A Computerized Test Battery to Study Pharmacodynamic Effects on the Central Nervous System of Cholinergic Drugs in Early Phase Drug Development
Summary
A validated computerized battery of neuropsychological and neurophysiological tests is used to study pharmacodynamic effects on the central nervous system of newly developed drugs in early phase development. To demonstrate the test battery, the acute effects of mecamylamine and the reversal of these effects by two agonist drugs are described.
Abstract
Investigating potential pharmacodynamic effects in an early phase of central nervous system (CNS) drug research can provide valuable information for further development of new compounds. A computerized and thoroughly validated battery of neuropsychological and neurophysiological tests has been shown to be sensitive to detect drug-induced effects of multiple new and existing compounds. The test battery covers the main CNS domains, which have been shown to respond to drug effects and can be repeatedly administered following drug administration to characterize the concentration-effect profile of a drug.
The standard tests in the battery are saccadic eye movement, smooth pursuit eye movement, the Bowdle visual analog scale (VAS), the Bond and Lader VAS, body sway, adaptive tracking, visual verbal learning, and quantitative electroencephalography (qEEG). However, the test battery is adaptive in nature, meaning that it can be composed and adjusted with tests fit to investigate specific drug classes, or even specific receptors.
Showing effects of new cholinergic drugs designed to have a pro-cognitive outcome has been difficult. The pharmacological challenge model is a tool for early proof-of-pharmacology. Here, a marketed drug is used to induce temporary and reversible disease-like symptoms in healthy subjects, via a pharmacological mechanism related to the disease that is targeted as indication for the new compound. The test battery was implemented to investigate the potential of the nicotinic receptor antagonist mecamylamine to be used as a challenge model for cholinergic dysfunction, as seen in neurodegenerative disorders.
A worsening of scores in a dose dependent manner on the visual verbal learning test (VVLT; a test for learning and memory abilities) and the adaptive tracking test (a measure of visuomotor control and arousal), in particular, showed that the test battery is sensitive to showing acute pharmacodynamic effect after administration of anti-cholinergic drugs.
Introduction
With human life expectancy steadily increasing over the last century the prevalence and incidence of diseases of the aging brain, such as dementia and other neurodegenerative processes, also grow. In parallel, the development of new drugs to treat these diseases is therefore expanding. However, many new drugs intended to be active in the CNS fail to reach the market due to lack of central effects or unwanted side effects in later phases of drug development1. In traditional phase 1 studies the objectives are to gain information on the pharmacokinetics, that is, the effect that the human body has on the drug (for example by metabolizing), as well as safety and tolerability of the new drug. Early proof of pharmacodynamic effect (the effect that the drug has on the body), however, may be even more important in decisions on moving forward in the clinical development of a new compound and may help avoid erroneous decision making with consequences at later phases of the development process2.
In the past two decades, the Centre for Human Drug Research (CHDR) has developed a computerized test battery of neuropsychological and neurophysiological measurements sensitive to CNS effects of drugs. This test battery is used repeatedly over the day to measure pharmacodynamic effects of a new compound. It thereby provides evidence of the drug's ability to have the desired effect, to penetrate the blood-brain barrier and enter the brain, or the lack thereof3. Also, outcomes of the test battery could provide information on the mechanism of action of a compound as the individual tests correspond to specific drug-responsive CNS domains. For example, if effects of the new drug are seen on the maze learning test, which is a test for visuospatial working memory, this could indicate that the drug acts on receptors in parts of the brain involved in visuospatial working memory. In addition, the test battery is used to screen for CNS side effects for compounds that are not designed to work in the CNS, and where CNS activation needs to be ruled out.
The test battery is made up of a large number of cognitive and neurophysiological tests, which have been shown to be sensitive to detect pharmacodynamic effects of CNS active drugs3,4,5,6. The core test battery comprises six neuropsychological domains: executive functioning, attention, memory, visuomotor functioning or coordination, motor skills, and subjective drug effects. The core tests are: saccadic eye movement7, smooth pursuit eye movement8, the Bowdle VAS9, the Bond and Lader VAS10, body sway, adaptive tracking11, visual verbal learning12, and qEEG, which cover the main cognitive and neurophysiological domains mentioned earlier. These tests have been shown to be able to measure changes in CNS functions as a result of administration of several types and classes of drugs (see below). The battery can be repeatedly administered (up to 12 times following drug administration) due to the 30-min total administration time, which is essential to characterize the concentration-effect profile of a drug. The test battery can be expanded and adjusted with different tests fit to investigate specific drug classes, or even specific receptors. The test battery has been validated in a wide range of drugs acting on different CNS systems (e.g., benzodiazepines, antipsychotics, ethanol, and cannabis12,13,14,15,16,17,18,19,20,21) to be able to reliably demonstrate drug related CNS effects.
While other computerized test batteries exist (described for example in Egerhazi et al.22 and Underwood et al.23), and are widely used in clinical trials, the test battery described in this paper stands out as it not only includes neuropsychological tests such as the VVLT and the VASs, but also neurophysiological measurements (e.g., EEG, eye movement tests), thereby combining different aspects of brain functioning in one test battery, and better reflecting the multimodal nature of cognitive behavior. Furthermore, as the test battery is computerized, the test results are generated electronically. This results in outcome values that are the same when used in different studies by different research staff, allowing for standardization of results, as well as values that are less error prone compared to scoring by hand. The outcome files can be easily uploaded into electronic database systems and can be used to generate interim reports of the pharmacodynamic effects of new drugs within a day.
There is at least one class of drugs where early proof of pharmacological effect in the brain has been difficult; the (pro)cholinergic drugs. Acetylcholine is one of the main neurotransmitters of the CNS and has been shown to play a key role in cognition, specifically in processes such as learning and memory24,25. Consequently, cholinergic dysfunction is indicated to underlie neurodegenerative processes such as Alzheimer's disease26. New compounds designed to enhance cognitive functioning, such as muscarinic and nicotinic receptor specific agonists, are now entering clinical studies.
As early phase studies are usually performed in healthy, often young subjects who cognitively perform at a normal level, it is difficult to study or even show proof of pharmacodynamic effect of a new drug intended to treat cognitive decline in patients with a disease of the brain.
Our group has therefore developed a tool that can be used for demonstrating early proof of pharmacology of a new drug: the pharmacological challenge model. An already approved and marketed drug is used to induce temporary and reversible disease-like symptoms in healthy subjects, via a pharmacological mechanism related to the disease that is targeted as indication for the new compound. In most cases this effect is an unwanted side effect of the drug, resulting from activation of receptors at a different location in the human body compared to the site where the drug is intended to work. For example, the muscarinic acetylcholine receptor antagonist scopolamine is used for the treatment of nausea and vomiting due to motion sickness. Side-effects resulting from antagonizing muscarinic acetylcholine receptors in the brain are the anti-cognitive effects such as reduced attention and memory resembling the deficits seen in Alzheimer's disease27.
Since scopolamine is used as a muscarinic acetylcholine challenge model to induce Alzheimer-like, yet temporary, cognitive effect in healthy subjects27, CHDR has developed and validated a pharmacological challenge model with mecamylamine. Mecamylamine is a non-competitive nicotinic acetylcholine receptor antagonist28 which results in cholinergic dysfunction, i.e., transient cognitive deficits, in healthy young males29,30.
The above mentioned computerized test battery has been used to investigate the potential of different dose levels of mecamylamine to show effects on the neurophysiological and cognitive tests. The expectation was that with increasing dose, the effects on the different tests would also increase. Subsequently these effects were related to the plasma concentrations of the drug, resulting in the plasma concentration-effects (pharmacokinetic-pharmacodynamic) relationship of mecamylamine29.
The tests incorporated in the design of this study were chosen based on the expected effects known from the literature and the pharmacological mechanism of action of mecamylamine on the nicotinic receptors:
Adaptive Tracking Test:
This is a pursuit-tracking task, for the measurement of visuomotor coordination and sustained attention. A circle of known dimensions moves randomly about a screen. The subject must try to keep a dot inside the moving circle by operating a joystick. If this effort is successful, the speed of the moving circle increases. Conversely, the velocity decreases if the test subject cannot maintain the dot inside the circle. In contrast to non-adaptive tracking methods, this leads to a constant and individually adapted challenge throughout the procedure. The adaptive tracking test used was developed by Hobbs & Strutt, according to specifications of Borland and Nicholson11.
Smooth Pursuit and Saccadic Eye Movement Tests:
The use of a computer for measurement of saccadic eye movements and smooth pursuit was originally described by Baloh et al.7, and for smooth pursuit by Bittencourt et al.8, and has been extensively validated at CHDR by Van Steveninck et al.19,20,21 The subject is required to follow a light source with the eyes, which moves horizontally on a screen at 58 cm distance. The light source moves continuously for measurement of smooth pursuit and jumps from side to side for measurement of saccadic eye movements.
VASs:
Assessment of subjective feelings of alertness, mood, and calmness was performed using a set of 16 visual analog lines as described by Norris (1971) and Bond and Lader10. Visual analog scores rely on the ability of subjects to semi-quantify a subjective state. Visual analog lines consist of 10-cm line segments. The subject is presented with 16 lines, 1 at a time, on the computer screen. At the two ends of the line, two opposing words representing states of mind (e.g., happy – sad, tense – relaxed) are presented. Subjects put a mark on a point on the line that best represents their subjective state corresponding to the condition tested. The result is a distance (mm) calculated from the mark on the line.
Body Sway:
A string originating from a potentiometer, which is incorporated into the test battery computer, is used to measure postural stability in a single plane while the subject stands still with the eyes closed (described in de Haas et al.12).
VVLT:
The VVLT is a word learning and memory test, described in more detail in de Haas et al.12 Subjects are presented with a series of 30 words, one by one on the computer screen. The words need to be pronounced and remembered. There are three immediate recall trials, one delayed free recall trial (i.e., without presentation of the words) after approximately 20 min and a recognition trial.
Pharmaco-EEG:
For the standard pharmaco-EEG, electrodes are limited to the midsagittal leads (Fz, Cz, Pz and Oz), two electrodes for recording eye movements (outer canthi), and a ground electrode placed 2 cm above the nasion. Changes in the amplitude of the following frequency bands are quantified by spectrum-analysis (i.e., fast Fourier transformation): ß-band (13.5-35 Hz), γ-band (35-48.9 Hz), α-band (7.5-13.5 Hz), and θ-and δ-bands (7.5 Hz or less).
Protocol
Each independent study using this test battery was approved by independent ethics committees, namely either the 'medical ethics committee of the Leiden University Medical Centre', Leiden, the Netherlands, or the 'Stichting Beoordeling Ethiek Biomedisch Onderzoek, Assen, the Netherlands.
1. Computerized Test Battery Assessments
NOTE: The test battery should be implemented under controlled conditions (e.g., light intensity, room temperature, and background noise) to minimize influence of exogenous factors on the subject's results. Tests that can be repeatedly performed should be administered at least once before drug administration to serve as baseline. The Table of Materials provides an overview of the materials and equipment of the test battery.
- Adaptive tracking test
- Switch on the power of the test battery computer and turn on the computer and screens.
- Seat the subject in front of the (subject) computer screen and joystick.
- Check which is the preferred hand of the subject and adjust the joystick accordingly.
- Instruct the subject to hold the joystick as a pen, with the arm resting on the table.
- Start the test script via the installed program.
- Fill out the requested specifics such as subject and study number.
- Execute the test by clicking 'start' on the test assistant screen.
- Monitor the performance of the subject on the test assistant screen and encourage the subject to keep the circle around the dot if the subject cannot exceed difficulty factor 2.
- Saccadic eye movement and smooth pursuit test
NOTE: The eye movement electrodes should be attached to the sites specified in the clinical study protocol based upon the 10-20 System of the International Federation of Societies for Electroencephalography and Clinical Neurophysiology.- Identify the outer canthus of the right eye (i.e., the angle at the outer end of the fissure between the eyelids).
- Repeat this procedure for the left eye.
- Identify the place for the ground electrode 2 cm above the nasion (i.e., the root of the nose).
- Thoroughly rub the sites of the eye electrodes using a cotton-wipe skin cleansing gel for bioelectrical measurement (see step 3.1) to decrease the skin impedance, and use a cotton-wisp stick.
- Be careful not to abrade the skin, but do not rub too softly. Wipe away the residual gel with a gauze.
- Apply the three self-adhesive electrodes at the prepared sites.
- Connect the wires to the eye electrodes. Put your hand behind the press-button of the electrode to prevent it from pushing into the skin.
- Direct the wires along the ears over the shoulder of the subject to prevent the wires from hanging before the eyes.
- Plug the three wires in the electrode impedance meter.
- Check the impedance on the display: if the impedance is over 5 kΩ, check the quality of the electrode-attachment.
- Connect the subject to the eye movement measurement system by plugging all electrodes into the telefector and connect its cable to the amplifier.
- Instruct the subject to place the head on the headrest and relax, to follow the light on the screen by moving the eyes, and to not move the head.
- Start the test script via the installed program. Fill out the requested specifics such as subject and study number.
- Start the test by pressing the spacebar upon the 'go' instruction on the test assistant screen.
- Bond and Lader VAS
- Instruct the subject to score how they are currently feeling by using the mouse to mark the visual analog line presented on the screen.
- Instruct the subject that the most extreme points on the line represent the most extreme sensation imaginable.
- Start the test script via the installed program. Fill out the requested specifics such as subject and study number.
- Instruct the subject to start the test by clicking the mouse.
- Body sway
NOTE: Subjects should wear flat shoes during this test. No instructions or other stimuli are presented on the computer screen.- Ask the subject to stand in front of the computer, with a distance between the feet of about 10 cm, and arms hanging alongside the body.
- Attach the string that originates from the potentiometer built into the test battery computer onto the waist of the volunteer (e.g., the belt, or pants) by using the clip at the end of the string.
- Adjust the height of the table with the computer on it until the string is horizontal; a maximum deviation of 5 ° is acceptable. Ask the subject to close his or her eyes.
- Start the test script via the installed program. Fill out the requested specifics such as subject and study number.
- Start the test by clicking on 'Start Body Sway Sampling Session' on the test assistant's computer screen.
- VVLT
NOTE: Volunteers are not allowed to write down words at any time during the whole test procedure.- Instruct the subject that during the following automatic (visual) presentation of the words, the subject should name the words when they appear and remember them, and that at the end of the list, all words that are recalled should be named, each word only once.
- Start the test script via the installed program. Fill out the requested specifics such as subject and study number.
- Instruct the subject to read the written instructions displayed on the screen.
- Tell the subject that the test will start when the subject presses the spacebar.
- Record the recalled words (correct, incorrect, and words mentioned multiple times) by clicking on the recalled words on the test assistant screen.
- Pharmaco-EEG
NOTE: The electrodes should be attached to the sites specified in the protocol, and locations are based on the 10-20 System of the International Federation of Societies for Electroencephalography and Clinical Neurophysiology.- Measure and identify the exact location of the electrodes on the subject's head.
- Thoroughly rub the site using a cotton-wipe stick and skin cleansing gel to decrease the skin impedance. Be careful not to abrade the skin, but do not rub too softly.
- Stand behind the subject and attach the electrodes to the cleansed sites. Work from behind to the front.
- Put the cap of the electrode through the box with paste and wipe away the remainder by striking the cap along the brim of the box.
NOTE: The cap should filled completely, but not overloaded with paste. - Press the electrode on the cleansed site by spreading scalp hair if necessary. Push the electrode on the skin and be careful that as little hairs as possible are under the electrode.
- Put the wire of the electrode over the shoulder of the subject into the subject's lap.
- Use a small piece of hair to fix the electrode with the paste (which appears from the opening of the electrode cap), and an additional piece of hair (at a right angle to the other piece) with some paste to further fix the electrode to the skin.
- Check whether the electrode impedances are below 5 kΩ and adjust if necessary.
- Use tape to bundle the wires and to fixate the bundle to the clothes of the subject.
- Attach the electrode wires to the recording equipment.
- Open the EEG program on the computer.
- Instruct the subject to relax and to not move or speak for the measurement period.
- Instruct the subject to close the subject's eyes.
- Start the test script via the installed program.
| Assessment | Domain | Description | Outcome values | Specifics | ||
| Adaptive tracking test | Visuo-motor coordination, vigilance | A circle moves randomly about the computer screen. The subject must try to keep a dot inside the moving circle by operating a joystick. If this effort is successful, the speed of the moving circle increases. The velocity is reduced if the test subject cannot maintain the dot inside the circle. | Percentage of time correctly tracked | Administration time: 4 minutes | ||
| Saccadic eye movement test | Saccadic eye movements | The subject is required to follow a light source with only the eyes, which moves horizontally on a screen at 58 cm distance. The light source jumps from side to side for measurement of saccadic eye movements. | Percentage of time the subject’s eyes are in smooth pursuit of the target, for each stimulus velocity and for each stimulus frequency | Administration time: 2 minutes. | ||
| Smooth pursuit test | Smooth pursuit | The subject is required to follow a light source with only the eyes, which moves horizontally on a screen at 58 cm distance. The light source moves continuously for measurement of smooth pursuit. | Peak velocity (deg/s), reaction time (s), jump size (deg), primary saccadic deflection (deg) and inaccuracy (%) are calculated for each saccadic eye movement | Administration time: 2 minutes. | ||
| Body sway test | Postural control in a single plane | The subject is asked to stand still, with eyes closed while attached to the meter by means of a cord. The feet should be approximately 10 cm part and the hands in a relaxed position alongside the body. | Antero-posterior movement in mm | Administration time: 2 minutes. | ||
| Visual analogue scales (B&L) | Subjective assessment of alertness, mood, calmness | Subjects are asked to indicate how they feel concerning a specific state by clicking on a line of 100 mm, flanked by two opposite adjectives (e.g. drowsy – awake). The test consists of 16 items (i.e. lines). | All scores are measured in mm, from the beginning of the line on the left side to the point where the mark produced by the subject crosses the line. The score represents the adjective on the right side of the line (e.g. a higher score on a scale marked awake – drowsy indicates that the subject feels drowsier). Composite scores for the three domains are computed: the composite score for alertness is composed of nine scores, mood of five, and calmness of two. | Administration time: 2 minutes. | ||
| Visual verbal learning test | Learning, short- and long term memory, retrieval | Subjects are presented 30 words in three consecutive word trials, i.e. word learning test. Each trial ends with a free recall of the presented words (Immediate Recall- a test to determine acquisition and consolidation of information). Approximately 30 minutes after start of the first trial, the subject is asked to recall as many words as possible (Delayed Recall- this test measures active retrieval from long term memory). Immediately thereafter, the subject undergoes a memory recognition test, which consists of 15 presented words and 15 ‘distractors’ (Delayed Recognition- testing memory storage). | Per trial a total number correct, total number incorrect and total number of doubles are recorded. For the recognition trial, total number correct, total number incorrect and reaction time (and SD of RT) are recorded. | Administration time: 10 minutes | ||
| Pharmaco-EEG | quantitative, cerebral EEG-activity | Subjects are asked to relax and depending on the protocol keep their eyes open or closed. | For each lead (frontal lead: frontal (Fz) – central (Cz), central lead: Cz – parietal (Pz), parietal lead: Pz – occipital (Oz)), fast Fourier transformation analysis is performed to obtain the sum of amplitudes in the delta- (2-4 Hz), theta (4-7.5 Hz), alpha- (7.5-13.5 Hz), beta- (13.5-35 Hz), and gamma-(35-48.9 Hz) frequency ranges | Administration time: 4 minutes | ||
Table 1: Description and specifics of the assessments. Description of the specifics of the individual tests, including a description of the domain that is tested, the administration time, and specific outcome variables.
Representative Results
The computerized test battery assessments generate standardized and electronic data files. See Table 1 for the specifics on outcome values per test.
The test battery is primarily used in early phase clinical drug studies investigating effects of novel compounds in comparison to a (non-active) placebo or (active) comparator drug. Therefore, the factor 'treatment' should be considered in the statistical analysis of the data. A pre-dose (i.e., drug-free) assessment should be performed for the majority of the tests used in the protocol, to serve as baseline data. The VVLT can only be performed at one time-point post-dose (often at the time-point where the concentration of drug is highest), without pre-dose measurement, the learning effects and the interference of the learning process for the pre-dose and post-dose different word lists are used. As most tests are performed multiple times following drug administration to characterize the time-profile of the drug effects, the effect of time should be considered in the statistical analysis of the data.
In the protocol here, the test results were analyzed with a mixed model analysis of covariance (ANCOVA) with subject, subject by treatment, and subject by time as random effects; and treatment, study period, and treatment by time as fixed effects. The average baseline value per test was taken as covariate, as baseline measurements were performed twice to prevent loss of baseline data if one of the assessments proved insufficient. Before implementing the mixed model, the data were inspected for normality of distribution by means of Q-Q plots. If necessary, data would be log-transformed to ensure normal distribution.The analysis is done using the least squared means (LSM) approach, where, per treatment in the analysis an estimate of the mean is calculated by the model (i.e., the LSM). The LSM is not the same as the raw data average for the treatment, because a correction for baseline took place, and missing values were estimated by the model and included in the analysis.
The analysis is presented in LSM graphs, which are based on the estimates of the analysis and are different from average graphs based on the raw data time profile. As LSMs do not have standard deviations, the graphs are made with 95% confidence interval error bars. To avoid overcrowding the graph, only the error bars of the treatment with the highest value are shown up and of the treatment with the lowest value are shown down.
The acute pharmacodynamic effects of a single oral dose of mecamylamine hydrochloride at 10 mg and 20 mg, a 15-min infusion of 0.5 mg scopolamine hydrobromide, and double placebo (oral and intravenous) are shown in Figure 1 (change from baseline LSM graph). As the VVLT is only performed once post-dose, the VVLT data are shown in a traditional box-plot fashion, with different boxes per treatment (see Figure 2).
The protocol described in this paper is part of a larger study described in published literature29,30 and an in press published paper. The results described below are an example of the results of two computerized battery tests, in 12 healthy young male subjects, in a four-way cross-over design. For further details on the study, please see Baakman et al.30
As expected, the performance on the adaptive tracking test (the percentage correctly tracked) was negatively influenced by the administration of the cholinergic antagonists mecamylamine and scopolamine. Both the mecamylamine 20 mg and the 0.5 mg scopolamine treatments significantly worsened the score compared to placebo administration. The overall treatment effect was F = (3,33) 43.25, p < 0.0001, the mecamylamine 20 mg estimated difference was -2.06% correctly tracked (95% confidence interval [CI]: -3.97, -0.15) with a p = 0.0355 and the scopolamine estimated difference was -10.4% correctly tracked (95% confidence interval [CI]: -12.4, -8.39) with p < 0.0001.
When looking at the VVLT, administered once post-dose at +3.5 h for the immediate recall trials and +5 h for the delayed and recognition trials, all treatments induced a poorer performance (i.e., less words remembered) on the third trial of immediate recall and the delayed recall trial (overall treatment effect was F = (3,33) 15.17, p < 0.0001 for the third immediate recall trial and F = (3,34) 9.98, p < 0.0001 for the delayed recall trial). The two dose levels of mecamylamine showed a dose related effect in that the 20 mg dose showed a larger decrease in total number correctly recalled compared to placebo than did the 10 mg dose compared to placebo. For the third immediate recall trial, the results are: on average -2.7 words (95% confidence interval [CI]: -5.1, -0.3), p = 0.0286 for the 10 mg mecamylamine administration, and on average -3.6 words (95% CI: -5.9, -1.4), p = 0.0025 for the 20 mg mecamylamine administration. For the delayed recall trial, the results are: on average -3.1 words (95% confidence interval [CI]: -5.8, -0.4), p = 0.0259 for the 10 mg mecamylamine administration, and on average -3.8 words (95% confidence interval [CI]: -6.4, -1.2), p = 0.0051 for the 20 mg mecamylamine administration. Administration of scopolamine 0.5 mg showed even stronger negative effects on word recall: on average -7.7 words (95% confidence interval [CI]: -10.1, -5.4), p < 0.0001 for the third immediate recall trial and on average -7.1 words (95% confidence interval [CI]: -9.8, -4.5), p < 0.0001 for the delayed recall trial, all compared to placebo.
Administration of scopolamine in healthy subjects is known to induce large negative effects on cognitive tests results, as was for example described in a large study in 90 healthy male subjects6. The above described results show that the tests of the computerized battery were also able to show this significant anti-cognitive effect of 0.5 mg intravenously administered scopolamine. Regarding administration of mecamylamine, literature reports that lower doses of up to 20 mg induce negative effects on cognitive test results31,32,33, even though the actual effect is much smaller compared to the effect of scopolamine30, which is also evident from the results in this protocol.
These results show that the tests from the computerized test battery are sensitive to show acute pharmacodynamic effects after single administrations of the investigated anti-cholinergic drugs. The tests can differentiate between administration of placebo and drug, and more importantly, can differentiate between the muscarinic antagonist scopolamine and the nicotinic antagonist mecamylamine. These effects are repeatedly shown in multiple tests, evident from the statistical results and the similar graphs with tests results (data presented in Baakman et al.30).
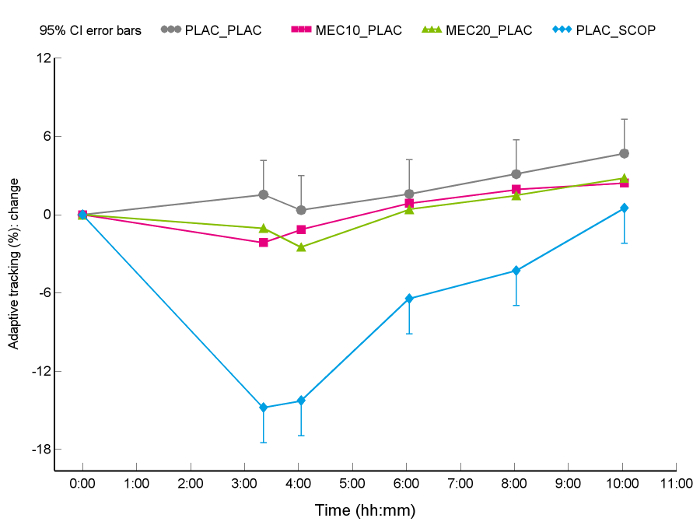
Figure 1: Effect of placebo, oral 10 mg and 20 mg mecamylamine, and intravenous 0.5 mg scopolamine on the adaptive tracking test in 12 healthy young males. Time course of mean values (and SD for highest and lowest scores) for the adaptive tracking test, measured at multiple time-points following drug administration (at t = 0), change from baseline data for 12 healthy male subjects. The percentage of correctly tracked is presented on the y-axis, time-point post-dose is presented on x-axis, with double placebo (oral and intravenous) results (grey circle), 10 mg mecamylamine results (magenta square), 20 mg mecamylamine results (green triangles) and 0.5 mg scopolamine (blue diamonds). This figure has been modified from Baakman et al.30 Please click here to view a larger version of this figure.
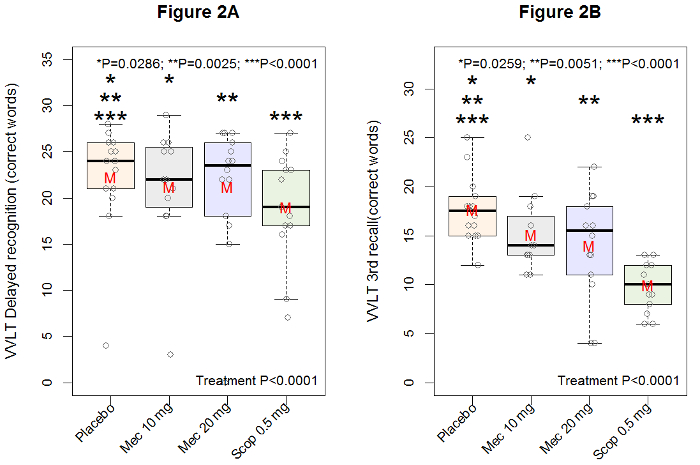
Figure 2: Effect of placebo, oral 10 mg and 20 mg mecamylamine, and intravenous 0.5 mg scopolamine on the visual verbal learning test in 12 healthy young males. Boxplot results of the VVLT delayed recognition trial (figure on the left) and third immediate recall trial, with the number of correctly remembered words on the y-axis and treatment on the x axis, for 12 healthy male subjects. The overall treatment effect is shown in the left bottom corner, the p-values of individual contrasts of treatment compared to placebo are depicted by means on the asterisks (*). The median is represented by the thick black line in the box. The mean is represented by the red 'M'. The grey circles represent actual data points (i.e., observations). This figure has been modified from Baakman et al.30 Please click here to view a larger version of this figure.
Discussion
Proof of pharmacodynamic effect is key in early phase drug development, as it warrants the next step of introducing a new drug in larger numbers of patients34. In the case of drugs developed to be active in the CNS it is especially important to show effects which indicate penetration of the blood-brain barrier35. Even though a lumbar puncture after a subject has received the drug is often chosen as a proxy for blood-brain barrier penetration, it is an invasive and burdensome technique and moreover, presence of the drug in the cerebrospinal fluid (CSF) does not equal activation of the drug by binding to its target(s) in the brain.
Phase I studies traditionally are data-intensive studies, with multiple series of assessments in close succession, to characterize the pharmacokinetic and pharmacodynamic profile of a new drug. Drugs that work in the CNS are likely to affect more than one neuropsychological and/or neurophysiological domain, as different receptors are often not just located in a single brain region. The main nicotinic receptors involved in cognition are located in the prefrontal, motor, and entorhinal cortices, and with lower density, in the cingulate and temporal cortex, thalamus and basal ganglia36. In addition, a single brain region is often connected to multiple other brain regions37.
Therefore, the computerized test battery core consists of a set of sensitive tests, of which the composition can be altered (i.e., tests can be added or removed from the battery) based on the expected CNS effects, to maximize the chance of positive results. This flexibility allows the battery to be suitable for use in studies with different types of drugs, but also in different populations. For example, in a study investigating a new drug in a small group of 24 patients with Huntington's disease (a neurodegenerative movement disorder), the core test battery was updated to include a test of fine motor skill (the finger tapping test, where in 5 consecutive trials of 10 s each, the spacebar needs to be tapped with the index finger of the dominant hand as quickly as possible), as one of the hallmarks of Huntington's disease are disturbances in fine motor skill38. Measurement of fine motor skills is not included in the core test battery, but is of importance to study potential changes in motor functioning in Huntington's disease. Nonetheless, the core tests have remained fairly stable over time, indicating the sensitivity of the battery for effects of a large number of drugs.
The number of tests in the battery should be kept concise to allow for multiple testing following drug administration, where test sessions should be planned such that the (presumed) pharmacokinetic profile of a drug is closely followed. This will result in information on pharmacodynamic effect coinciding with pharmacokinetic processes such as absorption, peak concentration, and elimination of the drug, information that could be combined in a pharmacokinetic-pharmacodynamic model, which was also developed for the protocol described in this paper29.
In some cases, the exact mechanism of action of an investigational compound is not yet fully understood from studies in animals. Over the past two decades the core tests from the computerized battery have been used to characterize the profile of effects of a large number of different investigational but also registered drugs from which the mechanism of action is known. This has resulted in a database of drug specific profiles, where for different drugs with the same mechanism of action, comparable test battery profiles are observed3. This allows for the profile of a new drug to be compared to the profiles of compounds of which the mechanism of action is known, and if a resemblance is found this could give insight into the mechanism of action of the investigational compound. The fact that comparable test profiles have been identified for different compounds with a similar mechanism of action provides strong proof for the sensitivity of the core tests of the test battery for CNS drug effects.
The potential for repeatability over a short period of time following drug administration is vital for the success of a battery like the computerized test battery described in this paper. The CNS is however influenced by both endogenous and exogenous factors, thereby altering a subject's test performance39. This highlights the importance of standardization of the conditions of the test environment, together with other subject specific factors. The exact conditions to be maintained during the execution of the tests should be specified in the study protocol and uniformly upheld in all subjects throughout the study. The lighting and room temperature should be kept constant over the testing period and the amount of distraction (noise, multiple persons in the room during testing, etc.) should be kept to a minimum. Other factors that could be controlled are certain aspects of the lifestyle of the subjects, such as diurnal rhythm, rest and fatigue, the intake of certain type of food and beverages, and the use of psychoactive substances.
Also, it is a known fact that neuropsychological test outcomes could be influenced by practice, or learning effects40, especially memory tests such as story and word list learning41 (e.g., VVLT test). Therefore, specific attention should be on allocated to the number of training sessions and test execution.
Other standardized, computerized test batteries have been developed and are widely used in drug development, with those described in Egerhazi et al.22 and Underwood et al.23 being among the most used in clinical trials. As mentioned before, the computerized test battery described in the current paper is different from these systems in that it also includes measurements of neurophysiological assessments (e.g., pupillometry, eye movement, EEG) by means of easy add-ons to the computer system, in addition to the more traditional neuropsychological tests such as the n-back test (described in Alvarez-Jimenez et al.29). The other systems however are portable computers, which makes testing at multiple sites feasible. Currently the set-up of the computerized test battery developed by CHDR is not suited for easy transportation between sites. A more portable version (i.e., laptop) has been designed and is currently being validated. This would allow for testing in multicenter clinical trials and possibly even at the home of, for example, patients who are unable to visit to the research institute due to mobility problems.
The computerized battery is a flexible battery, in the sense that other neuropsychological or physiological tests that have been shown to be sensitive to CNS drug effects can be incorporated in the system. Event related potentials (ERPs)42 are a recent example of this process: ERPs are gaining interest in clinical research and the demand for the inclusion of tests measuring different ERPs in clinical trials is growing. Ongoing validation of ERPs for implementation into the computerized test battery is currently being performed at CHDR.
In summary, the standardized, computerized test battery of neuropsychological and neurophysiological assessments described in this paper is designed to investigate pharmacodynamic effects of CNS active drugs in early phase drug development. The core tests have reliably and repeatedly shown to be sensitive to CNS effects, indicating penetration of the blood brain barrier and pharmacological activation of target sites in the CNS.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors have no acknowledgements.
Materials
| NeuroCart general computer hardware | |||
| Amplicon Impact E70 (=computer) | |||
| Medical insulation transformer | Thalheimer Trenntransformator | ERT 230/23/6G | |
| 24 inch widescreen | DELL | U2412M | for subject |
| PS2 Mouse | DELL | for subject | |
| PS2 Keyboard | DELL | for subject | |
| Photocamera | Canon | EOS 1100D | |
| EOS utility program | Canon | N.A. | photocamera software |
| 17 inch computer screen (research assistant) | DELL | 1708FP monitor | for research assistant |
| USB keyboard (research assistant) | DELL | for research assistant | |
| USB mouse (research assistant) | DELL | for research assistant | |
| Name | Company | Catalog Number | Comments |
| NeuroCart general computer software | |||
| Windows 7 or higher | Microsoft | ||
| E-prime 2.0 | Psychology Software Tools, Inc. (PST) | N.A. | every test has a custom, internally validated script |
| Name | Company | Catalog Number | Comments |
| EEG and eye electrodes hardware | |||
| Grass series Amplifier Systems | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15LT | amplifier for EEG electrodes |
| Quad, wide-band, high-gain, programmable AC amplifier | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15A54 | part of the 15LT ampyfier |
| Quad, high-gain, programmable AD amplifier | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15A94 | |
| Bioelectric Input Box, Electrode Board Model BIPOLA | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15LT | input box for electrodes |
| Electrode Impedance Meter | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | F-EZM5 | |
| A/ D converter | Cambridge Electronic Design (CED), Cambridge, UK | 1401 Mk1 and Mk2 | |
| Gold electrodes | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | Fx-E5GH | EEG electrodes |
| Ambu ECG electrodes | BlueSensor | N-OO-s/25 | Eye electrodes |
| EC2 cream | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | N.A. | electrode cream |
| Nuprep | Weaver and Company | N.A. | Skin prep gel |
| Name | Company | Catalog Number | Comments |
| EEG and eye electrodes software | |||
| Grass link 15 software | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | N.A. | |
| Spike 2 | Cambridge Electronic Design Limited | N.A. | every test has a custom, validated script |
| Name | Company | Catalog Number | Comments |
| Adaptive tracking materials (hard and software) | |||
| Adaptive tracking joystick | Job Kneppers Ontwerp en Realisatie B.V., Delft. | N.A. | custom built |
| TrackerUSB | Kevin Hobbs, CarbisDesign, UK | N.A. | Adaptive tracking software |
| Name | Company | Catalog Number | Comments |
| Bodysway hardware | |||
| Posturograph | Sentech BV | Celesco SP2 -50 | |
| Medical insulation transformer | Thalheimer Trenntransformator | ERT 230/23/6G | |
| Grass series Amplifier Systems | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15LT | |
| Quad, wide-band, high-gain, programmable AC amplifier | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15A54 | |
| Quad, high-gain, programmable AD amplifier | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15A94 | |
| Bioelectric Input Box, Electrode Board Model BIPOLA | Grass-Telefactor, An Astro-Med, Inc. Product Group/Natus | 15LT |
Riferimenti
- Alavijeh, M. S., Chishty, M., Qaiser, M. Z., Palmer, A. M. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx. 2 (4), 554-571 (2005).
- Peck, C. C. Postmarketing drug dosage changes. Pharmacoepidemiol Drug Saf. 12 (5), 425-426 (2003).
- Groeneveld, G. J., Hay, J. L., Van Gerven, J. M. Measuring blood-brain barrier penetration using the NeuroCart, a CNS test battery. Drug Discov Today Technol. 20, 27-34 (2016).
- Zuiker, R. G., et al. NS11821, a partial subtype-selective GABAA agonist, elicits selective effects on the central nervous system in randomized controlled trial with healthy subjects. J Psychopharmacol. 30 (3), 253-262 (2016).
- Chen, X., et al. Pharmacodynamic response profiles of anxiolytic and sedative drugs. Br J Clin Pharmacol. 83 (5), 1028-1038 (2017).
- Liem-Moolenaar, M., et al. Pharmacokinetic-pharmacodynamic relationships of central nervous system effects of scopolamine in healthy subjects. Br J Clin Pharmacol. 71 (6), 886-898 (2011).
- Baloh, R. W., Sills, A. W., Kumley, W. E., Honrubia, V. Quantitative measurement of saccade amplitude, duration, and velocity. Neurology. 25 (11), 1065-1070 (1975).
- Bittencourt, P. R., Wade, P., Smith, A. T., Richens, A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 15 (2), 259-262 (1983).
- Bowdle, T. A., et al. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology. 88 (1), 82-88 (1998).
- Bond, A., Lader, M. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 47 (3), 211-218 (1974).
- Borland, R. G., Nicholson, A. N. Visual motor co-ordination and dynamic visual acuity. Br J Clin Pharmacol. 18, 69S-72S (1984).
- de Haas, S. L., et al. The pharmacokinetic and pharmacodynamic effects of SL65.1498, a GABA-A alpha2,3 selective agonist, in comparison with lorazepam in healthy volunteers. J Psychopharmacol. 23 (6), 625-632 (2009).
- van Steveninck, A. L., et al. The sensitivity of pharmacodynamic tests for the central nervous system effects of drugs on the effects of sleep deprivation. J Psychopharmacol. 13 (1), 10-17 (1999).
- van Steveninck, A. L., et al. Pharmacodynamic interactions of diazepam and intravenous alcohol at pseudo steady state. Psychopharmacology (Berl). 110 (4), 471-478 (1993).
- Zoethout, R. W., Delgado, W. L., Ippel, A. E., Dahan, A., van Gerven, J. M. Functional biomarkers for the acute effects of alcohol on the central nervous system in healthy volunteers. Br J Clin Pharmacol. 71 (3), 331-350 (2011).
- de Visser, S. J., van der Post, J., Pieters, M. S., Cohen, A. F., van Gerven, J. M. Biomarkers for the effects of antipsychotic drugs in healthy volunteers. Br J Clin Pharmacol. 51 (2), 119-132 (2001).
- Dumont, G. J., de Visser, S. J., Cohen, A. F., van Gerven, J. M., Biomarker Working Group of the German Association for Applied Human, P. Biomarkers for the effects of selective serotonin reuptake inhibitors (SSRIs) in healthy subjects. Br J Clin Pharmacol. 59 (5), 495-510 (2005).
- Zuurman, L., Ippel, A. E., Moin, E., van Gerven, J. M. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 67 (1), 5-21 (2009).
- van Steveninck, A. L., et al. Effects of intravenous temazepam. I. Saccadic eye movements and electroencephalogram after fast and slow infusion to pseudo steady state. Clin Pharmacol Ther. 55 (5), 535-545 (1994).
- van Steveninck, A. L., et al. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 50 (2), 172-180 (1991).
- van Steveninck, A. L., et al. Effects of temazepam on saccadic eye movements: concentration-effect relationships in individual volunteers. Clin Pharmacol Ther. 52 (4), 402-408 (1992).
- Egerhazi, A., Berecz, R., Bartok, E., Degrell, I. Automated Neuropsychological Test Battery (CANTAB) in mild cognitive impairment and in Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 31 (3), 746-751 (2007).
- Underwood, J., et al. Associations between cognitive impairment and patient-reported measures of physical/mental functioning in older people living with HIV. HIV Med. 18 (5), 363-369 (2017).
- Jones, S., Sudweeks, S., Yakel, J. L. Nicotinic receptors in the brain: correlating physiology with function. Trends Neurosci. 22 (12), 555-561 (1999).
- Levin, E. D., McClernon, F. J., Rezvani, A. H. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl). 184 (3-4), 523-539 (2006).
- Kulshreshtha, A., Piplani, P. Current pharmacotherapy and putative disease-modifying therapy for Alzheimer’s disease. Neurol Sci. 37 (9), 1403-1435 (2016).
- Ebert, U., Kirch, W. Scopolamine model of dementia: electroencephalogram findings and cognitive performance. Eur J Clin Invest. 28 (11), 944-949 (1998).
- Webster, J. C., et al. Antagonist activities of mecamylamine and nicotine show reciprocal dependence on beta subunit sequence in the second transmembrane domain. Br J Pharmacol. 127 (6), 1337-1348 (1999).
- Alvarez-Jimenez, R., et al. Pharmacokinetics and pharmacodynamics of oral mecamylamine – development of a nicotinic acetylcholine receptor antagonist cognitive challenge test using modelling and simulation. J Psychopharmacol. 31 (2), 192-203 (2017).
- Baakman, A. C., et al. An anti-nicotinic cognitive challenge model using mecamylamine in comparison with the anti-muscarinic cognitive challenge using scopolamine. Br J Clin Pharmacol. , (2017).
- Newhouse, P. A., Potter, A., Corwin, J., Lenox, R. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology (Berl). 108 (4), 480-484 (1992).
- Newhouse, P. A., Potter, A., Corwin, J., Lenox, R. Age-related effects of the nicotinic antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology. 10 (2), 93-107 (1994).
- Thompson, J. C., Stough, C., Ames, D., Ritchie, C., Nathan, P. J. Effects of the nicotinic antagonist mecamylamine on inspection time. Psychopharmacology (Berl). 150 (1), 117-119 (2000).
- Miller, R., et al. How modeling and simulation have enhanced decision making in new drug development. J Pharmacokinet Pharmacodyn. 32 (2), 185-197 (2005).
- Mikitsh, J. L., Chacko, A. M. Pathways for small molecule delivery to the central nervous system across the blood-brain barrier. Perspect Medicin Chem. 6, 11-24 (2014).
- Paterson, D., Nordberg, A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 61 (1), 75-111 (2000).
- Li, Y., Richardson, R. M., Ghuman, A. S. Multi-Connection Pattern Analysis: Decoding the representational content of neural communication. Neuroimage. , (2017).
- Rao, A. K., Gordon, A. M., Marder, K. S. Coordination of fingertip forces during precision grip in premanifest Huntington’s disease. Mov Disord. 26 (5), 862-869 (2011).
- Taylor, L., Watkins, S. L., Marshall, H., Dascombe, B. J., Foster, J. The Impact of Different Environmental Conditions on Cognitive Function: A Focused Review. Front Physiol. 6, 372 (2015).
- Goldberg, T. E., Harvey, P. D., Wesnes, K. A., Snyder, P. J., Schneider, L. S. Practice effects due to serial cognitive assessment: Implications for preclinical Alzheimer’s disease randomized controlled trials. Alzheimers Dement (Amst). 1 (1), 103-111 (2015).
- Gavett, B. E., et al. Practice Effects on Story Memory and List Learning Tests in the Neuropsychological Assessment of Older Adults. PLoS One. 11 (10), e0164492 (2016).
- Luck, S. J. Direct and indirect integration of event-related potentials, functional magnetic resonance images, and single-unit recordings. Hum Brain Mapp. 8 (2-3), 115-201 (1999).

