Organic Structure-directing Agent-free Synthesis for *BEA-type Zeolite Membrane
Summary
A *BEA seed crystal was loaded on a porous α-Al2O3 support by the dip-coating method, and hydrothermally grown without using an organic structure-directing agent. A *BEA-type zeolite membrane having very few defects was successfully prepared by the secondary growth method.
Abstract
Membrane separation has drawn attention as a novel-energy saving separation process. Zeolite membranes have great potential for hydrocarbon separation in petroleum and petrochemical fields because of their high thermal, chemical, and mechanical strength. A *BEA-type zeolite is an interesting membrane material because of its large pore size and wide Si/Al range. This manuscript presents a protocol for *BEA membrane preparation by a secondary growth method that does not use an organic structure-directing agent (OSDA). The preparation protocol consists of four steps: pretreatment of support, seed preparation, dip-coating, and membrane crystallization. First, the *BEA seed crystal is prepared by conventional hydrothermal synthesis using OSDA. The synthesized seed crystal is loaded on the outer surface of a 3 cm long tubular α-Al2O3 support by a dip-coating method. The loaded seed layer is prepared with the secondary growth method using a hydrothermal treatment at 393 K for 7 days without using OSDA. A *BEA membrane having very few defects is successfully obtained. The seed preparation and dip-coating steps strongly affect the membrane quality.
Introduction
Membrane separation has drawn attention as novel-energy saving separation process. Many types of membranes have been developed for the past decades. Polymeric membranes have been widely used for gas separation, creating drinkable water from sea water1, and wastewater treatment2.
Inorganic membrane materials like silica3, carbon molecular sieve4, and zeolite have advantages for thermal, chemical, and mechanical strength compared with polymeric membranes. Therefore, inorganic membranes tend to be used under more severe conditions, such as hydrocarbon separation in petroleum and petrochemical fields.
Zeolite has unique adsorption and molecular sieving properties due to its micropores. In addition, zeolite has a cation exchange ability that contributes to control zeolite's adsorption and molecular sieving properties. The number of cations in zeolite is determined by the Si/Al ratio of the zeolite structure. Therefore, the size of the micropores and Si/Al ratio are key characteristics that determine the permeation and separation properties of zeolite membranes. For these reasons, zeolite is a promising type of inorganic membrane material. Some zeolite membranes have already been commercialized for dehydration of organic solvents due to their hydrophilicity and molecular sieving properties5,6,7,8.
*BEA-type zeolite is an interesting membrane material because of its large pore size and wide Si/Al range. *BEA has generally been prepared by hydrothermal treatment using tetraethylammonium hydroxide as organic structure-directing agent (OSDA). However, the synthesis method using OSDA has economic and environmental disadvantages. Recently, a seed-assisted method for *BEA synthesis without using OSDA was reported9,10.
*BEA is an intergrowth crystal of polymorph A and polymorph B. Thereby, "*" represents an intergrowth material. At present, no bulk materials consisting only of polymorph A or B is known.
We have successfully prepared *BEA membranes without using OSDA by a modified seed-assisted method11. The *BEA membrane had very few defects and exhibited high separation performance for hydrocarbons due to its molecular sieving effect. It is well known that calcination to remove OSDA after synthesis is one of the most common causes of defect formation in zeolite membranes12,13. Our *BEA membrane prepared without using OSDA showed good separation performance possibly because this calcination step was skipped.
The preparation of zeolite membranes is based on know-how and experience accumulated in the laboratory. Consequently, it is difficult for a beginner to synthesize zeolite membranes alone. Here, we would like to share a protocol for *BEA membrane preparation as a reference for everyone who wants to start membrane synthesis.
Protocol
1. Support preparation
- Pretreatment of support
- Cut out a 3 cm long tubular porous α-Al2O3 support (see Table of Materials).
- Wash the support with distilled water for 10 min. After that, wash the support with acetone for 10 min. Repeat this washing process 2x.
NOTE: Do not touch the outer surface of a support after the washing step. No other treatment was carried out (e.g., sonication, and rubbing by sandpaper, etc.) - Dry the washed support at 110 °C overnight prior to use for dip-coating.
NOTE: Measure the weight of the support piece after drying. The final membrane weight is calculated by the difference in the support weight before and after membrane synthesis.
2. *BEA seed crystal synthesis
- Preparation of seed crystal synthesis gel
- Add 26.2 g of colloidal silica (see Table of Materials) and 8.39 g of tetraethylammonium hydroxide (TEAOH) (see Table of Materials) into a 250 mL bottle made of polypropylene (Solution A). Stir the mixture using a magnetic stirrer for 20 min in a 50 °C in water bath. After that, stir the mixture using a magnetic stirrer for 20 min at room temperature.
- Add 8.39 g of TEAOH, 5.79 g of distilled water, 1.08 g of NaOH (see Table of Materials), and 0.186 g of NaAlO2 (see Table of Materials) into a Teflon beaker (Solution B). Stir the mixture by using a magnetic stirrer for 20 min at room temperature.
- Add solution B to solution A in the 250 mL bottle. The mixture of solution A and B will become milky. Cap the bottle and shake it vigorously by hand for 5 min. After that, stir the mixture using a magnetic stirrer for 24 h at room temperature. The gel obtained after 24 h of stirring is called the synthesis gel.
NOTE: The milky solution cannot be mixed with a magnetic stirrer at first because it forms a hard gel. The 5 min shaking by hand makes the milky solution soft and allows stirring with a magnetic stirrer. The final composition of the synthesis gel is 24Na2O: 1Al2O3: 200SiO2: 60TEAOH: 2905H2O.
- Crystallization
- Pour the synthesis gel into a Teflon-lined autoclave. Place the autoclave in an air oven at 100 °C for 7 days.
- Quenching
- Quench the autoclave with flowing water for 30 min after crystallization.
- Filtration
- Remove the white sediment in the autoclave by filtration. Wash the white sediment with 200 mL of boiling water to remove amorphous and uncrystallized materials. Dry the washed sediment at 110 °C overnight. The dried sediment is the seed crystal.
NOTE: A 200 nm mesh filter (see Table of Materials) was used to obtain the crystal. The Si/Al ratio of obtained crystal was ~19 as analyzed by energy dispersive X-ray spectrometry (EDX). About 2.3 g of seed crystal was obtained by a single synthesis. The preparation procedure refers to a previous report by Schoeman et al. with some modifications14.
- Remove the white sediment in the autoclave by filtration. Wash the white sediment with 200 mL of boiling water to remove amorphous and uncrystallized materials. Dry the washed sediment at 110 °C overnight. The dried sediment is the seed crystal.
- *BEA seed slurry preparation for dip-coating
- Add 0.50 g of seed crystals into 100 mL of distilled water to prepare a 5 g/L seed slurry. Carry out sonication of the seed slurry for 1 h to disperse the seed crystals.
3. Seeding on support by dip-coating
- Set up a support for the dip-coating equipment.
- Fix a tubular support with a stainless steel rod using Teflon tape to plug the inside of the support.
- Dip-coating
- Pour the seed slurry into a glass tube with a 19 mm diameter. Immerse the fixed support into the poured seed slurry and wait for 1 min. After that, withdraw the seed slurry vertically at ~3 cm/s. Dry the support at 70 °C for 2 h after dip-coating.
NOTE: The dip-coating process shown in 3.2.1 was run 2x. This protocol uses homemade equipment for dip-coating. One side of glass tube is plugged with a silicon cap with a tap from which the seed slurry can be withdrawn. The details about the dip-coating equipment are provided in the video.
- Pour the seed slurry into a glass tube with a 19 mm diameter. Immerse the fixed support into the poured seed slurry and wait for 1 min. After that, withdraw the seed slurry vertically at ~3 cm/s. Dry the support at 70 °C for 2 h after dip-coating.
- 3. Calcination
- Calcine the dip-coated support at 530 °C for 6 h.
NOTE: The calcination step was carried out to remove OSDA blocking the micropores of the seed crystals and to chemically bind the seeds onto the support surface. The increase and decrease temperature rates of the calcination step were 50 °C/min.
- Calcine the dip-coated support at 530 °C for 6 h.
- Measuring the weight of the seed crystal on the support
- After calcination, measure the weight of the support. The amount of seed crystal loaded is calculated by the difference in the support weight before and after dip-coating.
NOTE: The average weight of seeded crystal loaded on a support is ~17 mg.
- After calcination, measure the weight of the support. The amount of seed crystal loaded is calculated by the difference in the support weight before and after dip-coating.
4. *BEA membrane preparation by a secondary growth method
- Preparation for gel synthesis
- Add 92.9 g of distilled water, 9.39 g of NaOH, and 1.15 g of NaAl2O into a 250 mL polypropylene bottle. Stir the mixture using a magnetic stirrer for 30 min at 60 °C in a water bath. After that, add 81.6 g of colloidal silica in a stepwise manner into the mixture. Stir the mixture by using a magnetic stirrer for 4 h at 60 °C in a water bath. The gel that is obtained after stirring for 4 h is called the synthesis gel.
NOTE: Colloidal silica was slowly added at the rate of one drop (~0.05 g) per second. The final composition of the synthesis gel is 30Na2O: 1Al2O3: 100SiO2: 2000H2O. The preparation procedure of the synthesis gel is based on Kamimura et al. with some modifications9.
- Add 92.9 g of distilled water, 9.39 g of NaOH, and 1.15 g of NaAl2O into a 250 mL polypropylene bottle. Stir the mixture using a magnetic stirrer for 30 min at 60 °C in a water bath. After that, add 81.6 g of colloidal silica in a stepwise manner into the mixture. Stir the mixture by using a magnetic stirrer for 4 h at 60 °C in a water bath. The gel that is obtained after stirring for 4 h is called the synthesis gel.
- Crystallization
- Pour the synthesis gel into a Teflon lined autoclave in which the seeded support is placed vertically. The autoclave is placed in an air oven at 120 °C for 7 days.
- Quenching
- Quench the autoclave with flowing water for 30 min after crystallization.
- Washing and drying
- Wash the membrane in boiling water for 8 h and dry overnight. This is the *BEA membrane.
- Measuring the weight of the membrane
- After drying, measure the weight of the prepared membrane. The weight of the membrane is calculated by the difference in support weight before and after crystallization.
NOTE: The average weight of the *BEA membrane on each support is ~74 mg.
- After drying, measure the weight of the prepared membrane. The weight of the membrane is calculated by the difference in support weight before and after crystallization.
Representative Results
Figure 1 shows the preparation procedure of the *BEA seed crystal. Figure 2 shows the X-ray diffraction (XRD) pattern of synthesized *BEA seed crystal. Typical strong reflection peaks of (101) and (302) around 2q = 7.7 and 22.1° appeared. In addition, no obvious reflection peaks other than the *BEA-type zeolite were observed. These results showed that the pure phase of *BEA zeolite was successfully synthesized.
A typical FE-SEM image of the synthesized seed crystal is shown in Figure 3. Spherical seed crystals were observed and their size was uniformly ~200 nm. The Si/Al ratio of the obtained crystals was ~19 when analyzed by EDX.
Figure 4 and Figure 5 show the procedures of dip-coating and membrane preparation, respectively. Figure 6 shows the XRD pattern of synthesized *BEA membrane. As in the case of the seed crystals, typical strong reflection peaks of (101) and (302) around 2q = 7.7 and 22.1° appeared. In addition, reflection peaks of α-Al2O3 as support around 2q = 26, 35.5, and 38° were observed. As a result, we were able to confirm that the pure phase of *BEA was obtained as a membrane.
A typical field emission scanning electron microscope (FE-SEM) image of the synthesized membrane is shown in Figure 7. Crystals having truncated octahedral morphology uniformly covered the support surface. The distinct morphology seems to be very similar to typical *BEA crystals synthesized by the OSDA-free method previously reported9,10,15. The Si/Al ratio of the obtained membrane was ~5.1 analyzed by EDX.
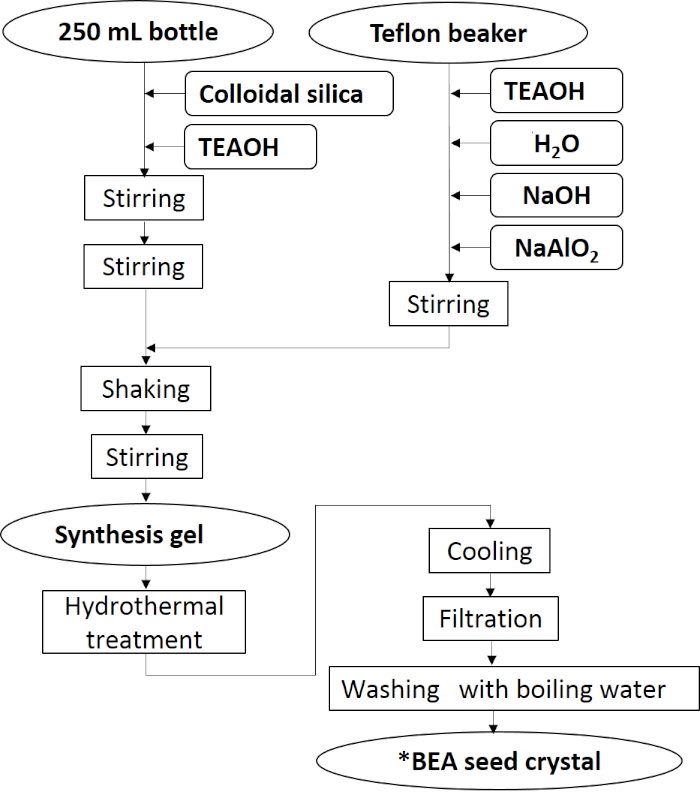
Figure 1: Preparation procedure of *BEA seed crystal. *BEA seed crystal was synthesized by typical hydrothermal treatment using OSDA. Please click here to view a larger version of this figure.
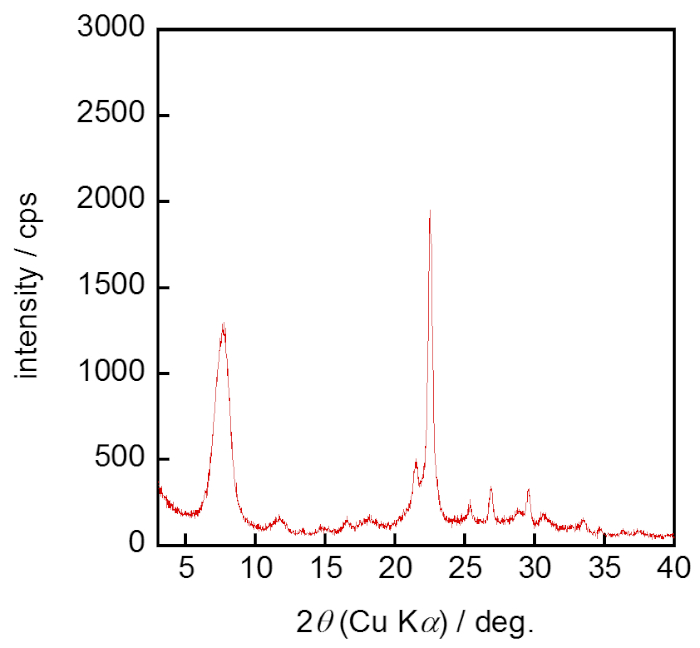
Figure 2: XRD pattern of *BEA seed crystals. The crystal phase of the sediment obtained was confirmed with the XRD pattern. Please click here to view a larger version of this figure.
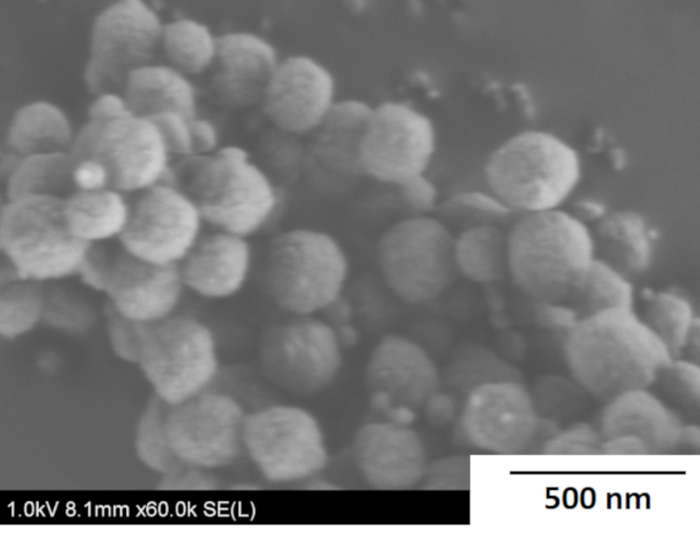
Figure 3: Typical FE-SEM image of seed crystals. Microscopic analysis was carried out to estimate the size of the seed crystals. Please click here to view a larger version of this figure.
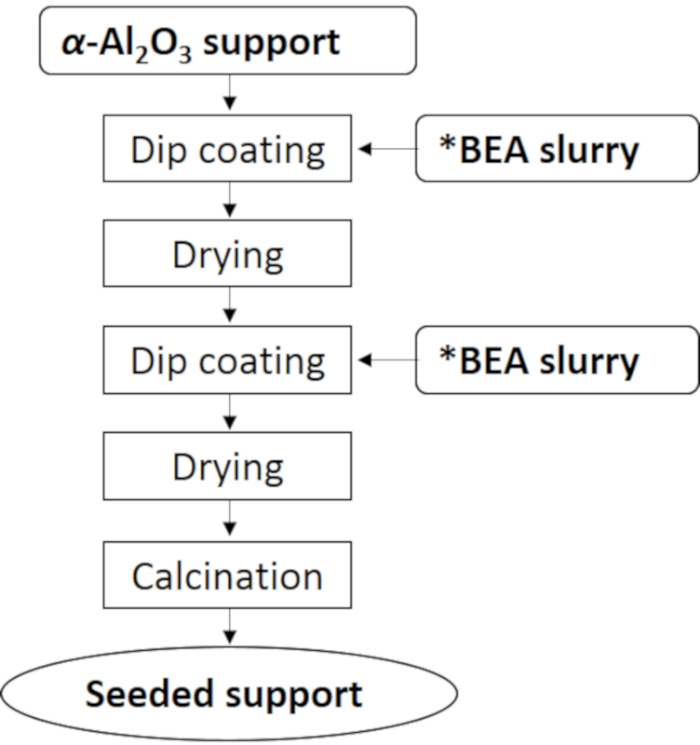
Figure 4: Dip-coating procedure. Seed crystals were loaded by the dip-coating method using the seed slurry. Please click here to view a larger version of this figure.
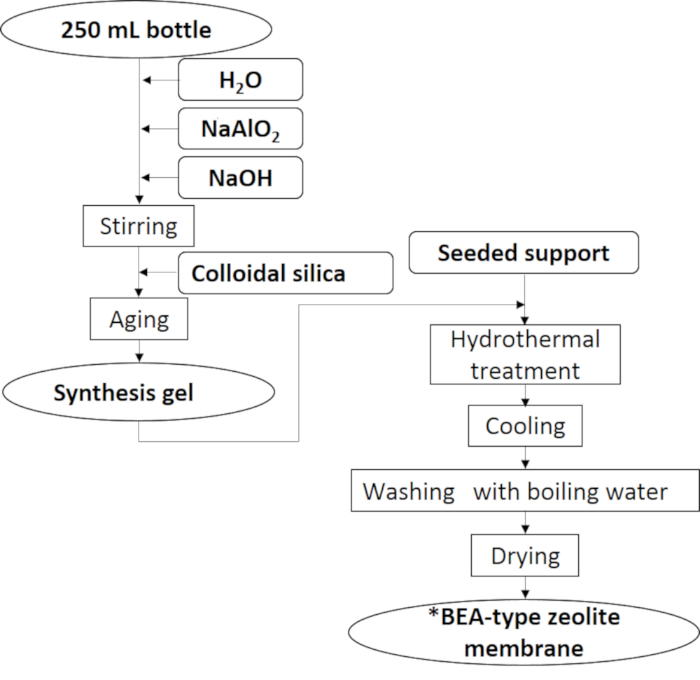
Figure 5: Preparation procedure of the *BEA membrane. The *BEA membrane was synthesized by the secondary growth method without using OSDA. Please click here to view a larger version of this figure.
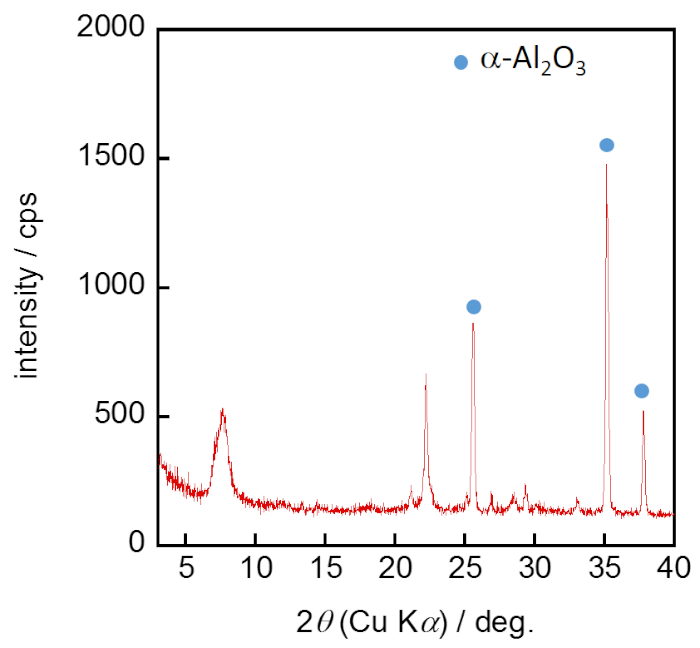
Figure 6: XRD pattern of the *BEA membrane. The crystal phase of the membrane obtained was confirmed from the XRD pattern. Please click here to view a larger version of this figure.
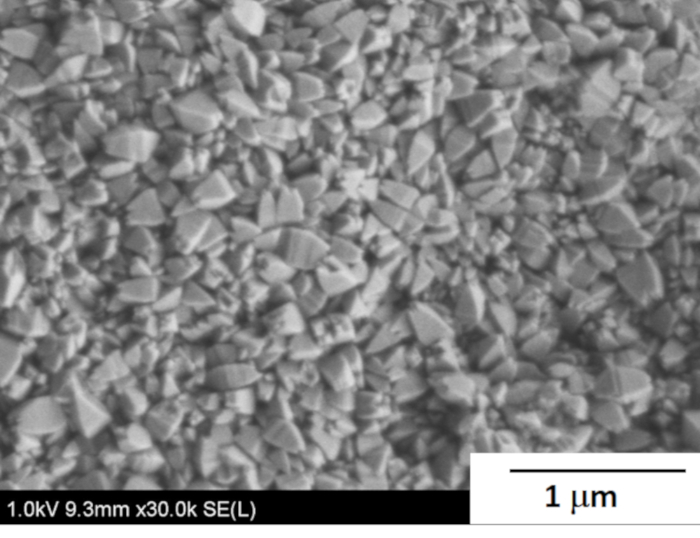
Figure 7: Typical FE-SEM image of the *BEA membrane. Microscopic analysis was carried out to investigate the membrane thickness and crystal morphology. Please click here to view a larger version of this figure.
Discussion
There are many kinds of Si and Al sources for zeolite synthesis. However, we cannot change raw materials for preparation of this *BEA-type membrane. If raw materials are changed, the phase of zeolite crystallized and/or growth rate may be changed.
Glass beakers cannot be used for synthesis gel preparation because the synthesis gel has high alkalinity. Bottles and beakers made of polyethylene, polypropylene, and Teflon can be used instead.
To prepare a higher quality *BEA membrane, uniform seed layer on the outer surface of tubular support is essential. The size of the seed crystals and their distribution are quite important to form a uniform seed layer by dip-coating. The required seed size is larger than that of the pore size of the support (150 nm) to stop the seed crystal from spreading into the support. In addition, a narrow distribution of the seed size is also required to prepare a uniform seed layer.
Crystallization conditions for membrane preparation such as temperatures and time periods are quite important. Changing the crystallization conditions easily shifts the phase of zeolite crystalized. Higher temperatures and longer time periods lead to crystallization of the MOR-type zeolite. If the MOR-type zeolite co-crystallizes in the *BEA membrane, large spherical crystal can be observed on the surface by microscopic observation.
Successfully synthesized *BEA membrane has very few defects and can be used for hydrocarbon separation11.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was partially supported by JST CREST (Japan Science and Technology agency, Create REvolutionary technological seeds for Science and Technology innovation program), Grant Number JPMJCR1324, Japan.
Materials
| a-Al2O3 support | Noritake Co. Ltd. | NS-1 | Average pore size, 150 nm; Outer diameter, 10 mm; Innar diameter, 7 mm |
| Colloidal silica | Nissan Chemical | ST-S | SiO2 30.5%, Na2O 0.44%, H2O 69.1% |
| Mesh filter (PTFE membrane) | Omnipore | JGWP04700 | Pore size, 200 nm |
| NaAl2O | Kanto Chemical | 34095-01 | Na2O 31.0-35.0%; Al2O3 34.0-39.0% |
| NaOH | Kanto Chemical | 37184-00 | 97% |
| Tetraethylammonium hydroxide | Sigma-Aldrich | 302929-500ML | 35 wt% solution |
References
- Ghaffour, N., Missimer, T. M., Amy, G. L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination. 309, 197-207 (2013).
- Hickenbottom, K. L., et al. Forward osmosis treatment of drilling mud and fracturing wastewater from oil and gas operations. Desalination. 312, 60-66 (2013).
- Kanezashi, M., Shazwani, W. N., Yoshioka, T., Tsuru, T. Separation of propylene/propane binary mixtures by bis(triethoxysilyl) methane (BTESM)-derived silica membranes fabricated at different calcination temperatures. Journal of Membrane Science. 415-416, 478-485 (2012).
- Xu, L., Rungta, M., Koros, W. J. Matrimid® derived carbon molecular sieve hollow fiber membranes for ethylene/ethane separation. Journal of Membrane Science. 380, 138-147 (2011).
- Morigami, Y., Kondo, M., Abe, J., Kita, H., Okamoto, K. The first large-scale pervaporation plant using tubular-type module with zeolite NaA membrane. Separation and Purification Technology. 25, 251-260 (2001).
- Kondo, M., Komori, M., Kita, H., Okamoto, K. Tubular-type pervaporation module with zeolite NaA membrane. Journal of Membrane Science. 133, 133-141 (1997).
- Hoof, V. V., Dotremont, C., Buekenhoudt, A. Performance of Mitsui NaA type zeolite membranes for the dehydration of organic solvents in comparison with commercial polymeric pervaporation membranes. Separation and Purification Technology. 48, 304-309 (2006).
- Kamimura, Y., Chaikittisilp, W., Itabashi, K., Shimojima, A., Okubo, T. Critical Factors in the Seed-Assisted Synthesis of Zeolite Beta and “Green Beta” from OSDA-Free Na+-Aluminosilicate Gels. Chemistry An Asian Journal. 5, 2182-2191 (2010).
- Majano, G., Delmotte, L., Valtchev, V., Mintova, S. Al-Rich Zeolite Beta by Seeding in the Absence of Organic Template. Chemistry of Materials. 21, 4184-4191 (2009).
- Sakai, M., et al. Formation process of *BEA-type zeolite membrane under OSDA-free conditions and its separation property. Microporous and Mesoporous Materials. 284, 360-365 (2019).
- Choi, J., et al. Grain Boundary Defect Elimination in a Zeolite Membrane by Rapid Thermal Processing. Science. 325, 590-593 (2009).
- Dong, J., Lin, Y. S., Hu, M. Z. -. C., Peascoe, R. A., Payzant, E. A. Template-removal-associated microstructural development of porous-ceramic-supported MFI zeolite membranes. Microporous and Mesoporous Materials. 34, 241-253 (2000).
- Schoeman, B. J., Babouchkina, E., Mintova, S., Valtchev, V. P., Sterte, J. The Synthesis of Discrete Colloidal Crystals of Zeolite Beta and their Application in the Preparation of Thin Microporous Films. Journal of Porous Materials. 8, 13-22 (2001).
- Sasaki, Y., et al. Polytype distributions in low-defect zeolite beta crystals synthesized without an organic structure-directing agent. Microporous and Mesoporous Materials. 225, 210-215 (2016).

