 1C). Press the Z limit to fix the position.
- Set the initial injection pressure to 4000 hPa and compensation pressure to 10 hPa. Set the duration of injection to 0.3 s. Lower the intensity of injection with each subsequent injection.
- Inject 0.5 µL of 99.96 mM neurotoxin 6-OHDA (or 0.9% w/v saline for sham control group) and let the microcapillary rest for 20 s. Continue to wet the fish with distilled water throughout the injection process to prevent drying out.
- Slowly remove the microcapillary and resuscitate the fish under running distilled water. Place the fish in an isolated recovery tank and remove any distractions that can potentially disturb the recovery process.
- Flush the microcapillary before the next injection to clear the blockage and ensure that the intensity of injection is sufficient to yield the desired volume of 0.5 µL of 6-OHDA.
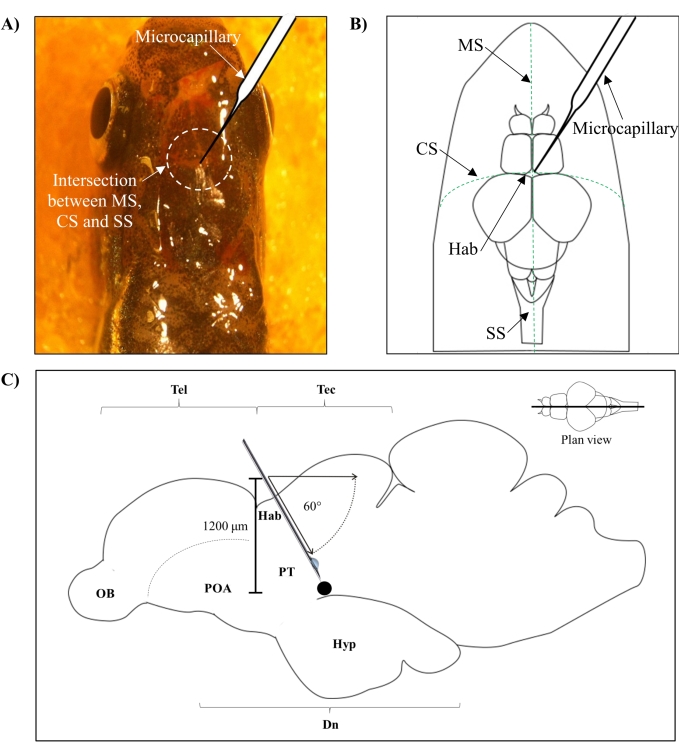
Figure 1: Injection site of neurotoxin, 6-OHDA. (A) The point of microcapillary entry is guided by the intersection between the metopic suture (MS), coronal suture (CS), and sagittal suture (SS) that connects the frontal and parietal skull of the zebrafish brain (plan view). (B) A schematic drawing (plan view) of the zebrafish skull and brain shows the microcapillary, which is lowered directly above the habenula (Hab), and its point of entry at the intersection between hemispheres. (C) A schematic drawing (sagittal section) of the zebrafish brain shows the angle of injection and depth of penetration. The black dot represents the lesioned site that is situated above the targeted area, the ventral diencephalon. Abbreviations: 6-OHDA: 6-hydroxydopamine, CS: coronal suture, Dn: diencephalon, Hab: habenula, Hyp: hypothalamus, MS: metopic suture, OB: olfactory bulb, POA: preoptic area, PT: posterior tuberculum, SS: sagittal suture, Tec: tectum, and Tel: telencephalon. Please click here to view a larger version of this figure.
3. Locomotor assessment
NOTE: Locomotor assessment of zebrafish (n = six / group; sham vs lesioned) was assessed individually via the open tank test using established protocols28,29 at day three and day 30 post-6-OHDA lesion.
- Video recording
- Place the experimental tank (length 20 cm, width 11.5 cm, height 13 cm) with its walls covered with white paper on a raised platform (Figure 2A).
- Illuminate the tank from the bottom using a light source. Fill the tank with distilled water (80%-90% full) and maintain the temperature at 28 ± 1.0 °C. Measure the temperature using a thermometer and regulate it using a commercial aquarium heater.
- After a minimum of 2 min of acclimatization, record the fish swimming behavior from a plan view on the 2-dimensional (2D) plane of the experimental arena using a video camera for 5 min (Figure 2B). To avoid inconsistency in the swimming behavior of the earlier and the last batch of recordings, do not exceed the acclimatization by 10 min30.
- Analyze the videos using video tracking software with the open-tank protocol for the acquisition of distance traveled (cm) and mean speed (cm/s) of each subject.
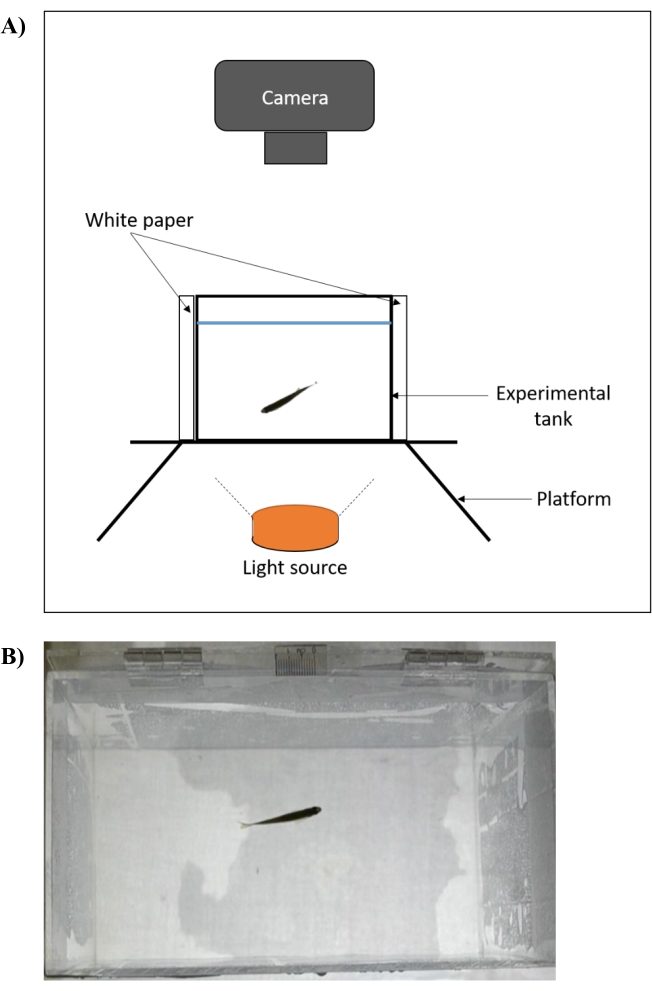
Figure 2: Experimental setup of an open tank test for assessment of zebrafish locomotor behavior. (A) The experimental tank (front view) is placed on a raised platform that is illuminated from below. The four walls of the tank are covered with white paper and the recordings are captured axially. The temperature is measured using a thermometer and regulated at 28 ± 1.0 °C using a commercial aquarium heater. (B) Screenshot (plan view) of video recording that is captured using the setup. Please click here to view a larger version of this figure.
- Data analysis
- Double click on the icon to open the video tracking software. Click on the File tab and select Create New Empty Experiment. This will allow the user to customize the experiment parameters according to the aims of the investigation.
- Click on the Protocol tab, select Video Sources, and click on Add New Video Source. Click on the available drop-down list and select the Video File option. This will prompt the file browsing pop-up from which the video recordings of interest can be selected.
- Click on the Apparatus subtab and select the Rectangular icon to set up the apparatus. Drag the rectangular icon to cover the whole experimental arena. Set the scale bar accordingly and input the numerical value of the scale measurement used in the length of the ruler section. The present experiment used a 10 mm scale for the open tank test.
- Set the animal color by selecting The Animals are Darker than The Apparatus Background. Leave the other available options in Tracking to the preset default settings.
- In the Zones subtab, click on the previously drawn apparatus. This zone is set as the standard zone of which the position is the same for all tests.
- Select the following options under test scheduling and test data report: Test Duration, Total Distance Traveled, and Average Speed. Other available tests on the list are optional and dependent on the researcher's investigative interest.
- In the Experiment tab, assign the animals according to their test group by typing in the group name under the Name section and the number of animals per group in the Number of Animals section.
- Switch to Tests tab to run the experiment. Click on the Start Test icon and wait until all the videos are analyzed.
- In the Risultati tab, click on the View the Report icon to view the locomotor data in text report form.
Locomotor Assessment of 6-Hydroxydopamine-induced Adult Zebrafish-based Parkinson’s Disease Model
Learning Objectives
The present experiment assessed the changes in adult zebrafish swimming behavior following ICV microinjection with 6-OHDA. The reason for using 6-OHDA as the neurotoxin of choice was due to its inability to cross the blood-brain barrier, which produced specific and targeted ablation of DpN in the area of interest-ventral diencephalon (Dn)16. The DpN subpopulation here holds anatomical resemblance to the DpN subpopulation in the human's substantia nigra pars compacta31.
As per our previous work22, the cellular effect of 6-OHDA ICV microinjection against DpN of adult zebrafish was confirmed through immunohistostaining of DpN marker-tyrosine hydroxylase (TH). The main brain region of interest was the Dn, made up of the preoptic area (POA), posterior tuberculum (PT), and hypothalamus (Hyp). It was found that 99.96 mM 6-OHDA resulted in a 100% survival rate of the adult zebrafish with the lowest number of TH-immunoreactive (TH-ir) in Dn. It was also found that more than 85% (p < 0.01) of TH-ir DpN in the Dn was ablated on day three postlesion. The number of TH-ir DpN then increased by more than 50% at day 14 postlesion before achieving full regeneration 30 days postlesion (Figure 3). This data supports the regenerative capabilities of DpN subpopulation in Dn of adult zebrafish following ablation32.
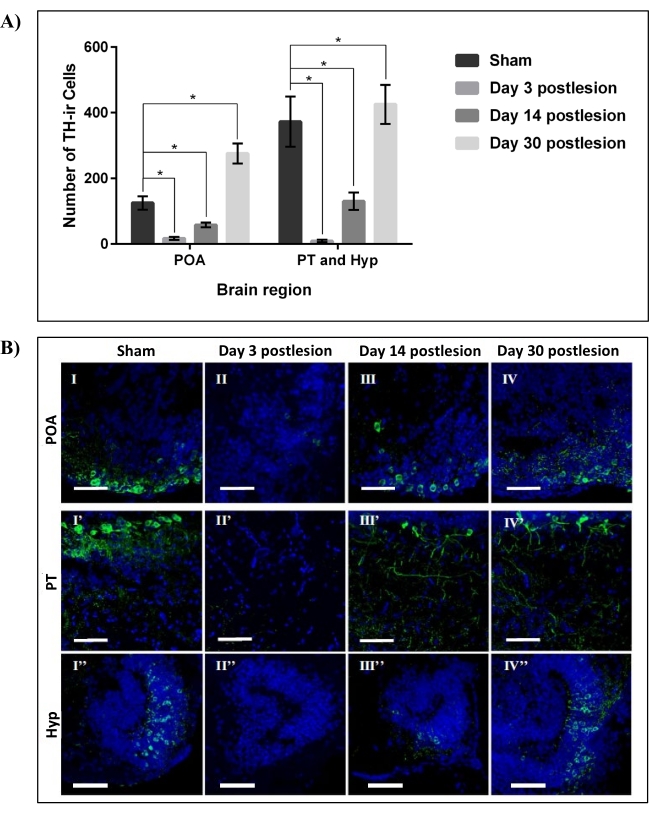
Figure 3: Regeneration of DpN in the Dn region of zebrafish lesioned by 99.96 mM 6-OHDA. (A) The number of TH-ir DpN in three main areas of the Dn region, POA, PT, and Hyp, over four data points: sham, 3, 14, and 30 days post-lesioning by 99.96 mM 6-OHDA neurotoxin. Each bar represents mean ± SD of n = 6 independent experiments; *p < 0.05. (B) Representative confocal microscope images of sagittally sectioned zebrafish brain of sham (I, I', and I''), 3 days post-lesioning (II, II', and II''), 14 days post-lesioning (III, III', and III''), and 30 days post-lesioning (IV, IV', and IV'') stained with TH (DpN; green) and DAPI (nuclei; blue). Scale bar = 50 µm. Abbreviations- DAPI: 4′, 6-diamidino-2-phenylindole, 6-OHDA: 6-hydroxydopamine, Dn: diencephalon, DpN: dopaminergic neurons, Hyp: hypothalamus, POA: preoptic area, PT: posterior tuberculum, SD: standard deviation, and TH-ir: tyrosine hydroxylase immunoreactive. Adapted from Vijayanathan et al.22. Please click here to view a larger version of this figure.
We then performed locomotor assessment using the open tank test to investigate changes in distance traveled (cm) and mean speed (cm/s) of adult zebrafish following ICV microinjection of 6-OHDA and sham. Experimental fish were then assessed on day three postlesion (least number of TH-ir DpN observed) and day 30 postlesion (fully restored DpN reported at lesion site). Analysis of zebrafish swimming behavior using a video tracking software indicated that both the mean speed (cm/s) and distance traveled (cm) of the lesioned group on day three postlesion were significantly reduced (p < 0.001) to <45% when compared to sham (Figure 4). The lesioned group exhibited recovery of motor function 30 days postlesion with no significant difference of both the mean speed (cm/s) and distance traveled (cm) when compared to sham.
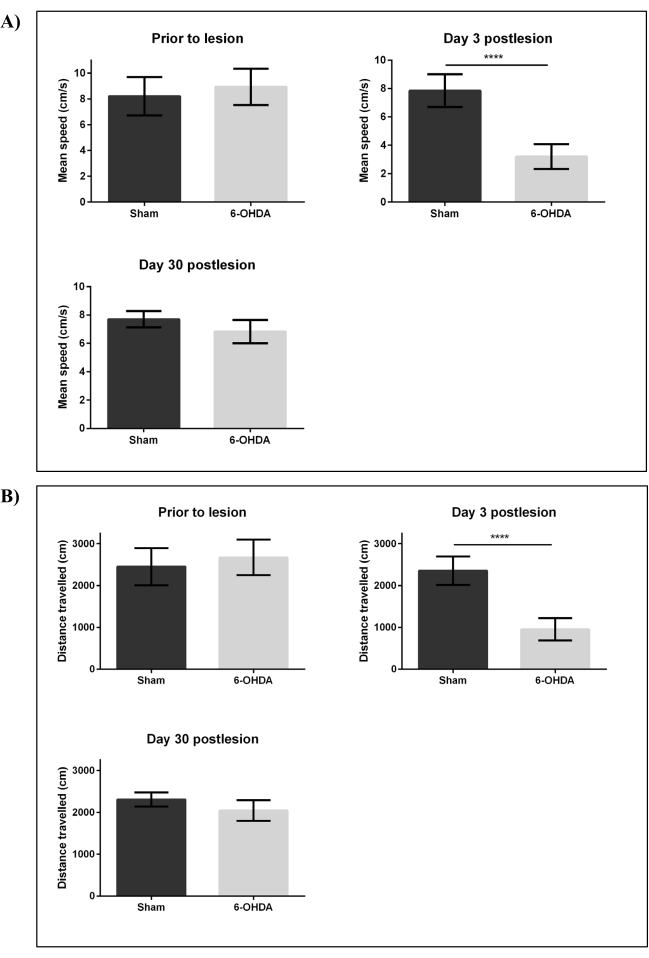
Figure 4: Changes in swimming behavior following intracerebroventricular injection by 6-OHDA. Swimming behavior of adult zebrafish was assessed before lesioning, on day three and day 30 postlesion by 99.96 mM 6-OHDA. Parameters that were assessed included: (A) mean speed (cm/s) and (B) distance traveled (cm). Each bar represents mean ± SD of six fish; ****p < 0.0001 (Student t-test). Abbreviations: 6-OHDA: 6-hydroxydopamine, SD: standard deviation. Please click here to view a larger version of this figure.
List of Materials
| Materials | |||
| 6-Hydroxydopamine (6-OHDA) | Sigma-Aldrich, Missouri, USA | 162957 | |
| Ascorbic acid | Thermo Fisher Scientific, California, USA | FKC#A/8882/53 | |
| Disposable pasteur pipette, 3 mL | Thermo Fisher Scientific, California, USA | FB55348 | |
| Microcentrifuge tube, 0.2 mL | Eppendorf, Hamburg, Germany | 30124332 | |
| Nice conical flask, 100 mL | Evergreen Engineering & Resources, Semenyih, Malaysia | SumYau0200 | |
| Phosphate buffered saline (PBS) | Sigma-Aldrich, Missouri, USA | P4417 | |
| Sodium bicarbonate | Sigma-Aldrich, Missouri, USA | S5761 | |
| Sodium chloride | Merck, Darmstadt, Germany | 106404 | |
| Stereomicroscope | Nikon, Tokyo, Japan | SMZ745 | |
| Tricaine methanesulfonate (MS-222) | Sigma-Aldrich, Missouri, USA | E10521 | |
| Equipment | |||
| ANY-maze software | Stoelting Co., Illinois, USA | – | version 7.0; video tracking software |
| Cubis II Micro Lab Balance | Sartorius, Göttingen, Germany | SE 2 | |
| FemtoJet IV microinjector | Eppendorf, Hamburg, Germany | 5192000035 | |
| Femtotip II, sterile injection capillary | Eppendorf, Hamburg, Germany | 5242957000 | |
| InjectMan 4 micromanipulator | Eppendorf, Hamburg, Germany | 5192000027 | |
| LED Portable Lamp | MR. DIY, Selangor, Malaysia | 9023251 | 20 mAh |
| PELCO Pro Superalloy, offset, fine tips | Ted Pella, California, USA | 5367-12NM | |
| Shanda aquarium heater | Yek Fong Aquarium, Selangor, Malaysia | SDH-228 | |
| Thermometer | Sera Precision, Heinsberg, Germany | 52525 | |
| Video camera | Nikon, Tokyo, Japan | D3100 |
Lab Prep
The limitations of current treatments in delaying dopaminergic neuronal loss in Parkinson's disease (PD) raise the need for alternative therapies that can restore these neurons. Much effort is currently directed toward a better understanding of neuroregeneration using preclinical in vivo models. This regenerative capability for self-repair is, however, inefficient in mammals. Non-mammalian animals like zebrafish have thus emerged as an excellent neuroregenerative model due to its capability to continuously self-renew and have a close brain homology to humans. As part of the effort in elucidating cellular events involved in neuroregeneration in vivo, we have established the 6-hydroxydopamine (6-OHDA)-induced adult zebrafish-based PD model. This was achieved through the optimized intracerebroventricular (ICV) microinjection of 99.96 mM 6-OHDA to specifically ablate dopaminergic neurons (DpN) in the ventral diencephalon (Dn) of zebrafish brain. Immunofluorescence indicated more than 85% of DpN ablation at day three postlesion and full restoration of DpN at lesioned site 30 days postlesion. The present study determined the impairment and subsequent recovery of zebrafish swimming behavior following lesion by using the open field test through which two parameters, distance traveled (cm) and mean speed (cm/s), were quantified. The locomotion was assessed by analyzing the recordings of individual fish of each group (n = 6) using video tracking software. The findings showed a significant (p < 0.0001) reduction in speed (cm/s) and distance traveled (cm) of lesioned zebrafish 3 days postlesion when compared to sham. The lesioned zebrafish exhibited full recovery of swimming behavior 30 days postlesion. The present findings suggest that 6-OHDA lesioned adult zebrafish is an excellent model with reproducible quality to facilitate the study of neuroregeneration in PD. Future studies on the mechanisms underlying neuroregeneration as well as intrinsic and extrinsic factors that modulate the process may provide important insight into new cell replacement treatment strategies against PD.
The limitations of current treatments in delaying dopaminergic neuronal loss in Parkinson's disease (PD) raise the need for alternative therapies that can restore these neurons. Much effort is currently directed toward a better understanding of neuroregeneration using preclinical in vivo models. This regenerative capability for self-repair is, however, inefficient in mammals. Non-mammalian animals like zebrafish have thus emerged as an excellent neuroregenerative model due to its capability to continuously self-renew and have a close brain homology to humans. As part of the effort in elucidating cellular events involved in neuroregeneration in vivo, we have established the 6-hydroxydopamine (6-OHDA)-induced adult zebrafish-based PD model. This was achieved through the optimized intracerebroventricular (ICV) microinjection of 99.96 mM 6-OHDA to specifically ablate dopaminergic neurons (DpN) in the ventral diencephalon (Dn) of zebrafish brain. Immunofluorescence indicated more than 85% of DpN ablation at day three postlesion and full restoration of DpN at lesioned site 30 days postlesion. The present study determined the impairment and subsequent recovery of zebrafish swimming behavior following lesion by using the open field test through which two parameters, distance traveled (cm) and mean speed (cm/s), were quantified. The locomotion was assessed by analyzing the recordings of individual fish of each group (n = 6) using video tracking software. The findings showed a significant (p < 0.0001) reduction in speed (cm/s) and distance traveled (cm) of lesioned zebrafish 3 days postlesion when compared to sham. The lesioned zebrafish exhibited full recovery of swimming behavior 30 days postlesion. The present findings suggest that 6-OHDA lesioned adult zebrafish is an excellent model with reproducible quality to facilitate the study of neuroregeneration in PD. Future studies on the mechanisms underlying neuroregeneration as well as intrinsic and extrinsic factors that modulate the process may provide important insight into new cell replacement treatment strategies against PD.
Procedura
The limitations of current treatments in delaying dopaminergic neuronal loss in Parkinson's disease (PD) raise the need for alternative therapies that can restore these neurons. Much effort is currently directed toward a better understanding of neuroregeneration using preclinical in vivo models. This regenerative capability for self-repair is, however, inefficient in mammals. Non-mammalian animals like zebrafish have thus emerged as an excellent neuroregenerative model due to its capability to continuously self-renew and have a close brain homology to humans. As part of the effort in elucidating cellular events involved in neuroregeneration in vivo, we have established the 6-hydroxydopamine (6-OHDA)-induced adult zebrafish-based PD model. This was achieved through the optimized intracerebroventricular (ICV) microinjection of 99.96 mM 6-OHDA to specifically ablate dopaminergic neurons (DpN) in the ventral diencephalon (Dn) of zebrafish brain. Immunofluorescence indicated more than 85% of DpN ablation at day three postlesion and full restoration of DpN at lesioned site 30 days postlesion. The present study determined the impairment and subsequent recovery of zebrafish swimming behavior following lesion by using the open field test through which two parameters, distance traveled (cm) and mean speed (cm/s), were quantified. The locomotion was assessed by analyzing the recordings of individual fish of each group (n = 6) using video tracking software. The findings showed a significant (p < 0.0001) reduction in speed (cm/s) and distance traveled (cm) of lesioned zebrafish 3 days postlesion when compared to sham. The lesioned zebrafish exhibited full recovery of swimming behavior 30 days postlesion. The present findings suggest that 6-OHDA lesioned adult zebrafish is an excellent model with reproducible quality to facilitate the study of neuroregeneration in PD. Future studies on the mechanisms underlying neuroregeneration as well as intrinsic and extrinsic factors that modulate the process may provide important insight into new cell replacement treatment strategies against PD.
