A New Optokinetic Testing Method to Measure Rat Vision
Summary
The Optokinetic Nystagmus (OKN) behavioral testing method is used for the assessment of visual acuity in rodents. Here a simple method is demonstrated that can be easily set up in research laboratories for a reliable assessment of visual function in both normal and experimental rats.
Abstract
Optokinetic nystagmus (OKN) is a reflexive eye movement initiated by the motion of visual stimuli in the field of vision. The head-tracking movement associated with OKN is commonly used as a measure of visual function in rodents. To record OKN responses in normal and experimental rats, a simple and inexpensive apparatus has been developed. This setup uses two tablet screens to display the OKN visual stimulus consisting of high contrast black and white stripes generated using the OKN Stripes Visualization Web Application, a freely available software. The rat is placed inside a clear Plexiglass holder that limits movement so that the rat's head continuously faces the OKN display screen. The position of the rat holder can be changed to adjust the distance between the rat and the display screen. A micro-camera positioned above the rat holder is used to record the rat's visual activities. These recordings can be used for quantitative assessments. Based on the presence or absence of clear head-tracking, the OKN responses at different spatial frequencies can be determined. The collected data demonstrates a novel technique for reliable measurement of visual acuity in normal and retinal degenerate rats.
Introduction
When the eye is exposed to sustained full-field visual motion, a distinct pattern of fast and smooth tracking eye movements and low-acceleration head movements emerge in the direction of visual motion, called optokinetic nystagmus (OKN)1,2. The neurological pathway of OKN passes from the retina to the lateral geniculate body, occipital lobe, and cerebellar flocculus and connects to the ocular motor neurons3. Neural damage anywhere along these neural pathways may lead to changes in the OKN responses. The OKN response is used as a tool to assess cerebral symmetry, psychogenic blindness, and visual acuity in human patients4,5. Visual acuity is assessed by quantifying functional responses, which can be integral in determining the success of treatments and experiments centered around restoring vision lost due to neurodegenerative diseases3,6,7. In animals, OKN responses can be used to accurately assess visual acuity, which provides researchers with the ability to collect both quantitative and qualitative data regarding visual function. In rodents, it is possible to measure the visual acuity of the left and right eyes independently based on the direction of rotation of the stripes both in the clockwise and counter-clockwise directions8. This counter-clockwise and clockwise motion exposes each eye to either naso-temporal (N-T) or tempo-nasal (T-N) motion9, respectively. The T-N stimulus results in significantly higher response compared to the N-T stimulus since rodents are more sensitive to the dangers coming from behind or from the side.
Previously, visual function in normal laboratory rats and retinal degenerate rats was tested using different OKN testing methods6,10,11,12,13. However, certain variabilities in the visual acuity scores are observed between different studies, including the data which is shown in the present investigation. This variability can be mostly attributed to the differences in the testing setup used. Differences in the size of the testing arena and the type of the OKN visual stimuli used6,10 can be the major factors. The stimuli used in these experiments include sinewave gratings for the appearance of a virtual cylinder14, interchangeable rotating cylinders15, and high contrast (black and white) stripes displayed on four computer monitors10. Major limitations associated with these OKN testing apparatus and methods include the large size of the equipment, movement of the animals in the testing arena, and frequent incidence of the animal falling from the testing platform7,11,12.
To minimize the above limitations, a new apparatus for OKN testing in rats has been developed. This apparatus is comparatively inexpensive, proven to be efficient, easy to operate, and enables assessment of visual function (Figure 1). The apparatus uses two tablet screens to display the OKN visual stimuli (visualization software) at different spatial frequencies. A micro-camera is used to record the animal's activities during testing for later analysis of the data. With the objective of making an easy-to-set up OKN apparatus in research laboratories, this new setup outlines critical modifications to the existing OKN testing apparatus. The OKN stimuli used here consists of black and white stripes at different spatial frequencies and different directions of rotation (left to right or right to left). The major component of the OKN testing apparatus includes two touchscreen tablet screens (7.9 inches) used to display the OKN stimuli (Figure 2). Two adjustable holders are used to hold the tablet screens in the desired position. The holders are securely attached to the edge of a procedure table that allows adjustment of its height and angle. The rats are placed in a rat holder that faces the display screens. The rat holders are made of transparent plastic (polymethyl methacrylate) tube. The holder is attached to a pedestal and a metal stand to ensure stable placement on the procedure table. The size of the holding tubes varies from 4 to 6 inches in length and 2.5 to 3 inches in diameter, depending on the size of the rats used. The distance between the rat and the display screen is adjusted by changing the position of the rat holder. The rat holder helps to maintain continuous exposure of the rat's head toward the display screens and reduces its movements during testing. A micro-camera is used to record the head tracking responses. The shortcomings of this new setup include different refresh rates for the screen and the possibility of optical illusions when using narrow stripes. However, these can be considered as common issues associated with a computer-based OKN setup. In addition to the above issues, in the current setup, rats are not tested using a virtual cylinder14 that influences the optimum OKN response. The novelty of this method lies within the technique and apparatus in which the method is employed. This technique can be easily set up in research laboratories for reliable visual acuity measurement in rodents.
Protocol
All animal procedures were performed in compliance with the experimental guidelines approved by the regional authorities and accepted by the Institutional Animal Care and Use Committee (IACUC) at the University of Southern California (USC), and conformed to the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research and the European directive 2010/63/EU on the protection of animals used for scientific purposes.
NOTE: The rats used in this study are pigmented retinal degenerate Royal College of Surgeons (RCS) rats and Long Evans (LE) rats. Figure 3 shows a schematic illustration showing different stages of OKN testing and analysis.
1. Procedures
- Preparation of the setup
- Keep the rat in the cage and for 30 min inside the testing room with the room lights turned off.
NOTE: This helps to minimize the stress that occurs during transportation and provides a uniform lighting condition for all experiments. - Set the position of the tablet screens facing each other at 155° to display the OKN Stripes Visualization Web Application.
- Place the rat holder in the middle of two tablet screens at 4.5 inches from the center.
NOTE: At this position, the rat's head will be about 3.5 inches away from the display screen. - Click on OKN Stripes Visualization Web Application from the desktop of the tablet to display visual stimuli (black and white stripes) starting with the lowest spatial frequency.
NOTE: Spatial frequencies 0.08, 0.15, 0.2, 0.24, 0.28, 0.33, 0.38 cycles per degrees (c/d) are used, starting at the lowest spatial frequency (0.08c/d) and changing to higher spatial frequencies (up to 0.38 c/d). At each spatial frequency, rats are tested for both clockwise and counter-clockwise directions in rotation of stripes (1 min each).
- Keep the rat in the cage and for 30 min inside the testing room with the room lights turned off.
- OKN testing procedure
- Select a rat holder of a suitable size.
- Bring the rat toward the opening of the rat holder and carefully guide the rat to the inside.
- Allow the rat to settle inside the holder (1-2 min) prior to testing.
NOTE: By proper handling, it is possible to maintain the rat inside the holder in most of the cases; when necessary, the rat holder is held high (up to 2 feet above the table) for a few seconds until the rat settles. The fear of the height is found to reduce the tendency for the rat to climb down from the rat holder. Additional time (1-2 min) is given for the rat to settle, if needed. - Insert the pedestal of the rat holder into the metal stand for its stable placement.
NOTE: This setup allows changing the rat's viewing distance from the tablet screen. - Turn on the camera to start video recording.
- Click on OKN Stripes Visualization Web Application from the desktop of the tablet screen and start running the program.
- Run the OKN stimuli in the left to right or right to left direction.
NOTE: Rotation of the stripes in the clockwise direction activates the left eye, whereas rotation in the counter-clockwise direction activates the right eye. The initial direction of rotation was chosen randomly to avoid potential habituation. - Observe the rat's head-tracking behavior.
- Start testing using the lowest spatial frequency (0.08 c/d), and then increase the spatial frequency in a step-wise (in the ascending) order.
- Notice the presence or absence of OKN responses.
- Continue testing, no resting period is given between different spatial frequencies.
- Stop video recording after completing all the spatial frequencies, and save the data using the rat identification number.
- Take the rat out of the holder and place it in the cage for approximately 30 min resting period between consecutive tests.
- Repeat the tests three times per rat.
2. Data analysis
- Review the recorded video and determine each rat's visual acuity by finding the spatial frequencies at which the rat responded.
- Record all responses (presence or absence of a clear head-tracking) in a spreadsheet.
- Find the highest spatial frequency to which a rat responded from the three tests. This is considered the final visual acuity.
NOTE: Since visual acuity measurement is subjective, data will be evaluated by two independent study personnel to confirm the visual acuity score for each rat12. The results are cross-checked until the final score is agreed on. A positive OKN response is defined as the presence of a clear and sustained head-tracking activity. Random head-tracking (unrelated to the OKN visual stimuli) or the absence of any head movement is considered a negative OKN response. - Conduct statistical analysis to compare groups or between the two eyes (left eye vs right eye).
NOTE: Review the video recordings to clarify that positive or negative responses is conducted as needed.
Representative Results
The OKN testing was performed using retinal degenerate (RD) Royal College of Surgeons (RCS) rats and age-matched normal Long Evans (LE) rats. The LE rats (n = 4), were used for establishing the baseline data to determine visual acuity scores in normal rats using the new setup. Statistical analysis was carried out using Microsoft excel (mean ± standard deviation). LE rats exhibited robust head-tracking at spatial frequencies from 0.15 c/d to 0.33 c/d. To evaluate the reliability and effectiveness of the new OKN apparatus in measuring visual acuity in RD rats, tests were performed using RCS rats (n = 9) at five different time points between the ages of postnatal (P) 35 and P100 (Figure 4). The RCS rats exhibited strong and sustained head-tracking responses up to the age of P50. After P50, there was considerable loss of visual acuity. After the age of P80, visual sensitivity in RCS rats decreased steeply. After P100, visual function in RCS rats was severely compromised, as is evident from the absence of head-tracking responses even at low spatial frequencies. Only weak and random head tracking responses were observed in RCS when tested at later time points. This pattern of visual acuity loss observed in the RCS rats progressed in a similar manner in both eyes. OKN testing using this new apparatus also suggested that visual acuity in rats can be influenced by the distance between the visual stimuli and the rat's eye. In our pilot experiments using LE rats, robust OKN responses were observed when the rat's head was positioned about 3.5 inches away from the display screen.
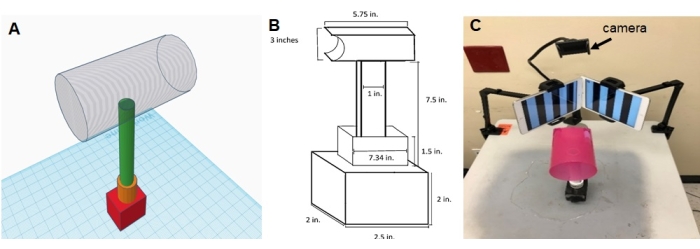
Figure 1: Diagrammatic sketch and representative image of the new OKN set-up. (A) diagrammatic sketch of the rat holder attached to a pedestal and a metal stand placed over a flat surface, (B) dimensions of rat holder and stand that are used for a 6-month-old rat, (C) an image of the new OKN setup. The tablet screens facing each other are placed at a 155° angle. Please click here to view a larger version of this figure.
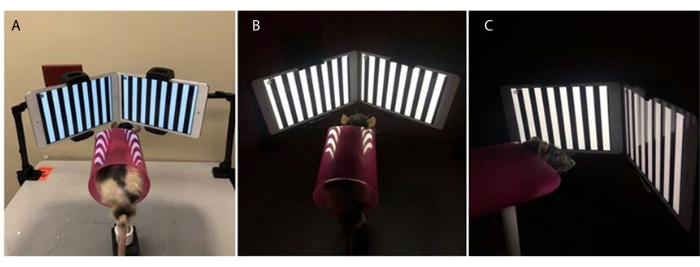
Figure 2: New OKN testing apparatus. Rat is held inside the transparent plastic tube for testing. (A–C) image taken from different angles showing the rat viewing the OKN stimuli. Please click here to view a larger version of this figure.
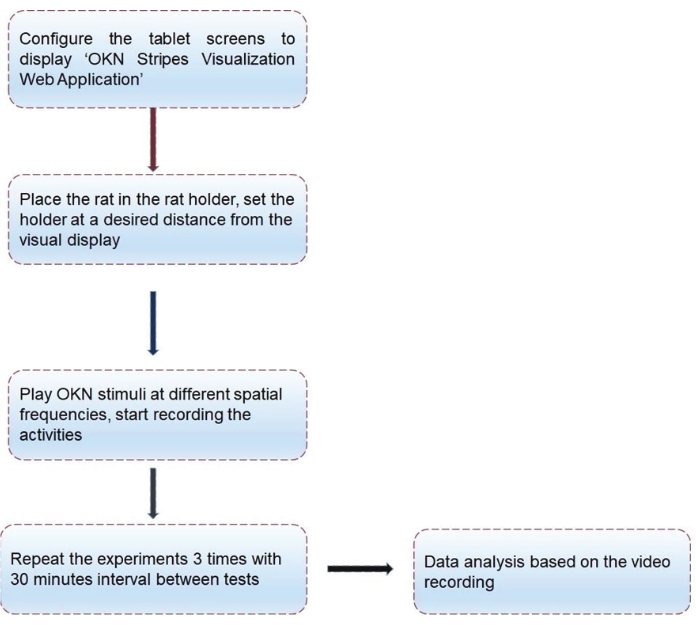
Figure 3: Schematic illustration showing different stages of OKN testing and analysis. Step-by-step instructions describing different stages of OKN testing procedure and data analysis using the new OKN apparatus. Please click here to view a larger version of this figure.
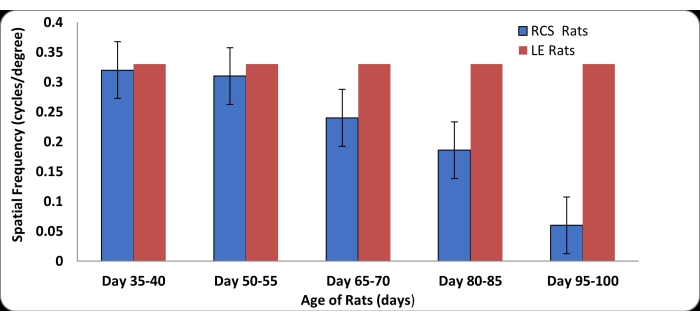
Figure 4: Visual acuity (±SD) measurements in normal LE rats and retinal degenerate (RD) Royal College of Surgeons (RCS) rats at different stages of the retinal degeneration disease. Normal LE rats showed the ability to track the stripes at all spatial frequencies (0.15 c/d to 0.33 c/d). RCS rats ages between P35 and P95 (n = 9) were subjected to OKN testing at five different time points. At P35, majority of the rats tracked at all the above spatial frequencies. A drastic decline in visual acuity was noticed in RCS rats between the age of 2 and 3 months. After the age of P100, none of the rats were able to show strong head tracking behavior. Please click here to view a larger version of this figure.
Discussion
OKN is a reflexive saw tooth motion of the eyes in response to a drifting stimulus, which is used as a tool to assess visual acuity in human subjects3. In animals, including primates and rodents, OKN testing is used as a quantitative measurement of visual function. The present study describes a novel, inexpensive OKN apparatus that can be easily set up in research laboratories for OKN behavioral testing in rats. OKN assessments in rodents were performed by different approaches. Previously, an interchangeable OKN drum attached to a motor for the rotation of the gratings was used6. More recently, OKN testing using computer screens to display the visual stimuli10, including those programmed to create a virtual cylinder, was used14. Commercially available OKN testing apparatuses have been used by various investigators to assess visual functional changes in normal and retinal degenerate animal models16,17,18. The animal's visual acuity is measured after placing them in a testing chamber equipped with four computer screens displaying the OKN stimuli7,15,19. Previously, the rat or mouse is placed atop a platform in the middle of the testing chamber. A micro-camera attached above records the movements of the rat. The data collected is evaluated and analyzed statistically for measures of head-tracking11,14,16 for various applications. A trained individual capable of identifying the head-tracking movements performs the data analysis in a masked fashion.
The current study demonstrated the reliability of using a new OKN testing apparatus, which is easy to construct in the laboratories for assessing visual function in rats. The apparatus is demonstrated to be suitable for assessing visual acuity in normal and retinal degenerate rats. Recent publications from this laboratory12,20 also demonstrated that data from this new OKN method is both reliable and comparable to data obtained from previously established OKN systems. In this new OKN testing setup, apart from the changes in the equipment design, testing procedures were mostly based on the description provided in the previous studies6,11,12,21. The critical steps needed are placement of the rats inside a semi-restrainer (to minimize the rat's movements during testing) and the initiation of the OKN Visualization Software in a random way (stripe rotation can be either left to right or right to left). In addition to changing the stripe width, the software also allows changing the contrast between the black and white stripes and speed of rotation of the stripes. In this study, only the OKN visual acuity scores (based on changing stripe width) were measured. For this, rats were tested using the highest contrast (black vs white stripes). Animals were tested at the maximum contrast level (305.50 cd/m2 vs 15.75 cd/m2 measured using a 371 R Optical Power Meter, Graseby Optronics, Orlando, FL). All experiments were performed in a dark room to minimize influence of external illumination in the OKN stimuli and to provide uniform testing environment for all experiments. During the tests, the black and white stripes at the following spatial frequencies: 0.08, 0.15, 0.2, 0.24, 0.28, 0.33, 0.38 were used. The direction of rotation of the stripes (right vs left or left vs right) was initiated in a random fashion to avoid potential habituation of the head-tracking behavior. Rats were allowed to rest between consecutive trials (about 30 min) to ensure that assessments are not influenced by the previous test. The video analysis require sufficient training for the investigator to distinguish between head-tracking responses vs random head movements.
In a majority of previous OKN apparatuses, the rats were placed on a platform that allowed their free movement (including the head). This may lead to constant variations in the viewing distance (distance between the eye and the visual stimuli). Since the rat's vision is sensitive to variation in the viewing distance22, its free movement inside the testing chamber may cause variations in the visual acuity scores. High variability in the visual acuity score within the same group of animals or in the same animal between different tests can cause difficulties in obtaining meaningful statistical inference. Based on this, in the present study, during testing, the rats were maintained in a semi-restrainer to minimize movements toward or away from the OKN stimuli. By limiting the free movement of the rat, variation in the viewing distance is minimized. Since the rat's head is directed toward the corner where the edges of the two tablets meet, both eyes are equally exposed to the stripes (viewing distance remains mostly constant). Overall, this new setup may provide the opportunity for researchers to obtain consistent visual functional data. Additional investigations based on changing the viewing distance and comparing the data with other setups are needed to make a strong conclusion of the above observations.
To establish the reliability of the new setup, both normal LE rats and retinal degenerate RCS rats were tested. Visual acuity measured using this new setup was 0.33 c/d in LE rats. In RCS rats, the visual acuity score changed based on the progression of the retinal degeneration disease. In RCS rats, photoreceptor degeneration occurs due to the dysfunction of retinal pigment epithelium (RPE) cells, leading to the accumulation of photoreceptor debris in the subretinal space that trigger the degenerative disease22,23. Several previous studies24,25 have established that the disease progression in RCS rats is slow in the beginning. This becomes more drastic by 2 months of age during the time when severe photoreceptor damage is reported. To validate the new setup, visual acuity tests were conducted in RCS at different postnatal time points (Figure 3). Based on this study, progressive loss of visual acuity in RCS rats occurs by the age of 2 months. By the age of 3 months, the OKN acuity in RCS rats was severely compromised, which is concomitant with the photoreceptor loss26,27.
The present study demonstrated a new, efficient, and economically advantageous design and apparatus for assessing OKN-based visual activities in normal and retinal degenerate rats. Since the distance between the rat eye and visual stimuli is an integral variable that can affect the accurate assessment of visual acuity in rats, this new equipment can minimize variation in the study results. In conclusion, this new OKN apparatus has proven to be a reliable screening technique for researchers to compare visual function in rats in a variety of research applications focused on therapeutic testing.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by the CIRM (California Institute for Regenerative Medicine) grants (DISC1-09912 PI- Thomas, DR3-07438- PI- Humayun), Unrestricted Grant to the Department of Ophthalmology from Research to Prevent Blindness, New York, NY, and support from Bright Focus Foundation (M2016186, Thomas, PI). Research reported in this publication was supported by the National Eye Institute of the National Institutes of Health under Award Number P30EY029220.
Materials
| iPad Mini | Apple | A1489 | Two iPad Mini's are used to display the OKN Stripes Visualization Software |
| Micro-camera/ Micro-Camera Attatchment | Lanon | B097H6WWDS | The micro-camera is used to record the experiment. The micro-camera attachment connects to the desk and holds the camera facing the rat. The head tracking responses are recorded and assessed at varying distances, spatial frequencies, and directions. |
| Plexiglass Tube/Rat Holder | Best acrylics | B07KMF31MC | The Plexiglass Tube is used to restrain the rat, with their head exposed, for the duration of the experiment. The tube is attached to another vertical plexiglass tube attachment to stabilize the rat holder during the experiment. The entire apparatus was designed and constructed in the lab. |
| Plexiglass Tube Attachment | Best acrylics | B07KMF31MC | This attachment holds the rat holder infront of the iPad screens, and allows the distance between the rat and iPad's to be manipulated. |
| Screen Holders | Kabcon | B08JLRPKQ1 | Two screen holders are used to hold the iPad's up, in order to display the OKN Stripes Visualization Software to the rat. |
| OKN Stripes Visualization Web Application | The MIT License (MIT) Copyright (c) 2016 Anton Yakushin | https://antonyakushin.github.io/okn-stripes-visualization/ | This application is a freely available softeware to display visual stimuli (black and white stripes) at different frequencies |
References
- Mustari, M. J., Ono, S. Optokinetic eye movements. Encyclopedia of Neuroscience. , 285-293 (2009).
- Gottlob, I. Ups and downs of optokinetic nystagmus. British Journal of Ophthalmology. 84, 445-447 (2000).
- Wester, S. T., Rizzo, J. F., Balkwill, M. D., Wall, C. Optokinetic nystagmus as a measure of visual function in severely visually impaired patients. Investigative Ophthalmology & Visual Science. 48 (10), 4542-4548 (2007).
- Daroff, R., Aminoff, M. . Encyclopedia of the Neurological Sciences. , (2014).
- Squire, L., et al. . Fundamental Neuroscience. , (2008).
- Thomas, B. B., Seiler, M. J., Sadda, S. R., Coffey, P. J., Aramant, R. B. Optokinetic test to evaluate visual acuity of each eye independently. Journal of Neuroscience Methods. 138 (1-2), 7-13 (2004).
- Cahill, H., Nathans, J. The optokinetic reflex as a tool for quantitative analyses of nervous system function in mice: Application to genetic and drug-induced variation. PLOS One. 3 (4), 2055 (2008).
- Segura, F., et al. Development of optokinetic tracking software for objective evaluation of visual function in rodents. Scientific Reports. 8, 10009 (2018).
- Lev-Ari, T., Katz, H., Lustig, A., Katzir, G. . Visual acuity and optokinetic directionality in the common chameleon (Chamaeleo chamaeleon). , (2017).
- Thomas, B. B., Shi, D., Khine, K., Kim, L. A., Sadda, S. R. Modulatory influence of stimulus parameters on optokinetic head-tracking response. Neuroscience Letters. 479 (2), 92-96 (2010).
- Shi, D. S., et al. Characterization of optokinetic response in normal and retinal degenerate rats and mice using a computer-based testing apparatus. Investigative Ophthalmology & Visual Science. 49 (13), 4422 (2008).
- Thomas, B. B., et al. Co-grafts of human embryonic stem cell derived retina organoids and retinal pigment epithelium for retinal reconstruction in immunodeficient retinal degenerate royal college of surgeons rats. Frontiers in Neuroscience. 15, 752958 (2021).
- Thomas, B. B., et al. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefe’s Archive for Clinical and Experimental Ophthalmology. 256 (11), 2113-2125 (2018).
- Prusky, G. T., Alam, N. M., Beekman, S., Douglas, R. M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investigative Ophthalmology & Visual Science. 45 (12), 4611-4616 (2004).
- Cameron, D., et al. The optokinetic response as a quantitative measure of visual acuity in zebrafish. Journal of Visualized Experiments. (80), e50832 (2013).
- Tabata, H., Shimizu, N., Wada, Y., Miura, K., Kawano, K. Initiation of the optokinetic response (OKR) in mice. Journal of Vision. 10 (1), 13 (2010).
- Huang, Y. -. Y., Neuhauss, S. C. F. The optokinetic response in zebrafish and its applications. Frontiers in Bioscience: A Journal and Virtual Library. 13, 1899-1916 (2008).
- Sirkin, D. W., Hess, B. J., Precht, W. Optokinetic nystagmus in albino rats depends on stimulus pattern. Experimental Brain Research. 61 (1), 218-221 (1985).
- Dietrich, M., et al. Using optical coherence tomography and optokinetic response as structural and functional visual system readouts in mice and rats. Journal of Visualized Experiments. (143), e58571 (2019).
- Rajendran Nair, D. S., et al. Long-term transplant effects of iPSC-RPE monolayer in immunodeficient RCS rats. Cells. 10 (11), 2951 (2021).
- Kretschmer, F., Sajgo, S., Kretschmer, V., Badea, T. C. A system to measure the Optokinetic and optomotor response in mice. Journal of Neuroscience Methods. 256, 91-105 (2015).
- Lin, T. -. C., et al. Assessment of safety and functional efficacy of stem cell-based therapeutic approaches using retinal degenerative animal models. Stem Cells International. 2017, 9428176 (2017).
- Ryals, R. C., et al. Long-term characterization of retinal degeneration in Royal College of Surgeons Rats using spectral-domain optical coherence tomography. Investigative Ophthalmology & Visual Science. 58 (3), 1378-1386 (2017).
- Di Pierdomenico, J., et al. Early events in retinal degeneration caused by rhodopsin mutation or pigment epithelium malfunction: Differences and similarities. Frontiers in Neuroanatomy. 11, 14 (2017).
- McGill, T. J., Douglas, R. M., Lund, R. D., Prusky, G. T. Quantification of spatial vision in the Royal College of Surgeons Rat. Investigative Ophthalmology & Visual Science. 45 (3), 932-936 (2004).
- Hetherington, L., Benn, M., Coffey, P., Lund, R. Sensory capacity of the Royal College of Surgeons rat. Investigative Ophthalmology & Visual Science. 41, 3979-3983 (2000).
- Sauvé, Y., Pinilla, I., Lund, R. D. Partial preservation of rod and cone ERG function following subretinal injection of ARPE-19 cells in RCS rats. Vision Research. 46 (8), 1459-1472 (2006).

.
