Granulocyte-dependent Autoantibody-induced Skin Blistering
Summary
In the animal model described in our present work, purified IgG antibodies against a stretch of 200 amino acids (aa 757-967) of collagen VII are injected repeatedly into mice reproducing the blistering phenotype as well as the histo- and immunopathological features characteristic to human epidermolysis bullosa acquisita (EBA)1.
Abstract
Autoimmune phenomena occur in healthy individuals, but when self-tolerance fails, the autoimmune response may result in specific pathology. According to Witebsky’s postulates, one of the criteria in diagnosing a disease as autoimmune is the reproduction of the disease in experimental animals by the passive transfer of autoantibodies. For epidermolysis bullosa acquisita (EBA), a prototypic organ-specific autoimmune disease of skin and mucous membranes, several experimental models were recently established. In the animal model described in our present work, purified IgG antibodies against a stretch of 200 amino acids (aa 757-967) of collagen VII are injected repeatedly into mice reproducing the blistering phenotype as well as the histo- and immunopathological features characteristic to human EBA 1. Full-blown widespread disease is usually seen 5-6 days after the first injection and the extent of the disease correlates with the dose of the administered collagen VII-specific IgG. The tissue damage (blister formation) in the experimental EBA is depending on the recruitment and activation of granulocytes by tissue-bound autoantibodies 2,-4. Therefore, this model allows for the dissection of the granulocyte-dependent inflammatory pathway involved in the autoimmune tissue damage, as the model reproduces only the T cell-independent phase of the efferent autoimmune response. Furthermore, its value is underlined by a number of studies demonstrating the blister-inducing potential of autoantibodies in vivo and investigating the mechanism of the blister formation in EBA 1,3,-6. Finally, this model will greatly facilitate the development of new anti-inflammatory therapies in autoantibody-induced diseases. Overall, the passive transfer animal model of EBA is an accessible and instructive disease model and will help researchers to analyze not only EBA pathogenesis but to answer fundamental biologically and clinically essential autoimmunity questions.
Protocol
1. Preparation of Pathogenic Antibody
Note: Purification and concentration of the antibodies should be performed on the same day, as antibodies are not to be stored in 0.1 M glycine pH 2.5-3 (elution buffer) over night (ON).
Affinity purification of IgG: use 25 ml of immune rabbit serum:
- Thaw the rabbit serum ON at 4 °C, mix it 1:1 with 1xPBS (running buffer) and centrifuge it at 1260 x g for 10 min at 20 °C. Optionally, a filtering step with filter paper might be included after centrifugation in case the serum is fatty.
- Meanwhile, wash the protein G matrix filled column with 10 bed volumes (bed volume=matrix volume) of 1XPBS (running buffer).
- Apply the diluted and filtered serum to the column and incubate 1 hr on the rocking platform at room temperature (RT).
- Collect the flow-through (FT) in a 50 ml Flacon tube. Do not discard it till the concentration and titer of the purified IgG is known.
- Wash the matrix with 5 bed volumes of 1XPBS and in the meantime prepare 50 ml Falcon tubes for collecting the eluted IgG fraction. Pipette 1 ml pH neutralizing buffer: 1 M TRIS pH 10 in each tube (otherwise the proportion of 1:20 TRIS buffer relative to the amount of eluate to be collected).
- Apply 100-150 ml elution buffer: 0.1 M glycine pH 2.5-3 to the column and collect 50 ml fraction(s). Flip the tube 2-3x, measure the pH and add neutralizing buffer until you’ve reached the pH 7.2-7.4.
- Collect few drops of IgG eluate and using the elution buffer as etalon measure the OD at 280 nm. If the OD is <0.1 stop collecting.
- Regenerate the protein G matrix and store it following the manufacturer’s instructions.
Concentration by ultrafiltration with Amicon tubes:
- Wash the Amicon Ultra (15 ml /30KDa) ultrafiltration tubes with 1XPBS by centrifuging 15-20 min at 3,220 x g at 4 °C. Discard the FT from the Amicon.
- Fill the Amicons with 15 ml of eluted IgG fraction and centrifuge for 25-30 min at 3,220 x g and 4 °C; centrifugation time always depends on the protein content of the eluate.
- Discard FT and repeat ultrafiltration till you have no eluted fraction left.
- Wash the concentrated IgG extensively, twice, with 1XPBS 25-30 min by centrifugation.
- Collect the “sticky”, yellowish antibody solution from the Amicon filter in a final volume of 1,500-2,000 μl of 1XPBS.
- Measure concentration of IgG preparation using a spectrophotometer or NanoDrop:
Conc. (mg/ml) = A(280 nm) x dilution factor /1,4 - Wash and store the Amicons following the manufacturer’s instructions.
2. Injection of Collagen VII-specific IgG into Mice
Before starting the actual experimental procedure, make sure that the experimental protocol is written and all materials are prepared for the experiment.
- Prepare the data sheets for the disease scoring, ELISAs, immunofluorescence (IF) analysis and myeloperoxidase (MPO) assay 4.
- Label the tubes for blood and organs, cryomolds as well as histology processing and embedding cassettes.
- The antibody solutions should be prepared fresh or thawed from stocks and characterized with regard to their reactivity (titer by IF microscopy and/or ELISA). The sterile filtered antibody solution should be diluted in PBS in a way that the required dose for injection varies between 250-1000 μl. Make sure that you have enough IgG to perform the entire experiment.
- Prepare fresh anticoagulant: heparin 20 U/ml and 8.7 mg/ml ketamine/ 1.3 mg/ml xylazine mixture for anesthesia. When sacrificing the mice have labeled tubes with 3.7% formaldehyde prepared.
- Sterilize surgical instruments (scalpel, scissors, fine point and curved forcipes) and prepare syringes (insulin syringes, 1 and 2 ml syringes), needles, digital camera and extra memory card.
Note: Perform all procedures on ketamine/xylazine or isoflurane narcotized mice. Isoflurane is the preferred anesthesia option for the daily skin checks, as mice recover quickly. However, when taking pictures, 87 mg/kg ketamine and 13 mg/kg xylazine is usually injected subcutaneously. For euthanasia the ketamine/xylazine dosage is increased to 130 mg/kg ketamine and 20 mg/kg xylazine.
- Check the already marked animals for their general health condition, skin and fur appearance, weigh them and measure their ear thickness (always stick to the same ear).
- Register observed values and changes in the clinical evaluation sheet.
- Collect 20-30 μl (2-3 drops) blood from the tail vein in a syringe containing the same volume of anticoagulant.
- Disinfect the fur and skin using 80% ethanol.
- Inject the antibody solution subcutaneously into the back using an insulin syringe. (For certain sites (e.g., the ears) only small volumes (10-50 μl) are feasible to be injected.)
Injections should be repeated every second day, 4 times. Balb/c and C57BL6 mice of a body weight of approximately 20 g start to develop the disease 3-4 days after the first administration of 400-500 up to 750 μg/g body weight/injection autoantibodies.
When finishing the experiment:
- Put the mice under deep narcosis by injecting 130 mg/kg and 20 mg/kg ketamine/xylazine subcutaneously, exsanguinate them from the posterior vena cava and cut the heart out.
- Collect organs/tissue samples such as skin: tail, ear, blood and esophagus and put them in previously labeled and PBS or 3.7% formaldehyde filled tubes.
When returning to the laboratory:
- Centrifuge the blood at 1,200 x g, 10 min at RT to separate plasma, transfer the plasma to properly labeled tubes and store them accordingly.
- Embed the skin biopsy in Optimal Cut Temperature medium using cryomolds, properly label, and store them at -80 °C.
- Place the tissue samples meant for histology in the labeled embedding molds and keep them in 3.7% formaldehyde till paraffin embedding.
3. Clinical Evaluation of Disease Severity
Mice should be examined daily for lesions of skin and mucosa and the findings should be recorded using appropriate forms. The criteria for early euthanasia is skin lesions affecting more that 20% of the body surface and a weight loss of 5-10% of the total body weight in three consecutive days, counting for a disease score of 5 (Table 1).
- Place the mouse on its belly. Start with the head area, namely with the right ear and check for blisters, erosions, crust on the inner as well as on the outer side. Continue the same way with the left ear.
- Right and left eyes are controlled for signs of erythema, alopecia, erosions and/or crust.
- Forehead and snout area are brushed through next, continuing with the ventral part of the snout, lip and the mucous membrane of the mouth.
- Examine the right front leg starting from the paw, moving upwards; switch to the left front leg. Same procedure with the right and left hind legs.
- Brush through the fur on the neck and back area with a curved fine point forceps.
- Turn the mouse on its back and check the ventral part as well as the ventral sides of the limbs the same way as previously.
- Check the tail.
- Take pictures of relevant body parts where the mice develop disease. Attention must be paid to the quality and quantity of the pictures.
- Calculate the clinical disease scores.
4. Analysis of Skin and Plasma Samples
- Prepare, store, and/or process all collected tissues according to the plan.
- Cut frozen tissue sections for IF detection of rabbit antibodies and mouse complement system components.
- Analyze the different protein and enzyme content (MPO assay) of frozen tissue and/or organ extracts.
- Section the paraffin embedded tissue and stain with H&E to visualize the extent of tissue damage.
- Analyze the plasma by ELISA, immunoblot.
Representative Results
The passive transfer of antigen specific antibodies results in a full blown disease in mice, resembling at clinical, histological and immunopathological levels the human EBA. Blisters, crusts, erosions and alopecia develop on the ears, snout, paws, legs, back and around the eyes of the mice. The first clinical signs of the disease will most probably appear on the ears and/or head area. Deposition of specific rabbit IgG, and mouse complement C3 is detected by direct IF at the dermal epidermal junction in cryosections of perilesional skin. In lesional skin subepidermal blisters and inflammatory infiltrate are seen by histology.
However, if the injection of pathogenic collagen VII-specific antibodies is discontinued, the disease activity gradually becomes lower, and within several weeks the lesions heal. Nevertheless, a certain degree of postinflammatory cicatricial alopecia may persist indefinitely.
Characterization of the recombinant autoantigen (and of pathogenic IgG)
Murine collagen specific IgG binds at the dermal -epidermal junction (Figure 2B). The specificity is assessed by immunoblot (Figure 2C, lanes 3, 4 and 7).
Scoring disease activity
The clinical evaluation of the disease is based on a scoring system we developed (Table 1): 0, no lesions; 1, less than 1% of the skin surface; 2, 1-5% of the skin surface; 3, 5-10% of the skin surface; 4, 10-20% of the skin surface is affected. IgG against murine collagen VII induces cutaneous lesions such as erythema, alopecia, blisters, erosions, crusts on the ears, eyes, snout, limbs and trunk of Balb/c mice (Figure 3).
Analysis of tissue-bound and circulating collagen-specific antibodies
Deposition of rabbit IgG, and mouse complement C3 are detected in frozen, perilesional tissue sections (Figure 4D and E respectively). The subepidermal blisters and the inflammatory infiltrate are seen in histological samples (Figure 4F). Circulating rabbit antibodies are analyzed by ELISA (Figure 5). Frozen tissue and/or organ extracts are analyzed by different assays for their protein and enzyme content (MPO assay).
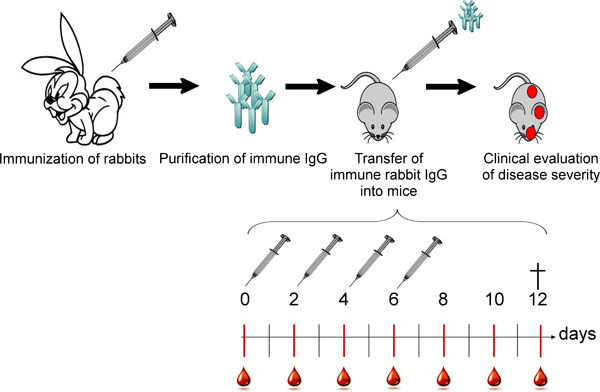
Figure 1. Overall scheme of the in vivo blistering induced by the passive transfer of collagen VII-specific antibodies. Rabbits are immunized with murine collagen VII and rabbit IgG is purified from the immune sera. Subsequently, the specific autoantibodies are injected subcutaneously into mice following an injection/bleeding schedule. Mice are being checked for general health condition and disease signs daily.
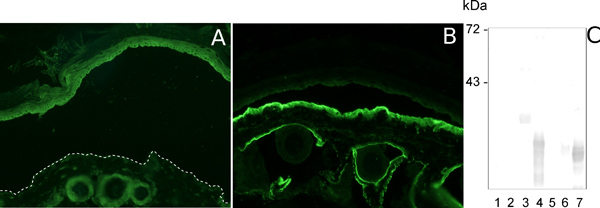
Figure 2. Characterization of pathogenic collagen VII-specific IgG. Indirect IF analysis of salt-split normal mouse skin sections incubated with pre-immune rabbit serum and with murine collagen VII-specific immune rabbit serum results in no deposition (A) and deposition of autoantibodies at the dermal epidermal junction (B), respectively. The specific antibodies recognize the antigen(s) they were raised against when immunoblot with a set of overlapping recombinant murine collagen VII fragments is performed (C, lanes 3, 4 and 7).
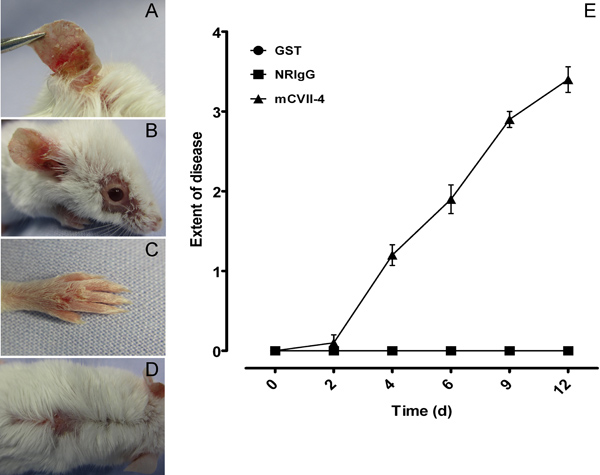
Figure 3. Clinical evaluation of mice. IgG to murine collagen VII induces cutaneous lesions such as alopecia, blisters, erosions, crusts on the ears, eyes, snout, limbs and trunk of Balb/c mice (A-D). Mice injected with specific autoantibodies reach a score of 4, whereas the ones injected with NRIgG or Abs against an indifferent protein had a score of 0 (E). The clinical score was calculated as follows: 0, no lesions; 1, less than 1% of the skin surface; 2, 1-5% of the skin surface; 3, 5-10% of the skin surface; 4, 10-20% of the skin surface is affected. Weight loss of 5-10% of the total body weight during three consecutive days counts as an extra point in the final score.
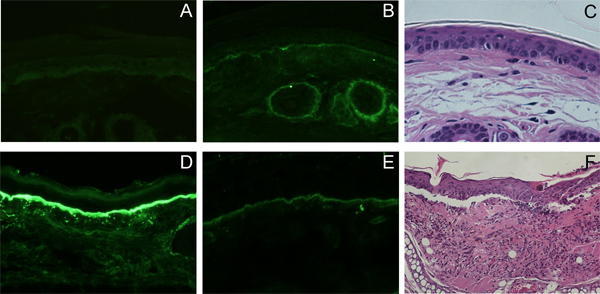
Figure 4. Histo- and immunopathological findings in mice injected with collagen VII-specific IgG. Deposition of rabbit IgG (D), and mouse complement C3 (E) is detected by direct IF at the dermal epidermal junction in cryosections of perilesional skin, in vivo. In lesional skin subepidermal blisters and inflammatory infiltrate is seen by histology (F). No deposition of rabbit IgG (A), mouse complement C3 (B), nor is subepidermal blister formation seen in the controls (C).
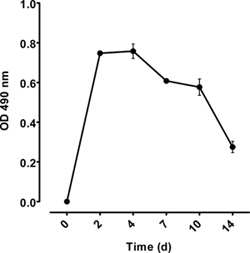
Figure 5. Immunoassay of plasma from mice injected with collagen VII-specific IgG. Plasma levels of circulating rabbit antibodies were measured by ELISA.
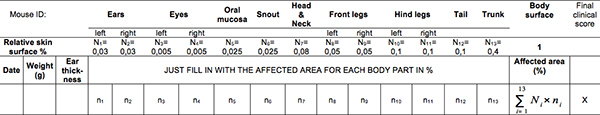
Table 1. Skin blistering disease scoring sheet. Click here to view larger table.
Scoring system:
- 0, no lesions;
- 1, less than 1% of the skin surface;
- 2, 1-5% of the skin surface;
- 3, 5-10% of the skin surface;
- 4, 10-20% of the skin surface is affected.
Extra hints when scoring:
- Weight loss of 5-10% of the total body weight during three consecutive days counts as an extra point in the final score 17.
- Erythema on the ears, around the eyes on paws are just the first signs of an ongoing immune reaction, if not paired with blisters or alopecia they are not quantified, just taken under observation.
- Marks on the tail could be bite or fight marks, so if they are present from the beginning their worsening might not necessarily be due to the disease. Consider carefully weather to count them!
Discussion
The passive transfer of autoantibodies into experimental animals is a major approach for demonstrating their pathogenicity 7, -12. Animal models obtained through this method, in addition to being the indirect evidence for autoimmunity 13, allow the investigation of the efferent phase of the pathogenic mechanism. The passive transfer model of antibody-induced granulocyte-dependent skin blistering of epidermolysis bullosa acquisita (EBA) was used to dissect the mechanisms of tissue damage in autoimmune dermatoses. It emerged also, as an exquisitely instructive model disease to study fundamental, biologically and clinically crucial aspects of antibody-mediated organ-specific autoimmune diseases that extend well beyond the limits of autoimmunity against collagen VII. Finally, this model may be used to study fundamental biological and pathophysiological processes, including basement membrane biology, granulocyte activation by immune complexes and complement activation.
Although several experimental settings, including ex vivo and animal models, are available for the autoimmune skin disease EBA 14, by far the most suitable to study the granulocyte-dependent inflammatory pathways is the passive transfer of autoantibodies into animals. In contrast to the ex vivo model, where the granulocytes are added to the skin sections previously incubated with the autoantibodies 15, here the infiltration of leukocytes is spontaneously reproduced by the mice. Furthermore, if we choose to work with specific antibodies raised against the antigen in other organisms, such as rabbit and goat, we avoid the problem of limited patient sera availability and we also have a better chance to reproduce complement activation and granulocyte recruitment 16.
For the successful reproduction of granulocyte-dependent blistering in the passive transfer model of EBA, it is important to be able to adequately score the signs of disease, starting with erythema and oedema through blisters, erosions with crusts and alopecia. The evaluation of mice is a process that needs to be previously learned. The scoring of the disease has to be performed by the same person, throughout the experiment and always seconded by a collaborator or aid person. The development and use of a standardized scoring system for each experimental disease is of the outmost importance. The enclosed scoring sheet should give the reader an insight how skin dermatoses are evaluated in mice.
When performed as shown in the video, the model of autoantibody-induced subepidermal blistering will greatly facilitate the further dissection of the granulocyte-dependent inflammatory pathways triggered by autoantibodies and resulting in tissue damage. Its value in medical research is supported by the promising perspective of developing and testing new antigen-specific T and B cell targeted anti-inflammatory therapies. Finally, it will help in answering fundamental biologically and clinically essential autoimmunity questions.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge support by grants from the Deutsche Forschungsgemeinschaft SI-1281/4-1 and BIOSS from the Medical Faculty of the Freiburg University (to CS).
Materials
| Name of the reagent | Company | Catalogue number | Comments |
| recombinant antigen | The plasmids encoding for the recombinant forms of murine collagen VII are available from the corresponding author. | ||
| immune rabbit serum | www.eurogentec.com | We had New Zealand White rabbits immunized with 200 μg of antigen, 3 times at 2 week intervals. For this purpose we have used the services of Eurogentec S.A., Belgium. | |
| Protein G agarose | Roche Applied Science | 11243233001 | |
| Balb/c mice | Charles River Laboratories | ||
| OCT compound, Tissue-Tek | Sakura Finetek | 4583 | OCT, optimal cutting temperature |
| Cryomold standard | Sakura Finetek | 4557 | 25 mm × 20 mm × 5 mm |
| Cryomold intermediate | Sakura Finetek | 4566 | 15 mm × 15 mm × 5 mm |
| Uni-Link-Einbettkassette | R. Langenbrinck | 09-0503 | Histology processing and embedding cassettes |
| Spezial-Tatowierfarbe Schwarz | H. Hauptnerund & Richard Herberholz GmbH & Co. KG | 71492000 | tattooing paste |
| Tatowierzange TZ1 | EBECO E. Becker & Co GmbH | tattooing device | |
| Heparin | Carl Roth GmbH & Co. | 7692.1 | |
| Formaldehyde 37% | Carl Roth GmbH & Co. | 7386 | |
| Ketamine hydrochloride | Sigma-Aldrich Chemie GmbH | K2753-1G | |
| Xylazine hydrochloride | Sigma-Aldrich Chemie GmbH | X1251-1G | |
| sterile PBS | Biochrom | L182-50 | |
| digital camera | Nikon | Coolpix 5400 | |
| Syringe driven filter unit 0.45 μm | Millipore | ||
| Caliper | Mitutoyo 7309 | 1667338 | Farnell (distributor) |
| disease scoring sheet | example enclosed |
References
- Sitaru, C., Mihai, S., Otto, C., Chiriac, M. T., Hausser, I., Dotterweich, B. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J. Clin. Invest. 115, 870-878 (2005).
- Kopecki, Z., Arkell, R. M., Strudwick, X. L., Hirose, M., Ludwig, R. J., Kern, J. S. Overexpression of the Flii gene increases dermal-epidermal blistering in an autoimmune ColVII mouse model of epidermolysis bullosa acquisita. J. Pathol. 225, 401-413 (2011).
- Kulkarni, S., Sitaru, C., Jakus, Z., Anderson, K. E., Damoulakis, G., Davidson, K. PI3Kβ plays a critical role in neutrophil activation by immune complexes. Sci. Signal. 4, 23-23 (2011).
- Chiriac, M. T., Roesler, J., Sindrilaru, A., Scharffetter-Kochanek, K., Zillikens, D., Sitaru, C. NADPH oxidase is required for neutrophil-dependent autoantibody-induced tissue damage. J. Pathol. 212, 56-65 (2007).
- Mihai, S., Chiriac, M. T., Takahashi, K., Thurman, J. M., Holers, V. M., Zillikens, D. The alternative pathway of complement activation is critical for blister induction in experimental epidermolysis bullosa acquisita. J. Immunol. 178, 6514-6521 (2007).
- Sesarman, A., Sitaru, A. G., Olaru, F., Zillikens, D., Sitaru, C. Neonatal Fc receptor deficiency protects from tissue injury in experimental epidermolysis bullosa acquisita. J. Mol. Med. 86, 951-959 (2008).
- Huang, X. R., Holdsworth, S. R., Tipping, P. G. Th2 responses induce humorally mediated injury in experimental anti-glomerular basement membrane glomerulonephritis. J. Am. Soc. Nephrol. 8, 1101-1108 (1997).
- Matsumoto, I., Staub, A., Benoist, C., Mathis, D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 286, 1732-1735 (1999).
- Oswald, E., Sesarman, A., Franzke, C., Wölfle, U., Bruckner-Tuderman, L., Jakob, T. The flavonoid luteolin inhibits Fcγ-dependent respiratory burst in granulocytes, but not skin blistering in a new model of pemphigoid in adult mice. PLoS One. 7, e31066 (2012).
- Liu, Z., Diaz, L. A., Troy, J. L., Taylor, A. F., Emery, D. J., Fairley, J. A. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J. Clin. Invest. 92, 2480-2488 (1993).
- Anhalt, G. J., Labib, R. S., Voorhees, J. J., Beals, T. F., Diaz, L. A. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N. Engl. J. Med. 306, 1189-1196 (1982).
- Toyka, K. V., Brachman, D. B., Pestronk, A., Kao, I. Myasthenia gravis: passive transfer from man to mouse. Science. 190, 397-399 (1975).
- Rose, N. R., Bona, C. Defining criteria for autoimmune diseases (Witebsky’s postulates revisited. Immunol. Today. 14, 426-430 (1993).
- Sitaru, C. Experimental models of epidermolysis bullosa acquisita. Exp. Dermatol. 16, 520-531 (2007).
- Sitaru, C., Kromminga, A., Hashimoto, T., Bröcker, E. B., Zillikens, D. Autoantibodies to type VII collagen mediate Fcgamma-dependent neutrophil activation and induce dermal-epidermal separation in cryosections of human skin. Am. J. Pathol. 161, 301-311 (2002).
- Sesarman, A., Oswald, E., Chiriac, M. T., Csorba, K., Vuta, V., Feldrihan, V. Why human pemphigoid autoantibodies do not trigger disease by the passive transfer into mice. Immunol. Lett. , (2012).
- Ishii, N., Recke, A., Mihai, S., Hirose, M., Hashimoto, T., Zillikens, D. Autoantibody-induced intestinal inflammation and weight loss in experimental epidermolysis bullosa acquisita. J. Pathol. 224, 234-244 (2011).

